1. Introduction
A consequence of rising day and night-time temperatures in warm grape-growing regions is the acceleration and uncoupling of ripening dynamics that separately lead to the accumulation of hexoses and phenolic in fruit [
1]. An additional negative consequence of these conditions is the hastening of ripening-time among cultivars previously having a larger separation. This creates a challenge for harvest-time decision making, as well as for transportation and winery logistics [
2].
Recent literature reviews have provided a detailed description of techniques utilized to delay ripening dynamics [
1,
3,
4]. These practices differ in application simplicity, duration of effects, economic suitability, and potential impacts on fruitfulness. Among them, winter pruning performed at the bud swollen stage can stop and/or delay bud development by a few days, which can also avoid a late spring frost event [
5,
6]. More recently, a number of publications [
7,
8,
9,
10,
11,
12] encompassing a wide range of genotypes, environments, and cultural practices have shown that retarding spur pruning up to the stage when, in the apical part of the still unpruned canes, shoots have already reached the 2–4 unfolded leaf stage of development, would delay bud burst from the basal nodes by over a month and postpone harvest up to 3 weeks.
Most promisingly, an understudied approach is double pruning/cropping, which was first tested by Dry in 1987 on Shiraz [
13]. This technique consisted of trimming canes in the summer to six nodes, and concurrently removing all laterals and clusters to force the dormant
n + 2 compound bud to push before entering endo-dormancy. This delay to the crop cycle positioned fruit ripening in a cooler period of the season and grape composition was greatly enhanced. The same technique was more recently applied by Gu et al. [
14], who, in the hot climate of Fresno (California), compared four dates of dormant bud forcing on Cabernet Sauvignon. Ripening was delayed by up to two months vs. the unforced control (for the hedging performed between 21 and 42 days after anthesis), and interestingly, berry flavonoids increased linearly until harvest in forced vines, while flavonoids in the control had peaked and began to decrease [
14]. In 2019, De Toda et al. [
15] tested different dates of forcing in Tempranillo and Maturana tinta cultivars, which consisted of trimming the primary shoot between 2
nd and 3
rd internode. A consistent delay in ripening was observed (up to two months vs. the unforced control) among all forced treatments; however, this came at the expense of a decrease in yield per vine and bud fruitfulness. Similar results were obtained by Martinez-Moreno et al. [
16] and by Lavado et al. [
17] in cv. Tempranillo.
Despite the effectiveness of bud forcing at maintaining fruit quality in some studies, the practice has a low likelihood of being adopted by the industry due to three main limitations: i) the drastic trimming of shoots and removal of all leaves on the primary shoot cause a strong source limitation that can severely impair current and next season’s yield; ii) the removal of all primary clusters and main and lateral leaves (needed to unlock the dormant compound buds) can be very time consuming; iii) most importantly, convincing a grape grower to drop the entire primary crop, trusting that a second crop will originate from the unlocked dormant compound buds is a very difficult task.
The objective of this work carried out on fruiting, potted Pinot noir grapevines was to provide proof of concept for a totally new forcing technique aimed at preserving also fruit quality of the primary crop. We hypothesized that severe early spring trimming of main shoots coupled with removal of developing laterals will promote growth of the compound bud. The originated forced shoots will warrant a second delayed crop, enhancing yield and shifting maturity into a much cooler period. Moreover, we predicted that maintaining the basal leaves on primary shoots will ensure fruitfulness at the basal nodes, therefore preserving next-year cropping potential.
2. Materials and Methods
2.1. Plant Material and Experimental Layout
The experiment was carried out in 2019 at the Department of Sustainable Crop Production (DIPROVES) of the Università Cattolica del Sacro Cuore in Piacenza (45°02′ N, 9°43′ E, 54 m asl), Italy using 16 three year old grapevines cv. Pinot Noir (clone VCR18 grafted on Kober 5BB) grown outside in 35 L pots filled with a mixture of sand, peat and loamy soil (30%, 20% and 50% by volume, respectively). Vines were spur-pruned to leave 4–5 two-count-node spurs per vine. After bud burst (BBCH 15 according to Lorenz et al. [
18]), vines were thinned to one shoot per node while cropping level was standardized removing any distal inflorescence on the primary shoots. Any shoots coming from base buds were also removed. From the initial group, each vine was randomly assigned to one of the following treatments (4 vines per treatment): Unforced control (UC), meaning that primary shoots were left to grow and trimmed to retain 13–14 main leaves only once they outgrew the top foliage wire; forcing one (F1) where all main shoots were trimmed above node 6 on DOY (Day Of the Year) 154, corresponding to the phenological stage BBCH 65 (full flowering with 50% of flower caps fallen according to Lorenz et al. [
18]); concurrently, any already developed lateral shoot was removed from the retained nodes. Forcing two (F2) and forcing three (F3) differed from F1 only in terms of date of application that was DOY 162 (corresponding to BBCH 69, end of flowering) and DOY 175 (corresponding to BBCH 73, groat (pea)-sized berries) for F2 and F3, respectively. In F1, F2 and F3 vines, developing lateral shoots were weekly removed until the end of June. After DOY 182, no lateral shoots developed anymore.
