Forecasting Yield and Lignocellulosic Composition of Energy Cane Using Unmanned Aerial Systems
Abstract
1. Introduction
2. Materials and Methods
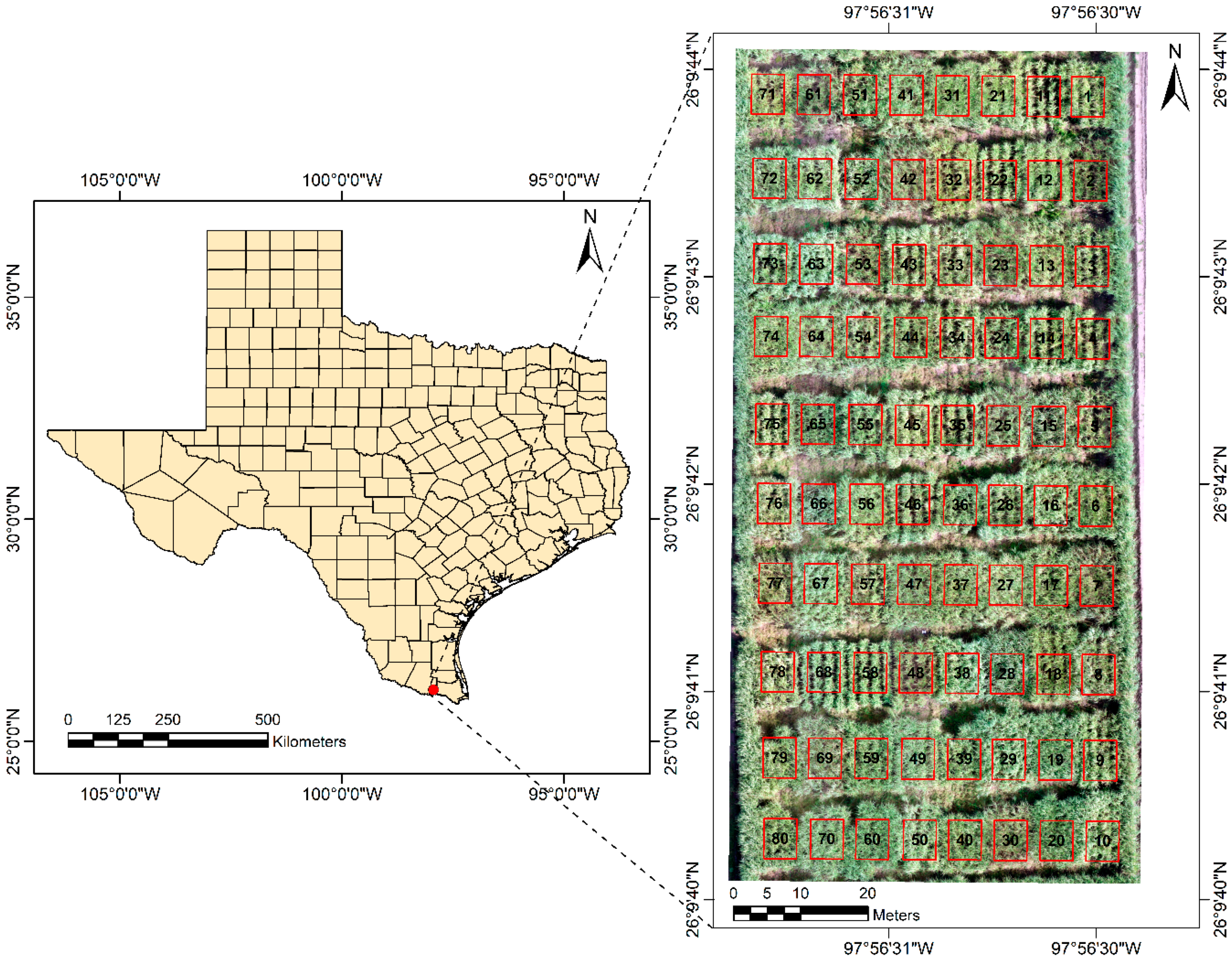
2.1. Study Site
2.2. Imagery Acquisition and Processing
2.3. Feature Extraction
2.3.1. Canopy Height
2.3.2. Canopy Cover
2.3.3. Normalized Difference Vegetation Index (NDVI)
2.3.4. Excess Green Index (ExG)
2.4. Cell Wall Composition Analysis
2.5. Harvest Data
2.6. Data Analysis
2.7. Yield and Total Cellulosic Content Models
3. Results
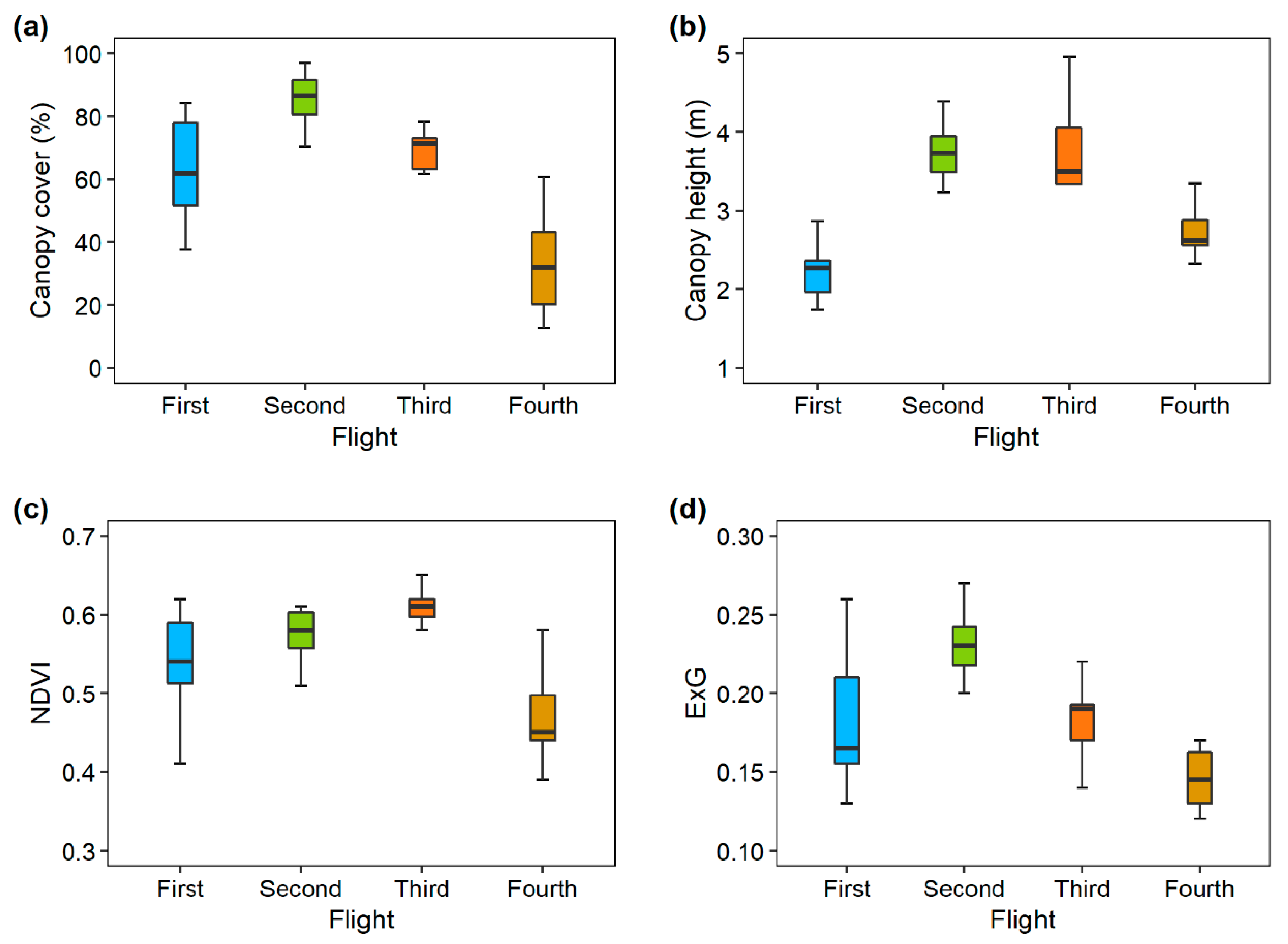
3.1. Statistics
3.2. Relationship Analysis
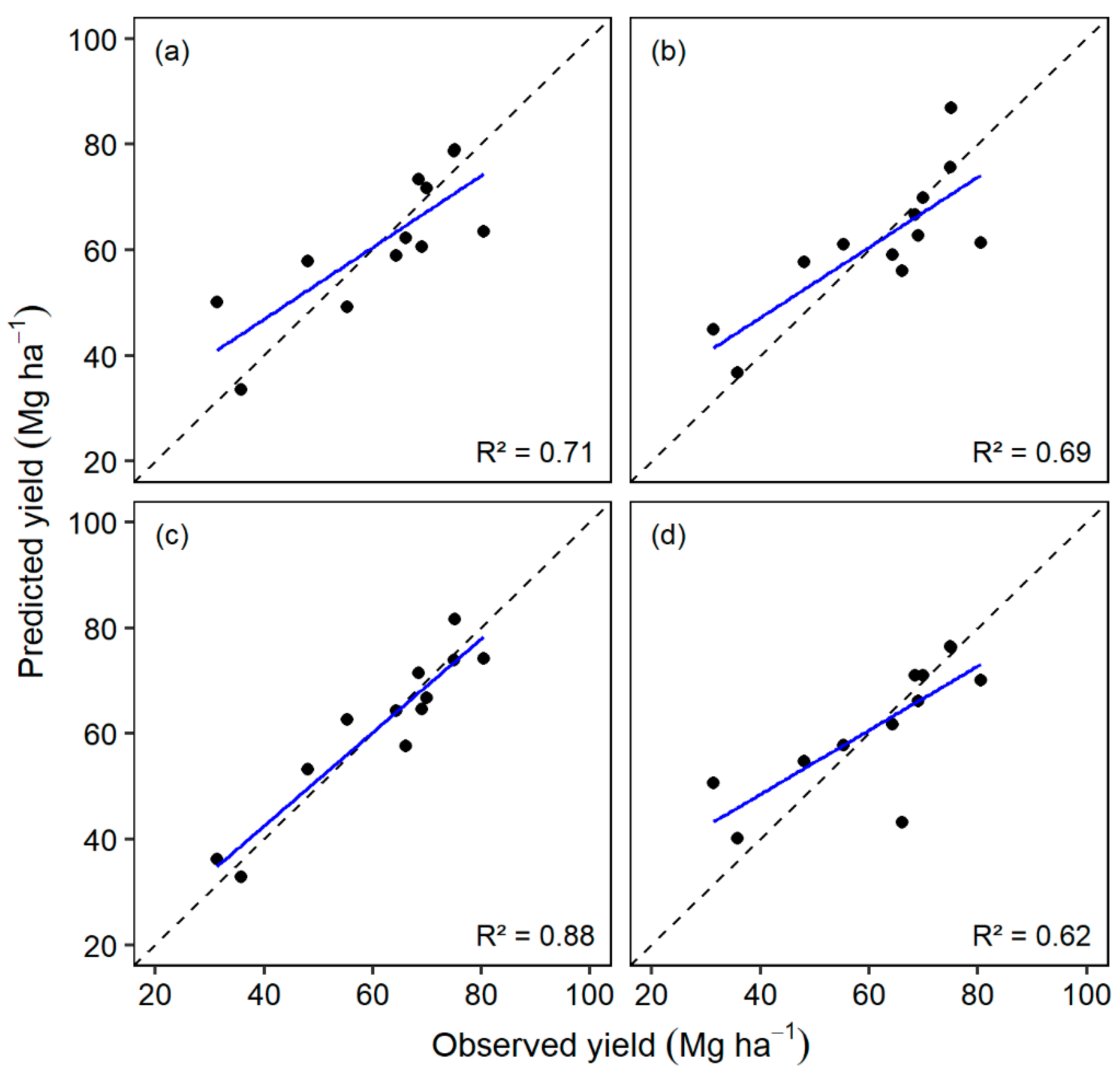
3.3. Energy Cane Yield and TCC Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Takeda, S.; Matsuoka, M. Genetic Approaches to Crop Improvement: Responding to Environmental and Population Changes. Nat. Rev. Genet. 2008, 9, 444–457. [Google Scholar] [CrossRef]
- Somerville, C.; Youngs, H.; Taylor, C.; Davis, S.C.; Long, S.P. Feedstocks for Lignocellulosic Biofuels. Science 2010, 329, 790–792. [Google Scholar] [CrossRef]
- Ellis, R.D.; Merry, R.E. Sugarcane Agriculture. In Sugarcane; Blackwell Publishing Ltd.: Oxford, UK, 2004; pp. 101–142. [Google Scholar] [CrossRef]
- Da Silva, J.A.G.; Sorrells, M.E.; Burnquist, W.L.; Tanksley, S.D. RFLP Linkage Map and Genome Analysis of Saccharum Spontaneum. Genome 1993, 36, 782–791. [Google Scholar] [CrossRef]
- Park, J.-W.; Benatti, T.R.; Marconi, T.; Yu, Q.; Solis-Gracia, N.; Mora, V.; Da Silva, J.A. Cold Responsive Gene Expression Profiling of Sugarcane and Saccharum Spontaneum with Functional Analysis of a Cold Inducible Saccharum Homolog of NOD26-like Intrinsic Protein to Salt and Water Stress. PLoS ONE 2015, 10, e0125810. [Google Scholar] [CrossRef]
- Scortecci, K.C.; Creste, S.; Calsa, T.; Xavier, M.A.; Landell, M.G.A.; Figueira, A.; Benedito, V.A. Challenges, Opportunities and Recent Advances in Sugarcane Breeding. In Plant Breeding; Abdurakhmonov, I., Ed.; InTech: Rijeka, Croatia, 2012; pp. 267–296. [Google Scholar]
