3.1. Forage Brassica N and S Effect on Leaf, Stem, and Root Yield
Kale and swede forage biomass yield (leaf and root/stem) increased linearly up to 200 kg N ha
−1, indicating that these species could actually have a response to greater N rates. The analysis of variance combined across three environments for Prosper in 2012 and 2014 and Walcott 2014, of leaf, root/stem, total biomass yield (leaf + root/stem), dead matter yield, total biomass yield, and total N concentration are presented in
Table 3. The triple interaction of species by N by S was significant for root/stem yield and leaf + root/stem yield (
Table 3). The double interactions N by S, S by species, and N by species were not significant for any parameter. The S main effect was not significant. Species main effect was significant for all parameters except leaf yield, total leaf N, and total root/stem N. The N main effect was significant for all parameters evaluated (
Table 3).
Leaf yield of kale and swede averaged across fertilization rates and environments ranged between 3.10 and 3.32 Mg ha
−1 respectively. Leaf yield across species, S rates and environments increased from 2.50 to 3.87 Mg ha
−1 with 0 to 200 kg N ha
−1 rates (
Table 4). Root/stem yield was much higher than leaf yield with a stronger response to N than leaf yield (
Table 4). Leaf area in swede ranged between was 27–32% of the total plant biomass without considering dead mass. In kale, leaf area ranged between 46–54%. Even dead matter accumulated during the season responded to N rates. This was likely due to plants with higher N rates had more leaves dying due to shading from leaves above.
The triple interaction N by S by species was mainly due to magnitude difference between swede and kale yields, rather than a true interaction of treatments; however, this significant interaction seems to indicate that S fertilization might reduce the need of N fertilization in swede, but not in kale (
Table 5). Swede had higher total root/stem yield and leaf + root/stem yield than kale for all N and S rates (
Table 5). This is explained because in swede the biomass from the enlarged root is much greater than that of kale stems. Swedes root yield is about 2/3 of the total plant biomass while in kale stem and leaf yield are similar. Root/stem yield in swede was highest with the 200 kg N ha
−1 rate and no S application. When sulfur was applied, highest root/stem yield was obtained at 150 kg N ha
−1, however, it was not significantly different than the 200 kg N ha
−1 rate. In kale, N did not increase root/stem yield regardless of sulfur rate. For the leaf + root/stem yield, both species had the highest yield with the 200 kg N ha
−1 rate. The addition of sulfur in swede produced the highest root/stem yield and leaf + root/stem yield with the 150 kg N ha
−1 rate, which might indicate that in the presence of S less N fertilizer might be needed to achieve same total forage yield goal.
The strong response of both species to N was observed across S rates (
Table 3). Most of the significant responses in root/stem yield and leaf + root/stem yield were between 0 kg N ha
−1 and 200 kg N ha