In all F treatments, normal crop from trimmed primary shoots was maintained. No trimming was performed on the shoots growing from the forced compound buds even if they outgrew the top wire, and clusters developed on the forced shoots were not thinned and/or standardized. In all treatments, the date of budburst was recorded as well as the veraison stage at 5% berry color change.
Pots were painted white to minimize possible overheating of the root system. Number and intensity of daily irrigation events was calibrated to assure pot water capacity not to decrease below 90%; pest management was primarily focused against downy and powdery mildews and followed sustainable local practices.
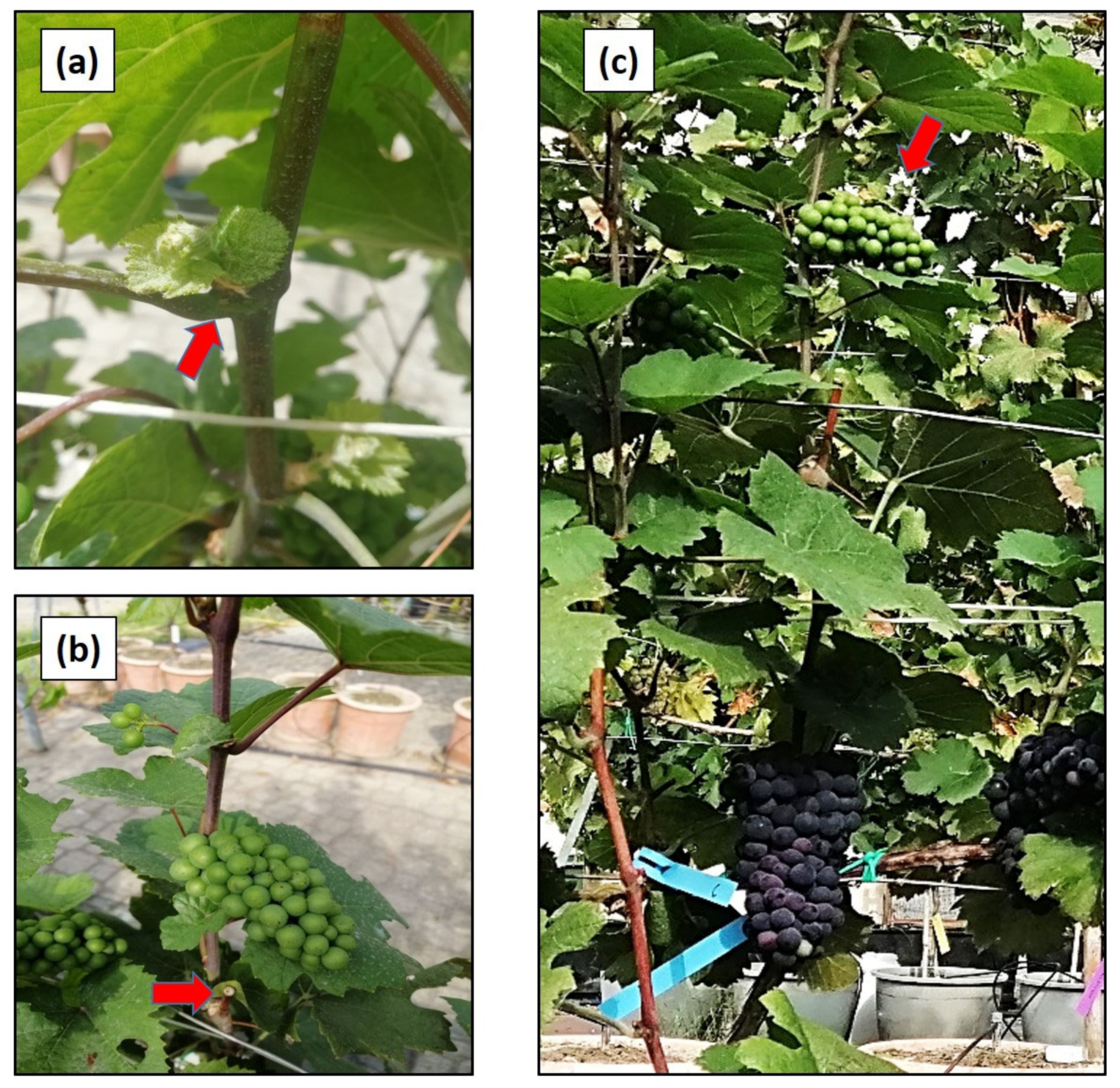
Figure 1 shows a detail of the dormant bud exiting dormancy (a), of the surface cut performed on the primary shoot with an already developed forced shoot (b) and of primary and forced crop bore on the same vine (c).