- Carvalho-Netto, O.V.; Bressiani, J.A.; Soriano, H.L.; Fiori, C.S.; Santos, J.M.; Barbosa, G.V.S.; Xavier, M.A.; Landell, M.G.A.; Pereira, G.A.G. The Potential of the Energy Cane as the Main Biomass Crop for the Cellulosic Industry. Chem. Biol. Technol. Agric. 2014, 1, 20. [Google Scholar] [CrossRef]
- Matsuoka, S.; Kennedy, A.J.; dos Santos, E.G.D.; Tomazela, A.L.; Rubio, L.C.S. Energy Cane: Its Concept, Development, Characteristics, and Prospects. Adv. Bot. 2014, 2014, 597275. [Google Scholar] [CrossRef]
- Levitt, J., II. Water, Radiation, Salt and Other Stresses. In Responses of Plants to Environmental Stresses; Academic Press: New York, NY, USA, 1980; p. 607. [Google Scholar]
- Salekdeh, G.H.; Reynolds, M.; Bennett, J.; Boyer, J. Conceptual Framework for Drought Phenotyping during Molecular Breeding. Trends Plant Sci. 2009, 14, 488–496. [Google Scholar] [CrossRef]
- Berger, B.; Parent, B.; Tester, M. High-Throughput Shoot Imaging to Study Drought Responses. J. Exp. Bot. 2010, 61, 3519–3528. [Google Scholar] [CrossRef]
- Casadesús, J.; Kaya, Y.; Bort, J.; Nachit, M.M.; Araus, J.L.; Amor, S.; Ferrazzano, G.; Maalouf, F.; Maccaferri, M.; Martos, V.; et al. Using Vegetation Indices Derived from Conventional Digital Cameras as Selection Criteria for Wheat Breeding in Water-Limited Environments. Ann. Appl. Biol. 2007, 150, 227–236. [Google Scholar] [CrossRef]
- Swain, K.C.; Thomson, S.J.; Jayasuriya, H.P.W. Adoption of an Unmanned Helicopter for Low-Altitude Remote Sensing to Estimate Yield and Total Biomass of a Rice Crop. Trans. ASABE 2010, 53, 21–27. [Google Scholar] [CrossRef]
- Yang, G.; Liu, J.; Zhao, C.; Li, Z.; Huang, Y.; Yu, H.; Xu, B.; Yang, X.; Zhu, D.; Zhang, X.; et al. Unmanned Aerial Vehicle Remote Sensing for Field-Based Crop Phenotyping: Current Status and Perspectives. Front. Plant Sci. 2017, 8, 1111. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Li, X.; Low, M.; Lobell, D.; Ermon, S. Deep Gaussian Process for Crop Yield Prediction Based on Remote Sensing Data. In Proceedings of the Thirty-First AAAI Conference for Artificial Intelligence (AAAI-17), San Francisco, CA, USA, 4–10 February 2017. [Google Scholar]
