Thermal Time as a Parameter to Determine Optimal Defoliation Frequency of Perennial Ryegrass (Lolium perenne L.) and Pasture Brome (Bromus valdivianus Phil.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Evaluated Variables and Sampling
2.4. Pasture Nutritive Value
2.5. Statistical Analysis
3. Results
3.1. Growth Dynamics
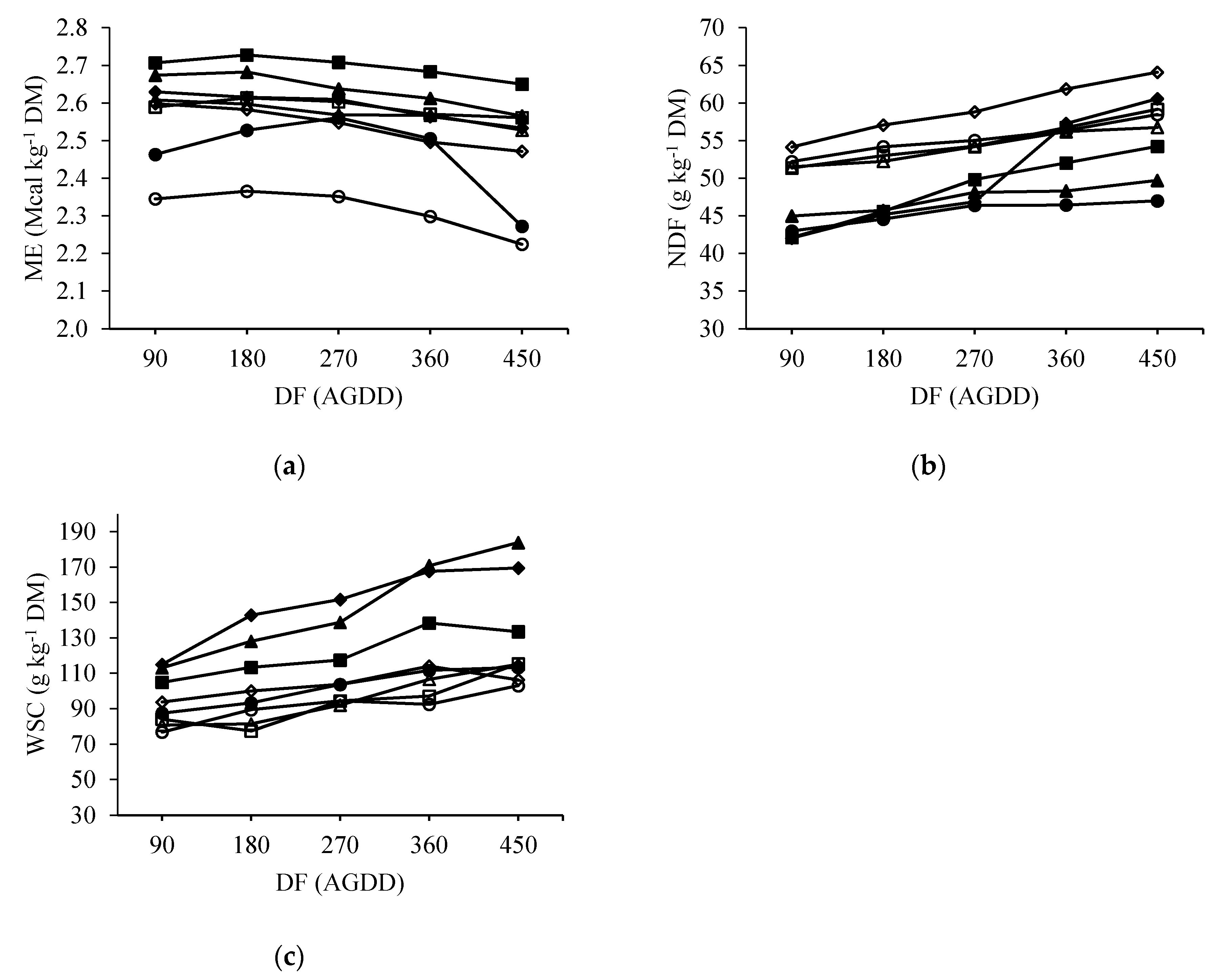
3.2. Nutritive Value
4. Discussion
4.1. Growth Dynamics
4.2. Nutritive Values
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Balocchi, O.A.; Solis, C.; Poff, J. Filocrono en una pradera de Lolium perenne L.: Efecto de la frecuencia de defoliación y fertilización nitrogenada. Agro Sur 2011, 39, 165–176. [Google Scholar] [CrossRef]
- Donaghy, D.J.; Turner, L.R.; Adamczewski, K.A. Effect of defoliation management on water-soluble carbohydrate energy reserves, dry matter yields, and herbage quality of tall fescue. Agron. J. 2008, 100, 122–127. [Google Scholar] [CrossRef]
- Acharán, F.; Balocchi, O.A.; López, I.F. Filocrono, producción de fitomasa y calidad nutritiva de una pradera de Lolium perenne L./Trifolium repens L. sometida a tres frecuencias e intensidades de defoliación. Agro Sur 2009, 37, 81–90. [Google Scholar]
- Wilhelm, W.; McMaster, G.S. Symposium on the phyllochron: Importance of the phyllochron in studying development and growth in grasses. Crop Sci. 1995, 35, 1–3. [Google Scholar] [CrossRef]
- Zaka, S.; Ahmed, L.Q.; Escobar-Gutiérrez, A.J.; Gastal, F.; Julier, B.; Louarn, G. How variable are non-linear developmental responses to temperature in two perennial forage species? Agric. For. Meteorol. 2017, 232, 433–442. [Google Scholar] [CrossRef]
- DeJong, T.M.; Da Silva, D.; Vos, J.; Escobar-Gutiérrez, A.J. Using functional–structural plant models to study, understand and integrate plant development and ecophysiology. Ann. Bot. 2011, 108, 987–989. [Google Scholar] [CrossRef]
- Dambreville, A.; Normand, F.; Lauri, P.-É. Plant growth co-ordination in natura: A unique temperature-controlled law among vegetative and reproductive organs in mango. Funct. Plant Biol. 2013, 40, 208–291. [Google Scholar] [CrossRef]
- Poff, J.A.; Balocchi, O.A.; López, I.F. Sward and tiller growth dynamics of Lolium perenne L. as affected by defoliation requency during autumn. Crop Pasture Sci. 2011, 62, 346–354. [Google Scholar] [CrossRef][Green Version]
- Castro, J.; Balocchi, O.A.; Alonso, M. Dinámica de crecimiento y calidad nutritiva de una pradera de Lolium perenne L. bajo diferentes frecuencias de defoliación: Periodo otoño-invierno. Agro Sur 2018, 46, 27–39. [Google Scholar]