2.2. Vine Measurements
At the time of each forcing, removed main and lateral leaves were separated and their fresh weight recorded. Then, samples were brought to the lab and leaf area was measured with a desk leaf area meter (LI-3000A leaf-area meter, LI-COR Biosciences, Lincoln, NE, USA). Three primary shoots per vine originating from the distal node of three different spurs were tagged to perform specific measurements. Total primary and forced shoot number per vine were recorded along with their fertility given as number of clusters per main or forced shoot. On each trimmed shoot, the node position originating a forced shoot from the compound or dormant bud was registered, together with the shoot diameter measured at the internode located between nodes 3–4. After the harvest of forced crop, in all treatments, primary tagged shoots and forced shoots were individually defoliated recording the number of leaves and their leaf area was separately measured. In UC, the contribution of laterals developed prior or after trimming was also added. Main single leaf areas for primary, forced and lateral shoots were calculated. Immediately after leaf fall, the number of nodes on primary and forced shoots as well as on UC lateral shoots was counted. The final leaf area for each type of shoot was estimated based on node counts and leaf blade areas. Total vine leaf area was then calculated as a sum of the three components.
2.3. Primary and Forced Crop Components
At the stage of separate flowers, all inflorescences on tagged and forced shoots were photographed against a dark background. In extra-vines of the same cultivar, pictures of 20 inflorescences were taken with the same methods before being sampled and brought to the lab, where the number of total flowers per inflorescence was destructively counted. A linear regression (y = 2.03×, R2 = 0.86) between the actual flower number (y) and the number of flowers counted on the photo (x) was calculated and used to estimate total flower number on the photographed inflorescences of tagged shoot and forced shoots of experimental vines. Fruit set was then calculated as the percentage of flowers developing into normal size berries at harvest.
Harvest of primary crop produced on each treatment was performed at different dates, based on fruit ripening progression. A sample of berries from the primary crop of each treatment was periodically taken to assess soluble solids concentration (TSS), pH and titratable acidity (TA). The primary crop was harvested when fruit of the specific treatment achieved a TA of ~ 8 g/L, identified as the optimal threshold for Pinot Noir premium sparkling wines. The weight of primary crop was measured with a field portable scale and the number of clusters recorded. Mean cluster weight was then calculated accordingly. The clusters from tagged primary shoots (three per vine) were sampled and used to determine rachis length, number of berries per cluster, berry weight and cluster compactness, calculated as unit (g) of fruit mass per unit (cm) of rachis.
To explore maximum ripening delay potential of the forcing technique, harvest date was the same (DOY 280, 7 October) for all the forced shoots. This was the latest date available before berries started to dehydrate and/or rot. Yield components were analyzed as previously described for the primary crop. Vine balance indexed as total leaf area-to-yield ratio was calculated for primary and forced shoots, as well as on a whole vine basis.
2.4. Fruit Composition
At harvest for both primary and secondary crop, a sample of 100 berries per vine and crop type was crushed to obtain a must. The TSS concentration was measured using a temperature-compensating RX 5000 refractometer (Atago, Bellevue, WA, USA) and must pH analyzed with a digital PHM82 pH meter (Radiometer Analytical, Villeurbanne, France). For the determination of titrable acidity (TA), 10 mL of juice solution was titrated against a standardized 0.1 N NaOH solution to a pH 8.2 end-point and expressed as g/L of tartaric acid equivalents using a Crison Compact Titrator (Crison, Barcelona, Spain). Tartaric acid and malic acid were quantified via HPLC after filtering juice through a 0.22 μm polypropylene syringe into auto-sampler vials. For this analysis, an Allure Organic Acid Column, 300 × 4.6 mm, 5 μm (Restek, Bellefonte, PA, USA) was used. Separation was performed in isocratic conditions using water, pH adjusted at 2.5 with ortho-phosporic acid. The column temperature was maintained at 30 ± 0.1 °C and 15 μL of sample was injected. The elution was monitored at 200–700 nm and detection by UV–vis absorption with DAD at 210 nm. Organic acids were identified using authentic standards and quantification was based on peak areas and performed by external calibration with standards.