- Khaki, S.; Wang, L.; Archontoulis, S.V. A CNN-RNN Framework for Crop Yield Prediction. Front. Plant Sci. 2020, 10, 1750. [Google Scholar] [CrossRef] [PubMed]
- Mathews, A.; Jensen, J. Visualizing and Quantifying Vineyard Canopy LAI Using an Unmanned Aerial Vehicle (UAV) Collected High Density Structure from Motion Point Cloud. Remote Sens. 2013, 5, 2164–2183. [Google Scholar] [CrossRef]
- Diaz-Varela, R.A.; Zarco-Tejada, P.J.; Angileri, V.; Loudjani, P. Automatic Identification of Agricultural Terraces through Object-Oriented Analysis of Very High Resolution DSMs and Multispectral Imagery Obtained from an Unmanned Aerial Vehicle. J. Environ. Manag. 2014, 134, 117–126. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Diaz-Varela, R.; Angileri, V.; Loudjani, P. Tree Height Quantification Using Very High Resolution Imagery Acquired from an Unmanned Aerial Vehicle (UAV) and Automatic 3D Photo-Reconstruction Methods. Eur. J. Agron. 2014, 55, 89–99. [Google Scholar] [CrossRef]
- Sanches, G.M.; Duft, D.G.; Kölln, O.T.; Luciano, A.C.D.S.; De Castro, S.G.Q.; Okuno, F.M.; Franco, H.C.J. The Potential for RGB Images Obtained Using Unmanned Aerial Vehicle to Assess and Predict Yield in Sugarcane Fields. Int. J. Remote Sens. 2018, 39, 5402–5414. [Google Scholar] [CrossRef]
- Chea, C.; Saengprachatanarug, K.; Posom, J.; Wongphati, M.; Taira, E. Sugar Yield Parameters and Fiber Prediction in Sugarcane Fields Using a Multispectral Camera Mounted on a Small Unmanned Aerial System (UAS). Sugar Tech 2020, 1–17. [Google Scholar] [CrossRef]
- Patrignani, A.; Ochsner, T.E. Canopeo: A Powerful New Tool for Measuring Fractional Green Canopy Cover. Agron. J. 2015, 107, 2312–2320. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring Vegetation Systems in the Great Plains with ERTS. In NASA Goddard Space Flight Center 3d ERTS-1 Symposium; NASA: Washington, DC, USA, 1974; pp. 309–317. [Google Scholar]
- Thenkabail, P.S.; Smith, R.B.; De Pauw, E. Hyperspectral Vegetation Indices and Their Relationships with Agricultural Crop Characteristics. Remote Sens. Environ. 2000, 71, 158–182. [Google Scholar] [CrossRef]
