Effects of Soft Rock and Biochar Applications on Millet (Setaria italica L.) Crop Performance in Sandy Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Design
2.2. Crop Management
2.3. Sample Collection
2.4. Net Photosynthetic Rate
2.5. Soil Properties Measurements
2.6. Data Analysis
3. Results
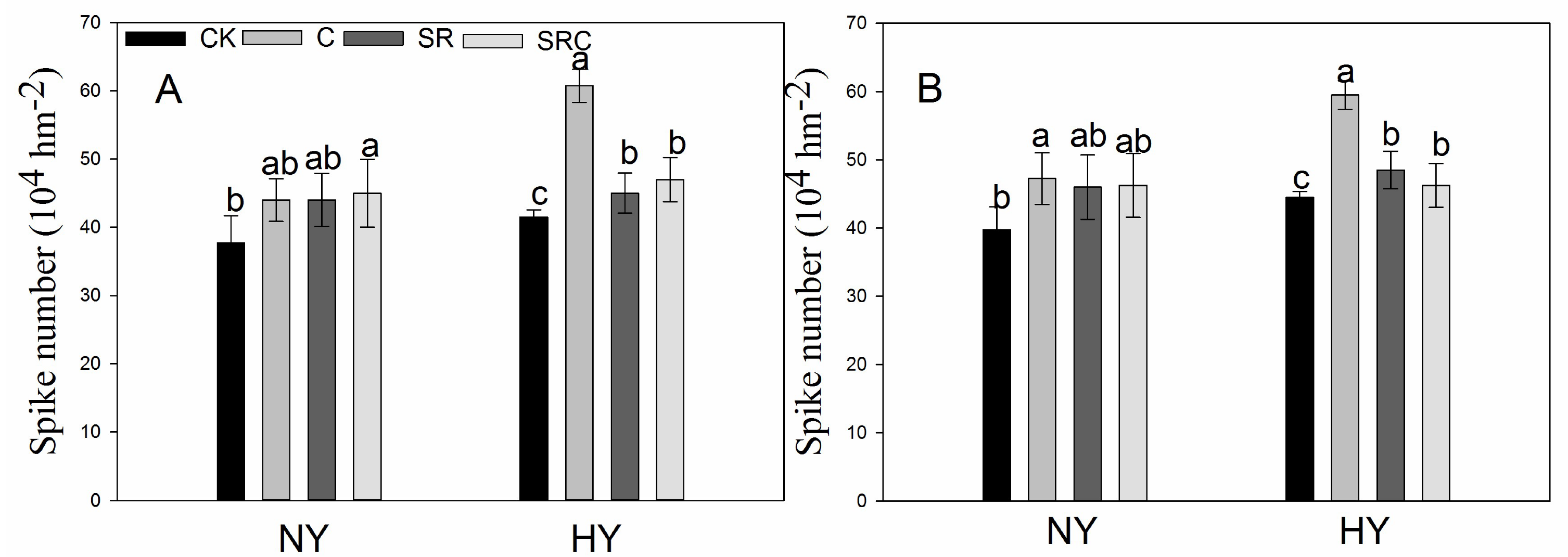
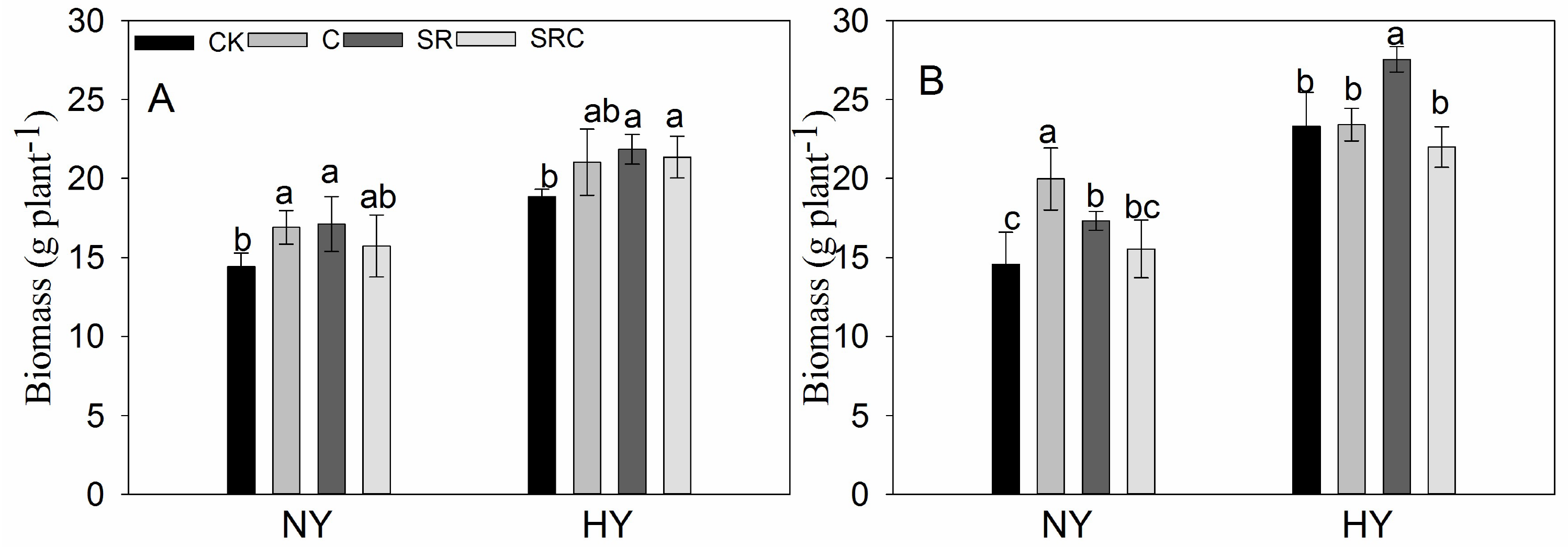
3.1. Grain Yield and Related Agronomic Traits
3.2. Soil Properties
3.3. Correlation Analysis
4. Discussion
4.1. Soft Rock Increases the Soils Ability to Retain Nutrients from Fertilizers
4.2. Soft Rock Increases Photosynthesis and Yield of Millet
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, J.-Y.; Gu, P.F.; Li, L.Y.; Zong, L.Y.; Zhao, W.-J. Changes of soil particle size fraction along a chronosequence in sandy desertified land: A fundamental process for ecosystem succession and ecological restoration. J. Soils Sediments 2016, 16, 2651–2656. [Google Scholar] [CrossRef]
- Jiang, L.; Jiapaer, G.; Bao, A.; Kurban, A.; Guo, H.; Zheng, G.; De Maeyer, P. Monitoring the long-term desertification process and assessing the relative roles of its drivers in Central Asia. Ecol. Ind. 2019, 104, 195–208. [Google Scholar] [CrossRef]
- D’Odorico, P.; Bhattachan, A.; Davis, K.F.; Ravi, S.; Runyan, C.W. Global desertification: Drivers and feedbacks. Adv. Water Resour. 2013, 51, 326–344. [Google Scholar] [CrossRef]
- Scherr, S.J. Soil Degradation: A Threat to Developing-Country Food Security by 2020? International Food Policy Research Institute: Washington, DC, USA, 1999. [Google Scholar]
- Kosmas, C.; Danalatos, N.; Cammeraat, L.H.; Chabart, M.; Diamantopoulos, J.; Farand, R.; Gutierrez, L.; Jacob, A.; Marques, H.; Martinez-Fernandez, J.; et al. The effect of land use on runoff and soil erosion rates under Mediterranean conditions. Catena 1997, 29, 45–59. [Google Scholar] [CrossRef]
- Yang, D.; Kanae, S.; Oki, T.; Koike, T.; Musiake, K. Global potential soil erosion with reference to land use and climate changes. Hydrol. Process. 2003, 17, 2913–2928. [Google Scholar] [CrossRef]
- Yu, F.C.; Yu, F.H.; Dong, M. Scale-Dependent Spatial Heterogeneity of Vegetation in Mu Us Sandy Land, a Semi-Arid Area of China. Plant Ecol. 2002, 162, 135–142. [Google Scholar]
- Sun, Z.; Han, J. Effect of soft rock amendment on soil hydraulic parameters and crop performance in Mu Us Sandy Land, China. Field Crops Res. 2018, 222, 85–93. [Google Scholar] [CrossRef]