- Manandhar, A.; Sinclair, T.R.; Rufty, T.W.; Ghanem, M.E. Leaf emergence (phyllochron index) and leaf expansion response to soil drying in cowpea genotypes. Physiol. Plant. 2017, 160, 201–208. [Google Scholar] [CrossRef]
- Bartholomew, P.W. Effect of varying temperature regime on phyllochron in four warm-season pasture grasses. Agric. Sci. 2014, 5, 1000–1006. [Google Scholar] [CrossRef]
- Davidson, A.; Da Silva, D.; Quintana, B.; DeJong, T.M. The phyllochron of Prunus persica shoots is relatively constant under controlled growth conditions but seasonally increases in the field in ways unrelated to patterns of temperature or radiation. Sci. Hortic. 2015, 184, 106–113. [Google Scholar] [CrossRef]
- Thiesen, L.A.; Diel, M.L.; Pinheiro, M.V.M.; Cocco, C.; Fontana, D.C.; Holz, E.; Caron, B.O.; Schmidt, D. Phyllochron and productive performance of strawberry cultivars: Impact of different regions of origin in a conventional cultivation system. J. Agric. Sci. 2018, 10, 167–178. [Google Scholar] [CrossRef]
- Nagelmuller, S.; Kirchgessner, N.; Yates, S.; Hiltpold, M.; Walter, A. Leaf Length Tracker: A novel approach to analyse leaf elongation close to the thermal limit of growth in the field. J. Exp. Bot. 2016, 67, 1897–1906. [Google Scholar] [CrossRef]
- Bonhomme, R. Bases and limits to using “degree.day” units. Eur. J. Agron. 2000, 13, 1–10. [Google Scholar] [CrossRef]
- Fulkerson, W.; Donaghy, D. Plant-soluble carbohydrate reserves and senescence-key criteria for developing an effective grazing management system for ryegrass-based pastures: A review. Aust. J. Exp. Agric. 2001, 41, 261–275. [Google Scholar] [CrossRef]
- Lee, J.M.; Donaghy, D.J.; Roche, J.R. Effect of defoliation severity on regrowth and nutritive value of perennial ryegrass dominant swards. Agron. J. 2008, 100, 308–314. [Google Scholar] [CrossRef]
- Turner, L.; Donaghy, D.; Lane, L.; Rawnsley, R. Effect of defoliation management, based on leaf stage, on perennial ryegrass (Lolium perenne L.), prairie grass (Bromus willdenowii Kunth.) and cocksfoot (Dactylis glomerata L.) under dryland conditions. 2. Nutritive value. Grass Forage Sci. 2006, 61, 175–181. [Google Scholar] [CrossRef]
- López, I.F.; Kemp, P.D.; Dorner, J.; Descalzi, C.A.; Balocchi, O.A.; García, S. Competitive strategies and growth of neighbouring Bromus valdivianus Phil. and Lolium perenne L. plants under water restriction. J. Agron. Crop Sci. 2013, 199, 449–459. [Google Scholar] [CrossRef]
- Keim, J.P.; López, I.F.; Balocchi, O.A. Sward herbage accumulation and nutritive value as affected by pasture renovation strategy. Grass Forage Sci. 2015, 70, 283–295. [Google Scholar] [CrossRef]
- Ordoñez, I.P.; López, I.F.; Kemp, P.D.; Donaghy, D.J. Pasture brome (Bromus valdivianus) leaf growth physiology: A six-leaf grass species. Agron. N. Z. 2017, 47, 13–22. [Google Scholar]