Fifty berries per vine for either primary and forced crop were used to determine total anthocyanins and phenolics. Berries were homogenized at 3584 ×
g with an Ultra-Turrax T25 (Rose Scientific Ltd., Edmonton, Canada) homogenizer for 1 min, then 2 g of the homogenate was transferred to a pre-tared centrifuge tube, enriched with 10 mL aqueous ethanol (50%, pH 5.0), capped and mixed periodically for 1 h before centrifugation at 959 × G for 5 min. A portion of the extract (0.5 mL) was added to 10 mL 1 M HCl, mixed and let stand for 3 h; absorbance was then measured at 520 nm and 280 nm on a JascoV-530 UV spectrophotometer (Jasco Analytical Instruments, Easton, MD, USA). Total anthocyanins and phenolics were expressed as mg per g of fresh berry mass [
19].
2.5. Gas Exchange Measurements
Leaf gas-exchange measurements were periodically taken during the season on primary and forced shoots of all the experimental vines. In more details, readings regarded the 3rd leaf of main shoots of all treatments and, on the forced shoots, the 3rd leaf of F1, F2 and F3 (only after their full expansion) was also used. Measurements were performed on DOYs 152, 161 and 174 (i.e., the days before F1, F2 and F3 application), DOY 206 (veraison of UC) and DOY 252. Readings were taken between 11:30 and 12:30, under saturating light conditions (PAR > 1400 mmol m–2 s–1), on one leaf for each shoot type per vine, using a portable, gas exchange open system, LCi infrared gas analyzer (ADC Bio Scientific Ltd., Hertz, UK). The system was equipped with a broad leaf chamber with a 6.25 cm2 window and all readings were taken at ambient relative humidity with an airflow adjusted to 350 mL min–1. The assimilation rate (leaf A), and stomatal conductance (leaf gs) were calculated from inlet and outlet CO2 and H2O concentrations.
2.6. Assessment of Bud Fruitfulness
The potential yield of the grapevine for the next season or potential fruitfulness is indicated by the number and size of the inflorescence primordia at the onset of dormancy [
20,
21]. At the end of the season, three canes per treatment were collected and transported to the lab. Subsequently, all dormant buds were dissected under a stereo microscope (40× magnification) as described by Sanchez and Dokoozlian [
22]. The number of inflorescence primordia in each primary bud was recorded and potential fruitfulness of buds according to their position on the cane was expressed as number of inflorescence primordia per primary bud.
2.7. Data Analysis
Data were subjected to a one-way ANOVA performed with XLSTAT statistical package (Addinsoft, New York, NY, USA) according to the completely randomized design that was used. In case of significant F test, means separation among UC vs. all forcing treatment was performed with a Student–Neumann–Keuls test, whereas, within each forcing treatment, separation between primary and forced shoots parameters was performed with a t-test. Repeated measures of the same parameters (A and gs rates) taken at different dates along the season were analyzed with the Repeated Measure analysis of variance routine embedded in the XLSTAT software package. The least squared (LS) mean method at p = < 0.05 was used for multiple comparisons within dates.
4. Discussion
This work is, to our knowledge, the first attempt to obtain a second harvest from a Vitis vinifera wine grape cultivar grown in a temperate climate. Shoots were forced in early summer by breaking compound bud dormancy without compromising the standard primary crop and the possibility to have intact and fruitful wood for the next year’s cropping. Our results confirm that such multiple goals can be achieved.