- Ni, H.; Zhang, L.; Zhang, D.; Wu, X.; Fu, X. Weathering of pisha-sandstones in the wind-water erosion crisscross region on the Loess Plateau. J. Mountain Sci. 2008, 5, 340–349. [Google Scholar] [CrossRef]
- Liang, Z.; Wu, Z.; Yao, W.; Noori, M.; Yang, C.; Xiao, P.; Leng, Y.; Deng, L. Pisha sandstone: Causes, processes and erosion options for its control and prospects. Int. Soil Water Conserv. Res. 2019, 7, 1–8. [Google Scholar] [CrossRef]
- Sun, Z.; Han, J.; Wang, H. Soft rock for improving crop yield in sandy soil in the Mu Us desert, China. Arid Land Res. Manag. 2019, 33, 136–154. [Google Scholar]
- Ma, W.; Zhang, X. Effect of Pisha sandstone on water infiltration of different soils on the Chinese Loess Plateau. J. Arid Land 2016, 8, 331–340. [Google Scholar] [CrossRef]
- Han, J.; Xie, J.; Zhang, Y. Potential role of feldspathic sandstone as a natural water retaining agent in Mu Us Sandy Land, Northwest China. Chin. Geograph. Sci. 2012, 22, 550–555. [Google Scholar] [CrossRef]
- Liu, X.J.; Zhang, Y.; Han, W.X.; Tang, A.H.; Shen, J.L.; Cui, Z.L.; Vitousek, P.; Erisman, J.W.; Goulding, K.; Christie, P.; et al. Enhanced nitrogen deposition over China. Nature 2013, 494, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. Chapter 2—A Review of Biochar and Its Use and Function in Soil. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2010; pp. 47–82. [Google Scholar]
- Qu, J.; Zheng, J.; Zheng, J.; Zhang, X.; Yu, X. Effects of wheat-straw-based Biochar on yield of rice and nitrogen use efficiency of late rice. J. Ecol. Rural Environ. 2012, 28, 288–293. [Google Scholar]
- Alburquerque, J.A.; Salazar, P.; Barrón, V.; Torrent, J.; Villar, R. Enhanced wheat yield by biochar addition under different mineral fertilization levels. Agron. Sustain. Dev. 2013, 33, 475–484. [Google Scholar] [CrossRef]
- Xu, C.Y.; Hosseini-Bai, S.; Hao, Y.; Rachaputi, R.C.N.; Wang, H.; Xu, Z.; Wallace, H. Effect of biochar amendment on yield and photosynthesis of peanut on two types of soils. Environ. Sci. Pollut. Res. 2015, 22, 6112–6125. [Google Scholar] [CrossRef]
- Sukartono Utomo, W.H.; Kusuma, Z.; Nugroho, W.H. Soil fertility status, nutrient uptake, and maize (Zea mays L.) yield following biochar and cattle manure application on sandy soils of Lombok, Indonesia. J. Trop. Agric. 2011, 49, 47–52. [Google Scholar]