- CIREN, Centro de Información de Recursos Naturales. Agrological Study X Region, Descriptions of Soils, Materials and Symbols; N° 123; Publication CIREN: Santiago, Chile, 2003. [Google Scholar]
- Biligetu, B.; Coulman, B. Responses of three bromegrass (Bromus) species to defoliation under different growth conditions. Int. J. Agron. 2010. [Google Scholar] [CrossRef]
- McMaster, G.S.; Wilhelm, W. Growing degree-days: One equation, two interpretations. Agric. For. Meteorol. 1997, 87, 291–300. [Google Scholar] [CrossRef]
- Deak, A.; Hall, M.H.; Sanderson, M.A.; Archibald, D.D. Production and nutritive value of grazed simple and complex forage mixtures. Agron. J. 2007, 99, 814–821. [Google Scholar] [CrossRef]
- Slafer, G.A.; Rawson, H. Sensitivity of wheat phasic development to major environmental factors: A re-examination of some assumptions made by physiologists and modellers. Funct. Plant Biol. 1994, 21, 393–426. [Google Scholar] [CrossRef]
- Bork, E.W.; Gabruck, D.T.; McLeod, E.M.; Hall, L.M. Five-year forage dynamics arising from four legume–grass seed mixes. Agron. J. 2017, 109, 2789–2799. [Google Scholar] [CrossRef]
- Amiard, V.; Morvan-Bertrand, A.; Cliquet, J.B.; Billard, J.P.; Huault, C.; Sandström, J.P.; Prud’homme, M.P. Carbohydrate and amino acid composition in phloem sap of Lolium perenne L. before and after defoliation. Can. J. Bot. 2004, 82, 1594–1601. [Google Scholar] [CrossRef]
- Sándor, R.; Picon-Cochard, C.; Martin, R.; Louault, F.; Klumpp, K.; Borras, D.; Bellocchi, G. Plant acclimation to temperature: Developments in the pasture simulation model. Field Crop. Res. 2018, 222, 238–255. [Google Scholar] [CrossRef]
- Alberda, T. The effects of cutting, light intensity and night temperature on growth and soluble carbohydrate content of Lolium perenne L. Plant Soil 1957, 8, 199–230. [Google Scholar] [CrossRef]
- Turner, L.; Donaghy, D.; Lane, P.; Rawnsley, R. Patterns of leaf and root regrowth, and allocation of water-soluble carbohydrate reserves following defoliation of plants of prairie grass (Bromus willdenowii Kunth.). Grass Forage Sci. 2007, 62, 497–506. [Google Scholar] [CrossRef]
- Gatti, M.L.; Ayala, A.T.; Cipriotti, P.A.; Golluscio, R.A. Effect of defoliation frequency and nitrogen fertilization on the production and potential for persistence of Dactylis glomerata sown in multispecies swards. Grass Forage Sci. 2016, 72, 489–501. [Google Scholar] [CrossRef]
- Frankowlindberg, B. Adaptation to winter stress in nine white clover populations: Changes in non-structural carbohydrates during exposure to simulated winter conditions and “spring” regrowth potential. Ann. Bot. 2001, 88, 745–751. [Google Scholar] [CrossRef][Green Version]