- Enciso, J.; Avila, C.A.; Jung, J.; Elsayed-Farag, S.; Chang, A.; Yeom, J.; Landivar, J.; Maeda, M.; Chavez, J.C. Validation of Agronomic UAV and Field Measurements for Tomato Varieties. Comput. Electron. Agric. 2019, 158, 278–283. [Google Scholar] [CrossRef]
- Woebbecke, D.M.; Meyer, G.E.; Von Bargen, K.; Mortensen, D.A. Color Indices for Weed Identification under Various Soil, Residue, and Lighting Conditions. Trans. ASAE 1995, 38, 259–269. [Google Scholar] [CrossRef]
- Berding, N.; Brotherton, G.A.; le Brocq, D.G.; Skinner, J.C. Near Infrared Reflectance Spectroscopy for Analysis of Sugarcane from Clonal Evaluation Trials: I. Fibrated Cane. Crop Sci. 1991, 31, 1017–1023. [Google Scholar] [CrossRef]
- Bischoff, K.P.; Gravois, K.A.; Schexnayder, H.P., Jr.; Hawkins, G.L. The Effect of Harvest Method and Plot Size on the Estimation of Sugarcane Yield. J. Am. Soc. Sugar Cane Technol. 2001, 21, 51–60. [Google Scholar]
- Richards, J. Computer Processing of Remotely-Sensed Images: An Introduction. Earth-Sci. Rev. 1990, 27, 392–394. [Google Scholar] [CrossRef]
- Yu, X.; Liu, Q.; Wang, Y.; Liu, X.; Liu, X. Evaluation of MLSR and PLSR for Estimating Soil Element Contents Using Visible/near-Infrared Spectroscopy in Apple Orchards on the Jiaodong Peninsula. Catena 2016, 137, 340–349. [Google Scholar] [CrossRef]
- Pinheiro Lisboa, I.; Melo Damian, J.; Roberto Cherubin, M.; Silva Barros, P.P.; Ricardo Fiorio, P.; Cerri, C.C.; Pellegrino Cerri, C.E. Prediction of Sugarcane Yield Based on NDVI and Concentration of Leaf-Tissue Nutrients in Fields Managed with Straw Removal. Agronomy 2018, 8, 196. [Google Scholar] [CrossRef]
- Zhou, X.; Zheng, H.B.; Xu, X.Q.; He, J.Y.; Ge, X.K.; Yao, X.; Cheng, T.; Zhu, Y.; Cao, W.X.; Tian, Y.C. Predicting Grain Yield in Rice Using Multi-Temporal Vegetation Indices from UAV-Based Multispectral and Digital Imagery. ISPRS J. Photogramm. Remote Sens. 2017, 130, 246–255. [Google Scholar] [CrossRef]