- Graber, E.R.; Meller Harel, Y.; Kolton, M.; Cytryn, E.; Silber, A.; Rav David, D.; Tsechansky, L.; Borenshtein, M.; Elad, Y. Biochar impact on development and productivity of pepper and tomato grown in fertigated soilless media. Plant Soil 2010, 337, 481–496. [Google Scholar] [CrossRef]
- Laird, D.; Fleming, P.; Wang, B.; Horton, R.; Karlen, D. Biochar impact on nutrient leaching from a Midwestern agricultural soil. Geoderma 2010, 158, 436–442. [Google Scholar] [CrossRef]
- Wang, H.; Huang, Y.; Shen, C.; Wu, J.; Yan, A.; Zhang, H. Co-transport of Pesticide Acetamiprid and Silica Nanoparticles in Biochar-Amended Sand Porous Media. J. Environ. Qual. 2016, 45, 1749–1759. [Google Scholar] [CrossRef]
- Trifunovic, B.; Gonzales, H.B.; Ravi, S.; Sharratt, B.S.; Mohanty, S.K. Dynamic effects of biochar concentration and particle size on hydraulic properties of sand. Land Degrad. Dev. 2018, 29, 884–893. [Google Scholar] [CrossRef]
- Yu, O.-Y.; Raichle, B.; Sink, S. Impact of biochar on the water holding capacity of loamy sand soil. Int. J. Energy Environ. Eng. 2013, 4, 44. [Google Scholar] [CrossRef]
- Hao, X.F.; Wang, J.Z.; Wang, G.Q.; Wang, X.Y.; Wang, L.Y. Analysis on the High Yield, Stable Yield and Adaptability of New Millet Varity Changsheng 07. J. Hebei Agric. Sci. 2011, 15, 1–3. [Google Scholar]
- Yan, J. Selection of Yield-Related Traits for Wheat Breeding in Semi-Arid Region. Int. J. Agric. Biol. 2018, 20, 569–574. [Google Scholar] [CrossRef]
- Bao, S. Soil Agrochemical Analysis; China Agriculture Press: Beijing, China, 2010. [Google Scholar]
- Huang, M.; Fan, L.; Jiang, L.; Yang, S.; Zou, Y.; Uphoff, N. Continuous applications of biochar to rice: Effects on grain yield and yield attributes. J. Integr. Agric. 2019, 18, 563–570. [Google Scholar] [CrossRef]
- Peng, X.; Tong, X.; Hao, L.; Wu, F. Applicability of biochar for limiting interrill erosion and organic carbon export of sloping cropland in a semi-arid area of China. Agric. Ecosyst. Environ. 2019, 280, 68–76. [Google Scholar] [CrossRef]
- Laghari, M.; Mirjat, M.S.; Hu, Z.; Fazal, S.; Xiao, B.; Hu, M.; Chen, Z.; Guo, D. Effects of biochar application rate on sandy desert soil properties and sorghum growth. Catena 2015, 135, 313–320. [Google Scholar] [CrossRef]
- Han, J.C.; Liu, Y.S.; Luo, L.T. Research on the Core Technology of Remixing Soil by Soft Rock and Sand in the Maowusu Sand Land Region. China Land Sci. 2012, 26, 87–94. [Google Scholar]