The first item that had to be resolved was to verify the degree of para-dormancy control exerted by the basal leaves of the growing shoot on the differentiating dormant buds. According to previous shoot and bud anatomy work, it is known that para-dormancy of a dormant bud during its first season of induction and differentiation can be released only if mature vegetative organs are left on the growing shoot [
23,
24,
25,
26]. In fact, regardless of the timing of forcing, shoot trimming above node 6 and the removal of any laterals has proved effective to break the dormancy of apical dormant buds. Concurrently, it has also been clarified that the presence, at the time of forcing, of green berries at varying stages of development on the primary clusters does not prevent dormant buds to exit para-dormancy. Then, at least three other major requirements have to be met to support the feasibility of the technique: i) the released dormant buds should preferably be those at the apical first or second node of each trimmed shoot to leave the first three basal dormant buds that will be retained after winter pruning undisturbed; ii) to provide suitable pruning wood for next year cropping these basal dormant buds are required to go through regular bud induction; iii) the unlocked dormant buds should be given enough time to undergo induction and differentiation of inflorescence primordia.
Data shown in
Table 3 as the number of forced shoots per primary shoot ratio indicate that the forcing energy was quite mild, especially in F1, setting at 0.49. Such a weak response can be explained by the lack of vigor. A basal diameter of 6–7 mm was required to push a forcing response (
Figure 3). Although vines were spur-pruned—a pruning technique that usually improves shoot uniformity compared to long-cane pruning [
27]—spurs left in the central part of the cordon tended to produce weaker shoots and some of them did not respond to forcing. It is our belief that being implemented on more vigorous, field grown spur-pruned plants might easily fix this potential drawback.
The suitable fruitfulness of the dormant buds located at the base of the trimmed shoot and to be used as next season fruiting spurs were also met, as their fertility ranged across 1.2 inflorescence primordia per shoot (
Table 3), which is adequate for the adoption of short pruning. Interestingly, no differences in IP occurred between the undisturbed shoots of UC and the primary shoots of the forcing treatments, suggesting either that bud induction had already been completed by the time forcing treatments were implemented or, likely in F1, preservation of the mature leaves (nodes 1–6) was sufficient to support induction. It could also be argued that IF values (
Table 2) were lower than current year shoot fruitfulness, setting at about 1.6–1.7 clusters/shoot. The weather pattern recorded in 2019 is a likely explanation for this difference; GGD and precipitation volume were abnormally low and high, respectively, during the month of May. Bud induction and initial differentiation typically take place during this time, so these negative conditions might have hindered the process [
28].
The third basic requirement of having sufficient fruitfulness for each forced shoot was also satisfied, as all forcing treatments reached (F2) or approached (F1 and F3) the ideal values of one cluster per shoot. On a more practical basis, this means that any forcing carried out between DOY 154 (full flowering of UC) and DOY 175 (pea-size of UC) is suitable. The later the forcing was implemented, the higher the area of source leaves was removed from shoots (
Table 2), and the higher the chance that the dormant bud induction process had already taken place. Moreover, similarly to IP observed in winter, forced shoots fruitfulness could have been negatively affected by the pre-flowering weather pattern. Theoretically, with more favorable temperatures in May, fruit could have set even closer to primary shoot fruitfulness levels.
An intriguing aspect of successfully implemented crop-forcing is the effects on yield components. Forcing provided 40%–50% additional yield (1 kg/vine) over UC vines, however, yield components varied substantially depending on the time of forcing. F1 forced clusters had a higher flower number than the other treatments that translated to a fruit set that was almost double that of F2 and F3 (
Table 4). The forced dormant buds undergo an uninterrupted cycle of induction and differentiation of floral organs, whereas in a normal biannual bud cycle, flower number is also affected by environmental conditions and vegetative growth gradient along the productive unit (either spur or cane) during springtime of the second year [
28]. The earliest forcing might have benefitted from either better environmental conditions upon dormant bud pushing and/or by the fact that main shoot trimming in F1 has removed a much lower LA than F2 and F3 (
Table 2), thereby making the developing buds a stronger sink. Despite the high berry number per cluster, F1 clusters did not show higher compactness than F3 for instance, as the effect due to the increased berry number was counteracted by a longer rachis.
Although the criteria for harvest were different for primary and forced clusters (primary clusters were harvested at a common TA, while forced clusters were harvested at the same date), the large differences observed in TSS, TA and total anthocyanins between the two cropping cycles is important for the potential implementation of this practice in temperate climates. The positive effects of crop-forcing on harvest grape composition in primary and forced clusters involve both the delay of key phenological stages and the dynamic changes in leaf function and vine balance. LA regrowth triggered by forcing allowed F2 to fully compensate LA, which was similar to UC harvest. Meanwhile, F1 and F3 had a lower final LA than UC. In F1, total LA was limited by a lower number of forced shoots; conversely, F3 regrowth upon forcing was insufficient to replenish previously removed LA. When also taking into account that F treatments assured additional yield, the final total LA/Y ratios for all F treatments were lower than the ratio calculated for UC, setting at 1.1 m
2/kg. In particular, LA/Y ratios calculated at harvest for F1 and F3, ranging near 0.5 m
2/kg, are much lower than the minimum threshold (1 m
2/kg) that, according to the literature, is supposed to be necessary to prevent limitations on sugar accumulation [
29]. However, comparing such final LA/Y ratios with the overall grape composition recorded in either primary or forced clusters make very clear that, under these circumstances, they are not fully reliable.