- Kyratzis, A.C.; Skarlatos, D.P.; Menexes, G.C.; Vamvakousis, V.F.; Katsiotis, A. Assessment of Vegetation Indices Derived by UAV Imagery for Durum Wheat Phenotyping under a Water Limited and Heat Stressed Mediterranean Environment. Front. Plant Sci. 2017, 8, 1114. [Google Scholar] [CrossRef]
- Portz, G.; Amaral, L.R.; Molin, J.P. Measuring Sugarcane Height in Complement To Biomass. In Proceedings of the 11th International Conference on Precision Agriculture, Indianapolis, IN, USA, 15–18 July 2012. [Google Scholar]
- De Souza, C.H.W.; Lamparelli, R.A.C.; Rocha, J.V.; Magalhães, P.S.G. Height Estimation of Sugarcane Using an Unmanned Aerial System (UAS) Based on Structure from Motion (SfM) Point Clouds. Int. J. Remote Sens. 2017, 38, 2218–2230. [Google Scholar] [CrossRef]
- Rahman, S. Growth, Yield and Quality of Plant and Ratoon Crops of Sugarcane as Affected by Plant Material and Management Practices. Ph.D. Thesis, University of Rajshahi, Rajshahi, Bangladesh, 2012. [Google Scholar]
- Trout, T.J.; Johnson, L.F.; Gartung, J. Remote Sensing of Canopy Cover in Horticultural Crops. HortScience 2008, 43, 333–337. [Google Scholar] [CrossRef]
- Geipel, J.; Link, J.; Claupein, W. Combined Spectral and Spatial Modeling of Corn Yield Based on Aerial Images and Crop Surface Models Acquired with an Unmanned Aircraft System. Remote Sens. 2014, 6, 10335–10355. [Google Scholar] [CrossRef]
- Fernandes, J.L.; Ebecken, N.F.F.; Esquerdo, J.C.D.M. Sugarcane Yield Prediction in Brazil Using NDVI Time Series and Neural Networks Ensemble. Int. J. Remote Sens. 2017, 38, 4631–4644. [Google Scholar] [CrossRef]
- Shafian, S.; Rajan, N.; Schnell, R.; Bagavathiannan, M.; Valasek, J.; Shi, Y.; Olsenholler, J. Unmanned Aerial Systems-Based Remote Sensing for Monitoring Sorghum Growth and Development. PLoS ONE 2018, 13, e0196605. [Google Scholar] [CrossRef]
- Maresma, Á.; Ariza, M.; Martínez, E.; Lloveras, J.; Martínez-Casasnovas, J. Analysis of Vegetation Indices to Determine Nitrogen Application and Yield Prediction in Maize (Zea Mays L.) from a Standard UAV Service. Remote Sens. 2016, 8, 973. [Google Scholar] [CrossRef]