- Lepore, B.J.; Thompson, A.M.; Petersen, A. Impact of polyacrylamide delivery method with lime or gypsum for soil and nutrient stabilization. J. Soil Water Conserv. 2009, 64, 223–231. [Google Scholar] [CrossRef]
- Ahmed, F.; Arthur, E.; Plauborg, F.; Razzaghi, F.; Kørup, K.; Andersen, M.N. Biochar amendment of fluvio-glacial temperate sandy subsoil: Effects on maize water uptake, growth and physiology. J. Agron. Crop Sci. 2018, 204, 123–136. [Google Scholar] [CrossRef]
- Saxton, K.E.; Rawls, W.J. Soil Water Characteristic Estimates by Texture and Organic Matter for Hydrologic Solutions. Soil Sci. Soc. Am. J. 2006, 70, 1569–1578. [Google Scholar] [CrossRef]
- She, X.; Zhang, X.; Wei, X. Improvement of water absorbing and holding capacities of sandy soil by appropriate amount of soft rock. Trans. Chin. Soc. Agric. Eng. 2014, 30, 115–123. (In Chinese) [Google Scholar]
- Kabirinejad, S.; Kalbasi, M.; Khoshgoftarmanesh, A.H.; Hoodaji, M.; Afyuni, M. Effect of incorporation of crops residue into soil on some chemical properties of soil and bioavailability of copper in soil. Int. J. Adv. Biol. Biomed. Res. 2014, 2, 2819–2824. [Google Scholar]
- Bouranis, D.L.; Vlyssides, A.G.; Drossopoulos, J.B.; Karvouni, G. Some characteristics of a new organic soil conditioner from the co-composting of olive oil processing wastewater and solid residue. Commun. Soil Sci. Plant Anal. 1995, 26, 2461–2472. [Google Scholar] [CrossRef]
- Zhen, G.; Han, J.; Li, J. Response of organic carbon mineralization and bacterial communities to soft rock additions in sandy soils. Peer J. 2020, 8, 8948. [Google Scholar]
- Sinclair, T.R.; Horie, T. Leaf Nitrogen, Photosynthesis, and Crop Radiation Use Efficiency: A Review. Crop Sci. 1989, 29, 90–98. [Google Scholar] [CrossRef]
- Richards, R.A. Selectable traits to increase crop photosynthesis and yield of grain crops. J. Exp. Bot. 2000, 51, 447–458. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.; Wang, N.; Chen, Y.; Zhang, S. Changes in the yield and associated photosynthetic traits of dry-land winter wheat (Triticum aestivum L.) from the 1940s to the 2010s in Shaanxi Province of China. Field Crops Res. 2014, 167, 1–10. [Google Scholar] [CrossRef]
- Jiang, G.M.; Sun, J.Z.; Liu, H.Q.; Qu, C.M.; Wang, K.J.; Guo, R.J.; Bai, K.Z.; Gao, L.M.; Kuang, T.Y. Changes in the rate of photosynthesis accompanying the yield increase in wheat cultivars released in the past 50 years. J. Plant Res. 2003, 116, 347–354. [Google Scholar] [CrossRef]