- Berone, G.; Agnusdei, M.; Colabelli, M. Evaluación de caracteres asociados al crecimiento invernal en cultivares de Lolium perenne y Bromus stamineus. Rev. Argent. Prod. Anim. 2017, 37, 1–7. [Google Scholar]
- Guy, C.; Kaplan, F.; Kopka, J.; Selbig, J.; Hincha, D.K. Metabolomics of temperature stress. Physiol. Plant. 2008, 132, 220–235. [Google Scholar] [CrossRef]
- Singh, V.; Nguyen, C.B.; van Oosterom, E.J.; Chapman, S.C.; Jordan, D.R.; Hammer, G.L. Sorghum genotypes differ in high temperature responses for seed set. Field Crop. Res. 2015, 171, 32–40. [Google Scholar] [CrossRef]
- Solomon, J.K.Q.; Macoon, B.; Lang, D.J. Harvest management based on leaf stage of a tetraploid vs. a diploid cultivar of annual ryegrass. Grass Forage Sci. 2017, 72, 743–756. [Google Scholar] [CrossRef]
- Oakes, J.; Heiniger, R.; Crozier, C.; Murphy, J.; Wilkerson, J. Phyllochron interval and yield response to planting date and fertility in wheat. CropForage Turfgrass Manag. 2016, 2, 1–9. [Google Scholar] [CrossRef]
- Berone, G.; Lattanzi, F.A.; Agnusdei, M.G.; Bertolotti, N. Growth of individual tillers and tillering rate of Lolium perenne and Bromus stamineus subjected to two defoliation frequencies in winter in Argentina. Grass Forage Sci. 2008, 63, 504–512. [Google Scholar] [CrossRef]
- Chaves, G.G.; Cargnelutti Filho, A.; Alves, B.M.; Lavezo, A.; Wartha, C.A.; Uliana, D.B.; Pezzini, R.V.; Kleinpaul, J.A.; Neu, I.M.M. Phyllochron and leaf appearance rate in oat. J. Appl. Anim. Res. 2018, 76, 73–81. [Google Scholar] [CrossRef]
- Allen, E.; Sheaffer, C.; Martinson, K. Forage nutritive value and preference of cool-season grasses under horse grazing. Agron. J. 2013, 105, 679. [Google Scholar] [CrossRef]
- Techio-Pereira, L.E.; Rodriguez, V.; Avanzi, J.C.; da Silva, S.C. Morphogenetic and structural characteristics of signal grass in response to liming and defoliation severity. Pesqui. Agropecuária Trop. 2018, 48, 1–11. [Google Scholar] [CrossRef]
- Berone, G.; Lattanzi, F.A.; Colabelli, M.; Agnusdei, M. A comparative analysis of the temperature-response of leaf elongation in Bromus stamineus and Lolium perenne plants in the field: Intrinsic and size-mediated effects. Ann. Bot. 2007, 100, 813–820. [Google Scholar] [CrossRef]
- Gastal, F.; Lemaire, G. Defoliation, shoot plasticity, sward structure and herbage utilization in pasture: Review of the underlying ecophysiological processes. Agriculture 2015, 5, 1146–1171. [Google Scholar] [CrossRef]
- Moyer, J.; Sweeney, D. Growth and forage quality responses of smooth bromegrass to nitrogen placement and timing. Agron. J. 2016, 108, 2453–2461. [Google Scholar] [CrossRef]