- Fu, Z.; Jiang, J.; Gao, Y.; Krienke, B.; Wang, M.; Zhong, K.; Cao, Q.; Tian, Y.; Zhu, Y.; Cao, W.; et al. Wheat Growth Monitoring and Yield Estimation Based on Multi-Rotor Unmanned Aerial Vehicle. Remote Sens. 2020, 12, 508. [Google Scholar] [CrossRef]
- Ashapure, A.; Oh, S.; Marconi, T.G.; Chang, A.; Jung, J.; Landivar, J.; Enciso, J. Unmanned Aerial System Based Tomato Yield Estimation Using Machine Learning. In Autonomous Air and Ground Sensing Systems for Agricultural Optimization and Phenotyping IV; Thomasson, J.A., McKee, M., Moorhead, R.J., Eds.; SPIE: Baltimore, MD, USA, 2019; Volume 11008, p. 22. [Google Scholar] [CrossRef]
- Roberts, D.; Roth, K.; Perroy, R. Hyperspectral Vegetation Indices. In Hyperspectral Remote Sensing of Vegetation; Thenkabail, P.S., Huete, A., Lyon, J.G., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 309–328. [Google Scholar] [CrossRef]
- Daughtry, C.S.T. Agroclimatology: Discriminating Crop Residues from Soil by Shortwave Infrared Reflectance. Agron. J. 2001, 93, 125–131. [Google Scholar] [CrossRef]
- Serrano, L.; Peñuelas, J.; Ustin, S.L. Remote Sensing of Nitrogen and Lignin in Mediterranean Vegetation from AVIRIS Data: Decomposing Biochemical from Structural Signals. Remote Sens. Environ. 2002, 81, 355–364. [Google Scholar] [CrossRef]

| DJI Phantom 4 Pro with RGB Sensor | Matrice 100 with SlantRange 3p Sensor | |
|---|---|---|
| Sensor resolution (pixels) | 5472 × 3648 | 1280 × 1024 |
| Spectral resolution | R, G, B | NIR, red edge, R, G |
| Weight (g) | 1388 | 2781 |
| N | Mean | Median | SD | Min | Max | |
|---|---|---|---|---|---|---|
| CC–1st | 12 | 61.35 | 61.85 | 16.03 | 37.75 | 84.02 |
| CC–2nd | 12 | 85.74 | 86.30 | 7.80 | 70.37 | 97.02 |
| CC–3rd | 12 | 69.78 | 71.25 | 5.96 | 61.69 | 78.34 |
| CC–4th | 12 | 33.18 | 31.84 | 16.93 | 12.59 | 60.79 |
| CH– 1st | 12 | 2.22 | 2.27 | 0.33 | 1.74 | 2.86 |
| CH– 2nd | 12 | 3.73 | 3.73 | 0.35 | 3.23 | 4.39 |
| CH–3rd | 12 | 3.60 | 3.50 | 0.89 | 2.03 | 4.96 |
| CH–4th | 12 | 2.76 | 2.62 | 0.34 | 2.32 | 3.35 |
| NDVI–1st | 12 | 0.54 | 0.54 | 0.06 | 0.41 | 0.62 |