−1. Forage brassicas often respond strongly to N but seldom to S [
1,
6].
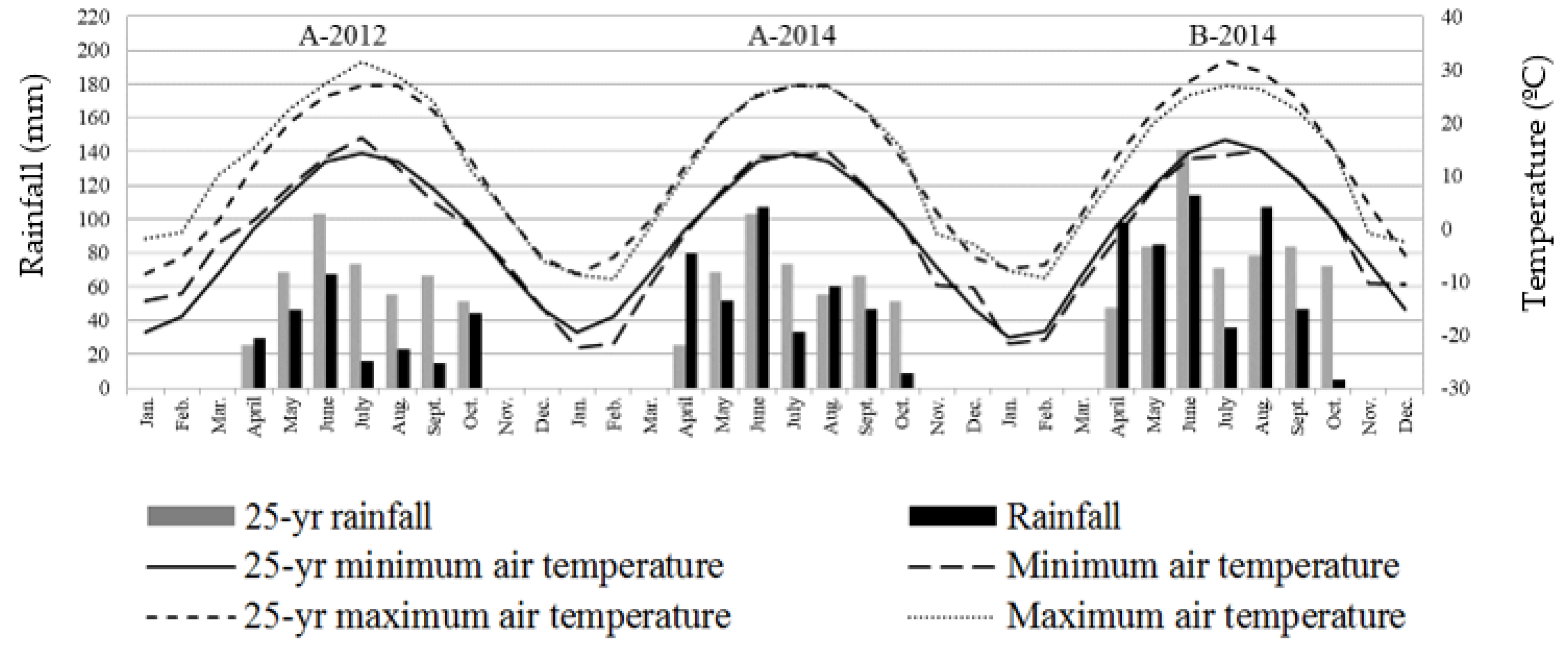
Leaf, stem and root yield of both crops were lower in Prosper in 2012 (data not shown). The most critical rainfall deficiency was in Prosper in 2012, with only 242 mm of rainfall during the growing season, compared with 445 mm of 25-yearrainfall average (
Figure 1). The rainfall in Walcott was slightly below the 25-year historical average, with 493 and 578 mm of rainfall during the growing season. In general, the rainfall between sowing and emergence time was adequate, with the lowest amount of rainfall (46.2 mm) in May, in Prosper in 2012 (
Figure 1).
The minimum and maximum temperature recorded during 2012 and 2014 were relatively similar to the 25-year average (
Figure 1). The highest minimum and maximum temperatures were observed in July and August, and the lowest minimum and maximum between December and February. The hottest summers were observed in 2012 in Prosper, with about 2.7 °C above average. The 2014 summer was similar to the average temperature in Prosper. In Walcott, the summer was the coolest, with 3 °C below average. These temperatures are adequate for plant growth of
Brassica spp. [
2].
The climatic parameters are highly important on forage brassica performance. Growing degree days (GDD, base temperature 0 °C) to maturity have a major effect on forage brassica biomass yield potential [
2]. Forage brassicas, although cool-season crops, are highly tolerant to heat, but heat in general speeds up reproductive development. High dry matter production with temperatures of about 32 °C have been reported [
37]. Forage brassicas accumulate about 1.1 Mg DM ha
−1 per each 100 GDD, base temperature 0 °C and maximum temperature 32 °C, without soil water constraints nor fertility [
2].
Water availability is the main environmental source of forage yield variation in brassica forages [
2], and water deficits during the growing season reduce forage yield [
38]. Swede needs 38 mm H
2O week
−1 during the season to maximize root yield [
39]. Kale and rape have more vigorous root systems than swede and turnip and, therefore, they can utilize stored water more efficiently [
2].
Regression analysis of yield response to N was conducted on relative values to account for magnitude differences among environments. Leaf and root/stem yield, leaf + root/stem) dead matter yield and total biomass yield (leaf + root/stem + dead matter biomass yield) showed linear response with increasing N rates (0, 50, 100, 150, and 200 kg N ha
−1) (
Figure 2;
Figure 3). These increments in forage yield with increased N rates are in accord with Chakwizira et al. [
41], who reported a linear response in forage yield of brassicas to N rates up to 200 kg N ha
−1. Other authors also reported increments in yield with increasing N rates [
42,
43]; however, these authors found quadratic response instead of the linear response observed in the present study. In most of the cases, the difference in biomass yield between the highest N rate (200 kg N ha
−1) and the lowest (0 kg N ha
−1) was between 15% for dead matter yield and 23% for leaf yield.