| DF (AGDD) | TLL (cm) | LER (cm d−1) | LAR (d L−1) | Phyllochron (AGDD L−1) | Accumulated Herbage Mass (kg DM ha−1 Year−1) |
|---|---|---|---|---|---|
| 1 (90) | 36.09 d | 0.49 c | 15.05 | 79.21 | 7576 e |
| 2 (180) | 38.24 cd | 0.51 bc | 15.32 | 79.23 | 8612 d |
| 3 (270) | 46.11 bc | 0.53 bc | 15.97 | 78.67 | 10463 c |
| 4 (360) | 51.24 ab | 0.59 ab | 15.32 | 82.79 | 11432 b |
| 5 (450) | 54.61 a | 0.63 a | 15.87 | 85.96 | 12600 a |
| m.s.e. | 2.055 | 0.020 | 0.611 | 2.320 | 227.264 |
| p-Value | <0.0001 | <0.0001 | 0.1132 | 0.1319 | <0.0001 |
| Sp | |||||
| L. perenne | 38.44 | 0.47 | 16.35 | 87.9 | 9497 |
| B. valdivianus | 52.47 | 0.63 | 14.66 | 74.44 | 10760 |
| m.s.e. | 1.299 | 0.013 | 0.386 | 1.467 | 35.934 |
| p-Value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Ssn | Accumulated Herbage Mass (kg DM ha−1 Ssn−1) | ||||
| Summer | 10.51 d | 0.19 d | 11.20 a | 86.00 ab | 1863 c |
| Autumn | 60.12 b | 0.68 b | 16.80 c | 81.90 b | 2371 b |
| Winter | 42.12 c | 0.45 c | 21.49 d | 72.86 c | 1440 d |
| Spring | 69.08 a | 0.87 a | 12.53 b | 92.87 a | 4454 a |
| m.s.e. | 1.838 | 0.018 | 0.547 | 2.075 | 50.818 |
| p- Value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Interaction | p-Value | ||||
| Ssn × Sp | 0.0063 | <0.0001 | <0.0001 | 0.0821 | <0.0001 |
| Ssn × DF | 0.1256 | <0.0001 | 0.2451 | 0.0504 | <0.0001 |
| Sp × DF | 0.7237 | 0.9235 | 0.9397 | 0.6682 | 0.3845 |
| DF × Sp × Ssn | 0.5962 | 0.7706 | 0.2191 | 0.6141 | 0.4024 |
| DF (AGDD) | CP (g kg−1) | ME (Mcal kg−1 DM) | NDF (g kg−1) | ADF (g kg−1) | WSC (g kg−1 DM) | DM (g kg−1) |
|---|---|---|---|---|---|---|
| 1 (90) | 221.4 a | 2.58 a | 476.7 c | 250.9 c | 94.46 c | 249.4 b |
| 2 (180) | 203.8 a | 2.59 a | 496.9 bc | 267.2 bc | 103.22 c | 235.1 b |
| 3 (270) | 189.0 ab | 2.57 a | 516.9 b | 287.4 b | 112.03 b | 243.9 b |
| 4 (360) | 167.0 b | 2.54 a | 543.9 a | 317.1 a | 124.83 a | 250.6 b |
| 5 (450) | 137.7 c | 2.48 b | 562.5 a | 333.6 a | 129.99 a | 288.0 a |
| m.s.e. | 1.46 | 0.004 | 1.65 | 1.51 | 1.178 | 5.05 |
| p-Value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Sp | ||||||
| L. perenne | 178.3 | 2.60 | 480.0 | 268.9 | 123.90 | 246.7 |
| B. valdivianus | 189.3 | 2.50 | 558.8 | 313.6 | 98.91 | 260.1 |
| m.s.e. | 0.92 | 0.003 | 1.04 | 1.07 | 0.745 | 3.19 |
| p-Value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0037 |
| Ssn | ||||||
| Summer | 186.2 b | 2.39 c | 503.6 b | 293.7 a | 96.55 c | 410.4 a |
| Autumn | 236.8 a | 2.64 a | 518.4 b | 282.5 ab | 107.59 b | 174.0 c |
| Winter | 172.3 b | 2.60 a | 507.8 b | 279.0 b | 121.04 a | 159.9 c |
| Spring | 139.9 c | 2.56 b | 547.9 a | 309.8 a | 126.44 a | 269.4 b |