| NDVI–2nd | 12 | 0.58 | 0.58 | 0.03 | 0.51 | 0.61 |
| NDVI–3rd | 12 | 0.61 | 0.61 | 0.02 | 0.58 | 0.65 |
| NDVI–4th | 12 | 0.47 | 0.45 | 0.05 | 0.39 | 0.58 |
| ExG–1st | 12 | 0.18 | 0.17 | 0.04 | 0.13 | 0.26 |
| ExG–2nd | 12 | 0.23 | 0.23 | 0.02 | 0.20 | 0.27 |
| ExG–3rd | 12 | 0.18 | 0.19 | 0.02 | 0.14 | 0.23 |
| ExG–4th | 12 | 0.12 | 0.13 | 0.04 | 0.05 | 0.17 |
| Yield (Mg ha−1) | 12 | 61.57 | 67.30 | 15.74 | 31.36 | 80.49 |
| TCC | 12 | 62.18 | 61.87 | 3.67 | 55.99 | 66.91 |
| CC 1 | CC 2 | CC 3 | CC 4 | CH 1 | CH 2 | CH 3 | CH 4 | NDVI 1 | NDVI 2 | NDVI 3 | NDVI 4 | ExG 1 | ExG 2 | ExG 3 | ExG 4 | Yield | TCC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CC 1 | 1.00 | |||||||||||||||||
| CC 2 | 0.46 | 1.00 | ||||||||||||||||
| CC 3 | 0.01 | 0.14 | 1.00 | |||||||||||||||
| CC 4 | 0.73 ** | 0.47 | −0.19 | 1.00 | ||||||||||||||
| CH 1 | 0.56 | 0.54 | −0.32 | 0.79 | 1.00 | |||||||||||||
| CH 2 | 0.56 | 0.63 * | −0.32 | 0.71 | 0.93 *** | 1.00 | ||||||||||||
| CH 3 | 0.55 | 0.83 *** | 0.04 | 0.55 | 0.78 ** | 0.83 *** | 1.00 | |||||||||||
| CH 4 | 0.56 | 0.71 ** | 0.00 | 0.53 | 0.73 ** | 0.83 *** | 0.76 ** | 1.00 | ||||||||||
| NDVI 1 | 0.29 | 0.81 ** | −0.01 | 0.33 | 0.72 ** | 0.79 ** | 0.85 *** | 0.85 *** | 1.00 | |||||||||
| NDVI 2 | 0.08 | 0.82 ** | 0.14 | 0.00 | 0.29 | 0.38 | 0.63 * | 0.48 | 0.76 ** | 1.00 | ||||||||
| NDVI 3 | −0.44 | −0.58 * | −0.06 | −0.16 | −0.13 | −0.21 | −0.47 | −0.19 | −0.31 | −0.56 | 1.00 | |||||||
| NDVI 4 | 0.35 | 0.48 | 0.18 | 0.53 | 0.71 ** | 0.66 * | 0.66 * | 0.67 * | 0.60 * | 0.36 | 0.09 | 1.00 | ||||||
| ExG 1 | 0.85 *** | 0.43 | −0.15 | 0.81 ** | 0.57 | 0.57 | 0.38 | 0.55 | 0.29 | 0.00 | −0.23 | 0.20 | 1.00 | |||||
| ExG 2 | 0.42 | 0.71 ** | 0.50 | 0.26 | −0.01 | 0.05 | 0.37 | 0.26 | 0.28 | 0.53 | −0.70 * | 0.02 | 0.39 | 1.00 | ||||
| ExG 3 | −0.09 | −0.08 | 0.45 | 0.12 | 0.11 | 0.00 | −0.04 | −0.06 | 0.08 | −0.12 | 0.25 | 0.14 | 0.10 | 0.05 | 1.00 | |||
| ExG 4 | 0.65 * | 0.49 | −0.09 | 0.89 *** | 0.82 ** | 0.73 ** | 0.64 * | 0.46 | 0.43 | 0.14 | −0.32 | 0.59 * | 0.63 * | 0.26 | 0.31 | 1.00 | ||