At the date of UC harvest, the primary clusters bore on forced vines were still lagging behind and required an additional 7–12-days to ripen. On a practical basis, having to differentiate also the picking dates of the primary clusters is time consuming, especially when considering that a second harvest for the forced crop is also scheduled. However, the F2 treatment had only a 7-d delay and such a gap can be easily negotiated by adjusting a common harvest dates with UC. F1 and F3 had a longer delay that matches quite well with the more limiting LA/Y at harvest. Even in this case, though, room of improvement can be easily found by rendering the response to forcing stronger (i.e., higher vigor of the trimmed shoots or environment allowing an even longer growing season). This is similar to the work of Poni and Giachino [
30], who imposed shoot trimming treatments at six nodes with or without lateral removal at fruit set using potted Cabernet Sauvignon vines. They found that when laterals were removed in addition to trimming, ripening was largely incomplete due to a limiting LA/Y ratio; whereas when laterals where untouched, TSS and color at harvest were only slightly limited as compared to the untrimmed control.
Traits of grape composition recorded in the forced clusters yielded a different and very interesting response. All forced treatments showed a great potential for continuing to accumulate sugar and anthocyanins until late in the season; higher values than those for regular crop picking were reached. Similar performances were also shown in forced crop of Cabernet Sauvignon [
14], and Tempranillo [
16]. As shown in
Table 5, LA/Y ratio of forced treatments, regardless of the unit of expression (single shoot or whole vine) were much higher than the corresponding ratios in primary shoots, which likely provided more potential for sugar and color accumulation. In addition to having a larger canopy in relation to crop level, the “quality” of foliage was also higher. Forced clusters were accompanied by a younger, and therefore, more functional canopy as compared to primary clusters during the transition from veraison to harvest. This effect is clearly manifested by post-veraison A rates measured on either primary and forced leaves at the same node (third from the base of the stem). Forced shoots had 4–6 µmol m
−2 s
−1 higher A rates than those measured in UC. A final reason explaining the better color in forced clusters is the climactic conditions during which ripening took place. Postponing the ripening process to a much cooler period of the season (
Table 1) is beneficial for either anthocyanins synthesis and preservation [
1].
An intriguing outcome in the grape composition of forced clusters was that, despite sugar and color accumulation being at or above optima, TA was still too high, oscillating around 13–14 g/L. This difference was reflected in the malic acid content, which was 5–6 g/L at harvest in forced clusters versus 2 g/L in primary clusters. It is well known that post-veraison malate degradation is enhanced by high temperatures [
31,
32], whereas this effect does not occur for tartaric acid [
31,
33]. Therefore, GDD/day from veraison onward for forced clusters (9.5–11.5) compared to the heat available for primary cluster over the same time span (~16 GDD/day) likely explain the difference in malic acid retention. While such desirable effect was somewhat expected, it is very meaningful that, in all forced cluster TSS and color from one side and TA from the other side were strongly decoupled; TSS and color responses were primarily controlled by LA/Y ratios and patterns of leaf senescence and function over the season, whereas TA (hence malate) response was mostly temperature driven. It is useless saying that such types of decoupling can turn out very useful in warm environments where maintenance of an adequate acidity level is crucial if sparkling wine types need to be elaborated.
Finally, the final grape composition described for the forced clusters also poses a challenging enological question as high TSS, good color, low pH and high acidity do seem to co-exist. Here, the matter seems to be redirected to growing conditions and length of the ripening season; pots might have exerted some limitation on the ripening potential whereas having a season providing a slightly longer ripening window could easily lead to a final grape composition of forced clusters that could also be used for a still, aged wine.
Although the technical specifications of the technique will need field growing conditions, it is a reasonable expectation that shoot trimming can be performed using an over-the-row mechanical trimmer. It is also possible that the same machine action might also remove some laterals that have already grown from the basal nodes, in that they facilitate the push of the dormant buds. Then, if mechanization is also a priority for a more sustainable management, the late season crop load can easily be mechanically harvested.