- Sangakkara, U.R.; Frehner, M.; Nösberger, J. Effect of Soil Moisture and Potassium Fertilizer on Shoot Water Potential, Photosynthesis and Partitioning of Carbon in Mungbean and Cowpea. J. Agron. Crop Sci. 2000, 185, 201–207. [Google Scholar] [CrossRef]
- Saikia, P.; Bhattacharya, S.S.; Baruah, K.K. Organic substitution in fertilizer schedule: Impacts on soil health, photosynthetic efficiency, yield and assimilation in wheat grown in alluvial soil. Agric. Ecosyst. Environ. 2015, 203, 102–109. [Google Scholar] [CrossRef]
- Li, D.; Tian, M.; Cai, J.; Jiang, D.; Cao, W.; Dai, T. Effects of low nitrogen supply on relationships between photosynthesis and nitrogen status at different leaf position in wheat seedlings. Plant Growth Regul. 2013, 70, 257–263. [Google Scholar] [CrossRef]
- Borchardt, M. Effects of flowing water on nitrogen- and phosphorus-limited photosynthesis and optimum N:P ratios by Spirogyra fluviatilis (Charophyceae). J. Phycol. 2004, 30, 418–430. [Google Scholar] [CrossRef]
- Vengavasi, K.; Pandey, R. Root exudation potential in contrasting soybean genotypes in response to low soil phosphorus availability is determined by photo-biochemical processes. Plant Physiol. Biochem. 2018, 124, 1–9. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, L.; Mclaughlin, N.B.; Mi, J.; Chen, Q.; Liu, J. Effect of synthetic and natural water-absorbing soil amendments on photosynthesis characteristics and tuber nutritional quality of potato in a semi-arid region. J. Sci. Food Agric. 2016, 96, 1010–1017. [Google Scholar] [CrossRef]
- Sarma, B.; Borkotoki, B.; Narzari, R.; Kataki, R.; Gogoi, N. Organic amendments: Effect on carbon mineralization and crop productivity in acidic soil. J. Cleaner Prod. 2017, 152, 157–166. [Google Scholar] [CrossRef]


| NY 90 kg N hm−2 and 45 kg P hm−2 | HY 270 kg N hm−2 and 135 kg P hm−2 | |
|---|---|---|
| CK | -- | -- |
| C | 4500 kg biochar hm−2 | 4500 kg biochar hm−2 |
| SR | sand: soft rocks = 3:1 at 0–20 cm | sand: soft rocks = 3:1 at 0–20 cm |
| SRC | 4500 kg biochar hm−2 and sand: soft rocks = 3:1 at 0–20 cm | 4500 kg biochar hm−2 and sand: soft rocks = 3:1 at 0–20 cm |
| Material | Texture | Sand (%) | Silt (%) | Clay (%) | BD (Mg m−3) | TN | TP | pH | Organic Matter (%) |
|---|---|---|---|---|---|---|---|---|---|
| Aeolian sandy soil | Sand | 94.6 | 4.3 | 1.1 | 1.57 | 0.08 | 0.24 | 7.4 | 0.153 |
| Soft rock | – | 18.9 | 32.1 | 49 | 1.33 | 0.02 | 0.71 | 8.1 | 0.165 |
| Biochar | – | – | – | – | – | 8.1 | 1.58 | 10.5 | 70.512 |