| Yield | 0.32 | 0.70 * | −0.09 | 0.44 | 0.75 ** | 0.79 ** | 0.90 *** | 0.63 * | 0.84 *** | 0.55 | −0.44 | 0.44 | 0.26 | 0.24 | 0.13 | 0.60 * | 1.00 | |
| TCC | 0.49 | 0.30 | −0.10 | 0.53 | 0.28 | 0.35 | 0.39 | 0.32 | 0.07 | −0.08 | −0.36 | 0.07 | 0.37 | 0.31 | −0.40 | 0.33 | 0.32 | 1.00 |
| Flight | Model | R2 | p-Value | RMSE |
|---|---|---|---|---|
| 07/17/18 | yield = 222.08 NDVI–58.39 | 0.71 | 0.0006 | 8.89 |
| 09/18/18 | yield = 30.66 CH + 148.40 NDVI − 138.34 | 0.69 | 0.0049 | 9.64 |
| 11/14/18 | yield = − 0.68 CC + 16.26 CH + 177.04 ExG + 18.25 | 0.88 | 0.0004 | 6.26 |
| 12/19/18 | yield = − 0.70 CC + 26.14 CH + 381.68 ExG − 32.95 | 0.62 | 0.0432 | 11.39 |
| Flight | Model | R2 | p-Value | RMSE |
|---|---|---|---|---|
| 07/17/18 | TCC = 0.11 CC + 55.26 | 0.24 | 0.1032 | 3.35 |
| 09/18/18 | TCC = 3.58 CH + 47.19 ExG + 38.00 | 0.21 | 0.3519 | 3.61 |
| 11/14/18 | TCC = 1.54 CH − 57.14 ExG + 67.15 | 0.30 | 0.1996 | 3.39 |
| 12/19/18 | TCC = 0.11 CC + 58.39 | 0.28 | 0.0779 | 3.27 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cholula, U.; da Silva, J.A.; Marconi, T.; Thomasson, J.A.; Solorzano, J.; Enciso, J. Forecasting Yield and Lignocellulosic Composition of Energy Cane Using Unmanned Aerial Systems. Agronomy 2020, 10, 718. https://doi.org/10.3390/agronomy10050718
Cholula U, da Silva JA, Marconi T, Thomasson JA, Solorzano J, Enciso J. Forecasting Yield and Lignocellulosic Composition of Energy Cane Using Unmanned Aerial Systems. Agronomy. 2020; 10(5):718. https://doi.org/10.3390/agronomy10050718
Chicago/Turabian StyleCholula, Uriel, Jorge A. da Silva, Thiago Marconi, J. Alex Thomasson, Jorge Solorzano, and Juan Enciso. 2020. "Forecasting Yield and Lignocellulosic Composition of Energy Cane Using Unmanned Aerial Systems" Agronomy 10, no. 5: 718. https://doi.org/10.3390/agronomy10050718
APA StyleCholula, U., da Silva, J. A., Marconi, T., Thomasson, J. A., Solorzano, J., & Enciso, J. (2020). Forecasting Yield and Lignocellulosic Composition of Energy Cane Using Unmanned Aerial Systems. Agronomy, 10(5), 718. https://doi.org/10.3390/agronomy10050718