3.2. Response of N and S Fertilization on N Accumulation
As for total forage yield, a strong interaction of N by S by species was observed for total root/stem N and total forage N accumulation, which was likely due to a magnitude effect. Total N accumulation was greater in swede roots than in kale stems at all N and S rates (
Table 6). This is likely related to the higher root yield in swedes compared with kale stems. Nitrogen accumulation increased as N rates increased. Nitrogen accumulation in root/stem and in total forage was significantly greater with S application only for the 150 kg ha
−1 rate (
Table 6). This might indicate that S interacted with N accumulation. Plants might accumulate more N in the presence of both nutrients, rather than just high N rates. In kale, the interaction of N by S was not significant for any N rate. Application of N fertilizer can lead to accumulation of nitrate (NO
3-N) in forage brassicas, particularly when N application rates exceed the requirement [
7]. This may result in potentially toxic NO
3-N (antinutritional compounds) content in grazeable plant tissues, leading to animal health issues and/or environmental pollution [
44].
Forage kale and rape (
Brassica napus L.), generally have higher NO
3-N concentration than turnip and swede. This is because roots, the major yield component of swede, have lower NO
3-N content than stems, which make up the bulk of yield in kale and rape [
7]. Additionally, Chakwizira et al. [
41] reported that NO
3-N contents were higher in kale stems and petioles (which included the midrib of the leaf) than in leaves. In a survey conducted in Nebraska, they determined that 48% of fresh brassica samples were considered at risk for causing nitrate toxicity as they exceeded the 2100 mg NO
3/kg DM threshold [
45].
On average, differences in total N between the highest and lowest N rate was higher in leaves (33%) than in roots and stems (20%). The trend observed in N as main effect could be explained because brassica forages respond to N fertilization up to 400 kg N ha
−1 [
23,
44]. The available N for forage brassicas (swede and kale) in the present experiment could have limited the growth to reach the maximum potential yield, thus brassicas showed a lineal response instead of typical quadratic response observed in experiments with different N rates [
44]. Another important factor that could explain the observed response to N fertilization is the soil N content. Prosper 2012 and Walcott 2014 had 44 and 31 kg ha
−1 of N-NO
3 in the soil at 0–60 cm (
Table 2). The soil N-NO
3 plus the highest N rate used in the present experiment would have been less than 250 kg N ha
−1 available for brassica growth, in these two environments (
Table 2), which is less than the recommendations of Chakwizira et al. [
45]. Conversely, Wilson and Manley [
46] did not find a response to N in kale because the NO
3-N content of the soil was high. According to Fletcher et al. [
47], kale can have null response to N in soils with high NO
3-N; conversely, in low N soils the response is usually significant. However, is important to know that forage brassicas can take up water and nutrients from 0.9 to 1 m deep [
6,
7]. Thus, the N available for plant growth could have been greater than 250 kg N ha
−1. Additionally, Vos and van der Putten [
48] reported that the response to N fertilizer in brassicas depends on the amount of residual and mineralized N in the soil, which is influenced by the cropping history.
Nitrogen response is also related with the water availability. The response can be greater when irrigation is used or at least adequate rainfall occurs during the growing season [
23,
49]. Brassicas must have at least 500 mm of water to avoid yield reduction by water deficit [
46]. Fletcher et al. [
6] mentioned that drought conditions of 100 mm rainfall produce half of yield compared with full irrigation (328 mm). However, in the environments evaluated in the present experiment the rainfall was low (210, 267, and 251 mm of rainfall in Prosper 2012 and 2014 and Walcott 2014, respectively) (
Figure 1). This agrees with de Ruiter et al. [
2], who reported water availability is the main environmental source of forage yield variation in brassicas.
Forage yield was composed of 31% leaves and 58% dead matter at the lowest N rate, but with the highest N rate applied, the percentage of both leaves and dead matter increased (
Figure 2 and
Figure 3), respectively. That probably occurred; because with limited N, the leaf area declined faster as leaves senesced mobilizing N to growing points and new leaves [
46]. The relative total N was higher for leaves than for root/stem, and as N rates increased total N increased. The total N rate was greater in leaves which can be observed in
Figure 4. These results are in accord with the information provided by Chakwizira et al. [
41], and Wilson and Maley [
46].