| m.s.e. | 1.30 | 0.004 | 1.48 | 1.69 | 1.054 | 4.51 |
| p-Value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Interaction | p-Value | |||||
| Ssn × Sp | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0023 |
| Ssn × DF | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Sp × DF | 0.0002 | 0.0002 | <0.0001 | 0.0022 | <0.0001 | 0.3453 |
| Ssn × Sp × DF | 0.0806 | <0.0001 | 0.0002 | 0.0792 | <0.0001 | 0.1996 |
| Nutrient | Ssn/DF | 90 | 180 | 270 | 360 | 450 | m.s.e. | p-Value |
|---|---|---|---|---|---|---|---|---|
| CP (g kg−1) | Autumn | 271.6 a | 255.5 b | 238.9 c | 221.4 d | 196.1 fgh | 2.915 | <0.001 |
| Winter | 216.0 de | 187.5 gh | 169.0 ij | 153.7 jk | 135.3 l | |||
| Spring | 193.6 fgh | 166.2 j | 149.0 kl | 109.9 m | 80.5 m | |||
| Summer | 204.2 ef | 206.1 def | 199.0 fg | 182.9 hi | 138.8 kl | |||
| ADF (g kg−1) | Autumn | 243.4 l | 262.0 ijk | 283.9 fgh | 304.3 de | 318.5 cd | 3.381 | <0.001 |
| Winter | 245.9 kl | 266.1 hij | 277.7 ghi | 299.9 ef | 305.2 de | |||
| Spring | 250.2 jkl | 268.8 hi | 296.0 ef | 355.6 b | 378.2 a | |||
| Summer | 263.9 ijk | 271.7 hi | 291.8 efg | 308.5 de | 332.3 c | |||
| DM (g kg−1) | Autumn | 191.6 e | 153.9 e | 160.8 e | 181.7 e | 181.8 e | 10.092 | <0.001 |
| Winter | 156.7 e | 161.2 e | 157.1 e | 164.4 e | 159.9 e | |||
| Spring | 245.2 d | 245.8 d | 258.3 d | 258.6 d | 338.9 c | |||
| Summer | 404.0 b | 379.3 bc | 399.3 b | 397.6 b | 471.4 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvache, I.; Balocchi, O.; Alonso, M.; Keim, J.P.; F. López, I. Thermal Time as a Parameter to Determine Optimal Defoliation Frequency of Perennial Ryegrass (Lolium perenne L.) and Pasture Brome (Bromus valdivianus Phil.). Agronomy 2020, 10, 620. https://doi.org/10.3390/agronomy10050620
Calvache I, Balocchi O, Alonso M, Keim JP, F. López I. Thermal Time as a Parameter to Determine Optimal Defoliation Frequency of Perennial Ryegrass (Lolium perenne L.) and Pasture Brome (Bromus valdivianus Phil.). Agronomy. 2020; 10(5):620. https://doi.org/10.3390/agronomy10050620
Chicago/Turabian StyleCalvache, Iván, Oscar Balocchi, Máximo Alonso, Juan Pablo Keim, and Ignacio F. López. 2020. "Thermal Time as a Parameter to Determine Optimal Defoliation Frequency of Perennial Ryegrass (Lolium perenne L.) and Pasture Brome (Bromus valdivianus Phil.)" Agronomy 10, no. 5: 620. https://doi.org/10.3390/agronomy10050620
APA StyleCalvache, I., Balocchi, O., Alonso, M., Keim, J. P., & F. López, I. (2020). Thermal Time as a Parameter to Determine Optimal Defoliation Frequency of Perennial Ryegrass (Lolium perenne L.) and Pasture Brome (Bromus valdivianus Phil.). Agronomy, 10(5), 620. https://doi.org/10.3390/agronomy10050620