| Year | NY | HY | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CK | C | SR | SR+C | CK | C | SR | SR+C | ||
| Grain Yield (kg/hm2) | 2018 | 2109.0 ± 76.9b | 2882.0 ± 199.5a | 2220.3 ± 122.5b | 2140.3 ± 202.3b | 3439.7 ± 237.7c | 5024.7 ± 240.8a | 4018.3 ± 76.8b | 3349.8 ± 170.7c |
| 2019 | 2722.7 ± 57.6c | 3914.0 ± 137.8a | 3226.3 ± 93.1b | 3555.7 ± 287.5ab | 3168.5 ± 78.5c | 4898.75 ± 87.1a | 4452.0 ± 333.3ab | 4092.3 ± 281.4b | |
| Biomass (kg/hm2) | 2018 | 8664.3 ± 250.0b | 11,125.0 ± 620.2a | 10,076.8 ± 882.3ab | 9344.5 ± 757.4ab | 11,519.5 ± 410.9c | 16,757.3 ± 982.1a | 14,258.3 ± 601.7ab | 12,772.3 ± 594.0b |
| 2019 | 8454.5 ± 341.2c | 11,987.5 ± 740.4a | 9969.7 ± 75.1b | 11,541.5 ± 1280.6ab | 9181.8 ± 185.6c | 16,042.0 ± 390.8a | 13,717.3 ± 626.5b | 11,294.5 ± 1387.1bc | |
| HI (%) | 2018 | 24.3 ± 0.2a | 25.9 ± 0.8a | 22.2 ± 0.8b | 22.8 ± 0.3b | 29.8 ± 1.1ab | 30.0 ± 0.3a | 28.3 ± 1.1bc | 26.2 ± 0.1c |
| 2019 | 32.3 ± 0.27a | 32.8 ± 1.2a | 31.6 ± 2.3a | 31.4 ± 2.5a | 34.5 ± 0.3b | 30.5 ± 0.4b | 32.3 ± 1.0b | 37.0 ± 2.1a | |
| Probability level of ANOVA | |||||||||
| Grain yield | Biomass | HI | |||||||
| Y | *** | ns | *** | ||||||
| F | *** | *** | *** | ||||||
| T | *** | *** | ns | ||||||
| Y × F | *** | * | ** | ||||||
| Y × T | ** | ns | ** | ||||||
| F × T | * | ** | ns | ||||||
| Y × F × T | ns | ns | * | ||||||
| Soil Depth (cm) | 2018 | 2019 | |||
|---|---|---|---|---|---|
| NY (%) | HY (%) | NY (%) | HY (%) | ||
| CK | 0–20 | 11.73 ± 0.05 | 8.67 ± 0.52 | 9.10 ± 0.64 | 7.69 ± 1.13 |
| 21–40 | 6.47 ± 0.11 | 4.84 ± 0.13 | 7.93 ± 0.65 | 5.80 ± 0.68 | |
| C | 0–20 | 9.12 ± 0.07 | 9.71 ± 0.92 | 11.02 ± 0.72 | 9.56 ± 0.31 |
| 21–40 | 13.5 ± 0.52 | 13.12 ± 0.18 | 14.36 ± 1.06 | 13.31 ± 0.31 | |
| SR | 0–20 | 11.2 ± 0.16 | 9.65 ± 1.12 | 11.36 ± 0.70 | 10.76 ± 1.51 |
| 21–40 | 14.5 ± 0.25 | 9.06 ± 0.19 | 13.07 ± 0.67 | 10.94 ± 0.67 | |
| SRC | 0–20 | 11.0 ± 0.36 | 9.65 ± 0.14 | 11.23 ± 0.69 | 10.01 ± 0.29 |
| 21–40 | 12.3 ± 0.19 | 12.54 ± 0.13 | 13.63 ± 1.64 | 12.24 ± 0.18 | |
| 2018 | 2019 | |||||||
|---|---|---|---|---|---|---|---|---|
| AP (mg/kg) | AN (mg/kg) | Organic Matter (%) | AP (mg/kg) | AN (mg/kg) | Organic Matter (%) | |||
| Flowering | NY | CK | 0.038 ± 0.005c | 7.000 ± 0.001c | 0.069 ± 0.000c | 0.040 ± 0.001c | 8.050 ± 0.350b | 0.274 ± 0.010c |
| C | 0.039 ± 0.001c | 6.998 ± 0.001c | 0.241 ± 0.035a | 0.039 ± 0.001c | 7.350 ± 0.350b | 0.389 ± 0.058a | ||