3.3. Forage Nutritive Value
Forage nutritive value of leaves was not affected by N or S rates or species main effect but there was a significant interaction between species and S for crude protein (CP) and acid detergent lignin (ADL) (
Table 7). Crude protein was greater in swede fertilized with 40 kg S ha
−1 compared with kale at 40 kg S ha
−1 (
Table 8). Crude protein concentration in kale and swede leaves ranged from 95 to 138 g kg
−1. Crude protein concentration in swede and kale in this study were above those needed by beef cattle. A gestating cow in the mid-1/3 of pregnancy and weighing 540 kg requires 10 kg of dry matter intake with 71 g kg
−1 of crude protein [
50] Several studies have reported that CP increases in leaves and stems with N fertilization but the response depends on N fertilization and weather conditions [
43,
51]. Higher CP can be explained by higher leaf:stem ratio which is promoted by N availability [
51]. Conversely, other authors did not find effect of increasing N rates on leaf and root yield of turnip [
5]. The lack of response of forage nutritive value to N in this study was likely due to high soil N or high mineralization rate of residues of the previous crop.
Forage brassicas are more succulent and higher in nutritive value than almost any other type of forage [
37] and provide forage when the majority of warm- and cool-season grasses and legumes are unproductive [
52,
53,
54,
55]. However, the nutritional content of brassica crops is variable and depends on environment but also of the degree of maturity of the plant at harvest time [
56]. Forage brassicas are highly productive, digestible forbs that contain relatively high levels of CP and digestible carbohydrates [
57]. Crude protein concentration in leaves was higher in swede than in kale at 40 kg S ha
−1 (
p ≤ 0.05) (
Table 8). Crude protein values in forage brassicas ranges from 130 to 280 g kg
−1 varying with species, harvest maturity, and environment [
10,
58,
59]. Crude protein content in shoots and roots depends on the cultivars used [
53]. Additionally, N fertilization and weather conditions influence CP content [
60]. The null response to increasing N rates on CP in this study can be explained by soil water deficiency because 2012 was a very dry environment (
Figure 1) or due to the high N content in the soils used in these experiments, mainly in Prosper 2014 (
Table 2).
Acid detergent lignin in leaves tended to be higher (
p < 0.05) with 40 kg S ha
−1 rates than 0 without S fertilization, and also in kale compared with swede (
Table 8). However, ADL was statistically higher in swede with no S applied compared with ADL in kale with 40 kg S ha
−1. These results agree with Jung et al. [
43] and Kunelius at al. [
61], who mentioned that kale usually has lower digestibility and higher fiber, due to their longer and fibrous stems.
Total digestible nutrients were higher (
p < 0.05) in swede than kale, at both S rates averaged across all N rates (
Table 8). Kale had a TDN of 700–710 g kg
−1 compared with 800 g kg
−1 TDN in swede. This can be explained by the higher concentration of water soluble carbohydrates in swede compared with kale. Additionally, kale usually has lower dry matter digestibility than other forage brassicas due to their longer and fibrous stems [
42,
61]. The N by S interaction was significant for NDF, ADF, ADL, NDFD, and TDN for root/stems averaged across species and environments (
Table 9). Fiber components, NDF and ADF, were lower when only S fertilizer was applied compared with no S or N fertilizer application. Oppositely, at the highest N rate, NDF and ADF were higher when S was applied than without application. In addition, fiber digestibility and TDN were significantly higher when S was applied in the absence of N fertilizer compared with no S application (
Table 10). At higher N rates, plants grow faster and taller changing the proportion of root/stems and leaves. Fiber digestibility is higher in leaves than in stems and roots. Without N fertilization, S fertilizer application (40 kg S ha
−1) reduced NDF, ADF, and ADL content increasing digestibility and TDN in comparison to no S application. Sulfur, similarly to N, has a role in protein synthesis, including many enzymes in cell wall components synthesis. Sulfur, likely substituted N functions in part when N was a limiting factor, allowing the plant to mobilize carbon resources away from fiber synthesis and secondary pathways leading to lignin formation. In addition sulfur mobility in the plant is limited, thus in the treatment with no N or S application, sulfur could not complement N functions [
13,
14].