| SR | 0.131 ± 0.016a | 14.00 ± 7.00Ea | 0.251 ± 0.068a | 0.134 ± 0.101a | 13.650 ± 1.050a | 0.342 ± 0.035ab | ||
| SRC | 0.074 ± 0.003b | 8.401 ± 0.002b | 0.155 ± 0.017b | 0.073 ± 0.001b | 7.450 ± 0.349b | 0.309 ± 2.50Eb | ||
| HY | CK | 0.065 ± 0.005c | 10.50 ± 0.001c | 0.171 ± 0.034c | 0.068 ± 0.001c | 10.50 ± 5.25Ec | 0.301 ± 0.011c | |
| C | 0.063 ± 0.008c | 14.00 ± 0.002b | 0.286 ± 0.017a | 0.052 ± 0.001d | 14.50 ± 0.002b | 0.412 ± 0.012a | ||
| SR | 0.150 ± 0.035b | 17.50 ± 0.002a | 0.271 ± 1.07Ea | 0.158 ± 0.101b | 18.50 ± 0.348a | 0.359 ± 6.00Eb | ||
| SRC | 0.418 ± 0.221a | 14.00 ± 0.003b | 0.221 ± 0.034b | 0.446 ± 0.041a | 15.50 ± 0.348b | 0.368 ± 2.50Eb | ||
| Harvest | NY | CK | / | / | / | 0.019 ± 0.009a | 6.710 ± 0.642b | 0.317 ± 0.014c |
| C | / | / | / | 0.025 ± 0.009b | 7.173 ± 0.107b | 0.351 ± 0.014b | ||
| SR | / | / | / | 0.075 ± 0.019a | 10.896 ± 0.849a | 0.385 ± 0.014ab | ||
| SRC | / | / | / | 0.064 ± 0.019a | 10.111 ± 0.354a | 0.400 ± 0.034a | ||
| HY | CK | / | / | / | 0.094 ± 0.019c | 12.330 ± 1.190b | 0.381 ± 0.014b | |
| C | / | / | / | 0.102 ± 0.009bc | 12.936 ± 0.383b | 0.403 ± 0.014ab | ||
| SR | / | / | / | 0.149 ± 0.009a | 16.415 ± 0.428a | 0.400 ± 0.014ab | ||
| SRC | / | / | / | 0.119 ± 0.039ab | 14.155 ± 0.678b | 0.420 ± 0.024a | ||
| 2018 | 2019 | ||||
|---|---|---|---|---|---|
| Jointing Stage (µmol CO2 m−2 s−1) | Flowering Stage (µmol CO2 m−2 s−1) | Jointing Stage (µmol CO2 m−2 s−1) | Flowering Stage (µmol CO2 m−2 s−1) | ||
| NY | CK | 24.58 ± 0.77 | 12.14 ± 0.27 | 20.90 ± 0.46 | 13.9 ± 0.26 |
| C | 27.74 ± 1.16 | 16.55 ± 0.92 | 21.70 ± 0.28 | 16.12 ± 0.80 | |
| SR | 25.74 ± 1.34 | 16.94 ± 0.40 | 21.14 ± 0.86 | 16.58 ± 0.64 | |
| SRC | 28.01 ± 0.40 | 14.25 ± 0.10 | 22.62 ± 0.53 | 17.83 ± 0.50 | |
| HY | CK | 23.94 ± 1.79 | 16.76 ± 0.24 | 23.18 ± 0.71 | 15.41 ± 0.31 |
| C | 33.62 ± 1.22 | 26.19 ± 0.56 | 19.43 ± 1.12 | 21.62 ± 1.05 | |
| SR | 27.58 ± 1.32 | 20.86 ± 0.75 | 22.43 ± 1.05 | 18.78 ± 0.64 | |
| SRC | 23.06 ± 1.72 | 23.27 ± 0.63 | 22.95 ± 0.48 | 16.76 ± 0.20 | |
| GY | BH | BF | TKW | APF | APH | OMF | OMH | ANF | ANH | TPF | TPH | TNF | TNH | SN | PnJ | PnF | SWC20 | SWC40 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GY | 0.89 ** | 0.74 ** | −0.06 | 0.16 | 0.60 | 0.71 ** | 0.62 | 0.55 * | 0.60 | 0.84 ** | 0.75 * | 0.72 * | 0.73 * | 0.58 * | 0.01 | 0.69 ** | −0.23 | 0.17 | |
| BH | 0.63 ** | −0.25 | 0.11 | 0.49 | 0.41 | 0.53 | 0.57 * | 0.49 | 0.76 * | 0.66 | 0.66 | 0.69 | 0.77 ** | 0.27 | 0.87 ** | −0.13 | 0.26 | ||
| BF | 0.10 | 0.35 | 0.815 * | 0.55 * | 0.53 | 0.74 ** | 0.82 * | 0.75 * | 0.71 * | 0.69 | 0.82 * | 0.51 * | −0.10 | 0.48 | −0.33 | −0.02 | |||
| TKW | 0.14 | 0.23 | 0.16 | 0.01 | 0.24 | 0.23 | −0.18 | −0.08 | −0.05 | −0.25 | −0.03 | 0.01 | −0.16 | −0.30 | 0.07 | ||||
| APF | 0.54 | 0.14 | 0.59 | 0.53 * | 0.54 | 0.41 | 0.47 | 0.47 | 0.10 | 0.23 | −0.13 | 0.26 | 0.00 | 0.17 | |||||
| APH | 0.34 | 0.82 * | 0.90 ** | 1.00 ** | 0.75 * | 0.85 ** | 0.84 ** | 0.69 | 0.58 | 0.27 | 0.57 | −0.04 | −0.03 | ||||||
| OMF | 0.47 | 0.28 | 0.34 | 0.81 * | 0.64 | 0.66 | 0.68 | 0.34 | −0.44 | 0.16 | −0.15 | 0.30 | |||||||
| OMH | 0.63 | 0.82 * | 0.78 * | 0.94 ** | 0.83 ** | 0.67 | 0.72 * | 0.27 | 0.67 | 0.20 | 0.35 | ||||||||
| ANF | 0.89 ** | 0.72 * | 0.68 | 0.84 ** | 0.59 | 0.66 ** | 0.04 | 0.55 * | −0.05 | 0.03 | |||||||||
| ANH | 0.75 * | 0.84 ** | 0.84 ** | 0.69 | 0.58 | 0.27 | 0.58 | −0.04 | −0.03 | ||||||||||
| TPF | 0.91 ** | 0.81 * | 0.77 * | 0.78 * | −0.18 | 0.79 * | 0.01 | 0.33 | |||||||||||
| TPH | 0.87 ** | 0.83 * | 0.78 * | 0.15 | 0.74 * | 0.10 | 0.32 | ||||||||||||
| TNF | 0.82 * | 0.87 ** | 0.05 | 0.72 * | 0.43 | 0.47 | |||||||||||||
| TNH | 0.79 * | 0.10 | 0.69 | 0.20 | 0.32 | ||||||||||||||
| SN | 0.25 | 0.69 ** | 0.19 | 0.55 * | |||||||||||||||
| PnJ | 0.38 | −0.04 | 0.03 | ||||||||||||||||
| PnF | −0.10 | 0.22 | |||||||||||||||||
| SWC20 | 0.47 | ||||||||||||||||||
| SWC40 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Zhang, N.; Yan, J.; Zhang, S. Effects of Soft Rock and Biochar Applications on Millet (Setaria italica L.) Crop Performance in Sandy Soil. Agronomy 2020, 10, 669. https://doi.org/10.3390/agronomy10050669
Sun Y, Zhang N, Yan J, Zhang S. Effects of Soft Rock and Biochar Applications on Millet (Setaria italica L.) Crop Performance in Sandy Soil. Agronomy. 2020; 10(5):669. https://doi.org/10.3390/agronomy10050669
Chicago/Turabian StyleSun, Yingying, Ningning Zhang, Jiakun Yan, and Suiqi Zhang. 2020. "Effects of Soft Rock and Biochar Applications on Millet (Setaria italica L.) Crop Performance in Sandy Soil" Agronomy 10, no. 5: 669. https://doi.org/10.3390/agronomy10050669
APA StyleSun, Y., Zhang, N., Yan, J., & Zhang, S. (2020). Effects of Soft Rock and Biochar Applications on Millet (Setaria italica L.) Crop Performance in Sandy Soil. Agronomy, 10(5), 669. https://doi.org/10.3390/agronomy10050669






