Evaluation of Glycine max and Glycine soja for Resistance to Calonectria ilicicola
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Plant Preparation
2.3. Pathogen Culture
2.4. Pathogen Inoculation
2.5. Visual Evaluation of Disease Severity
2.6. Genomic DNA Preparation
2.7. Relative Fungal Growth Determination by Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
2.8. Experimental Design and Data Analysis
3. Results
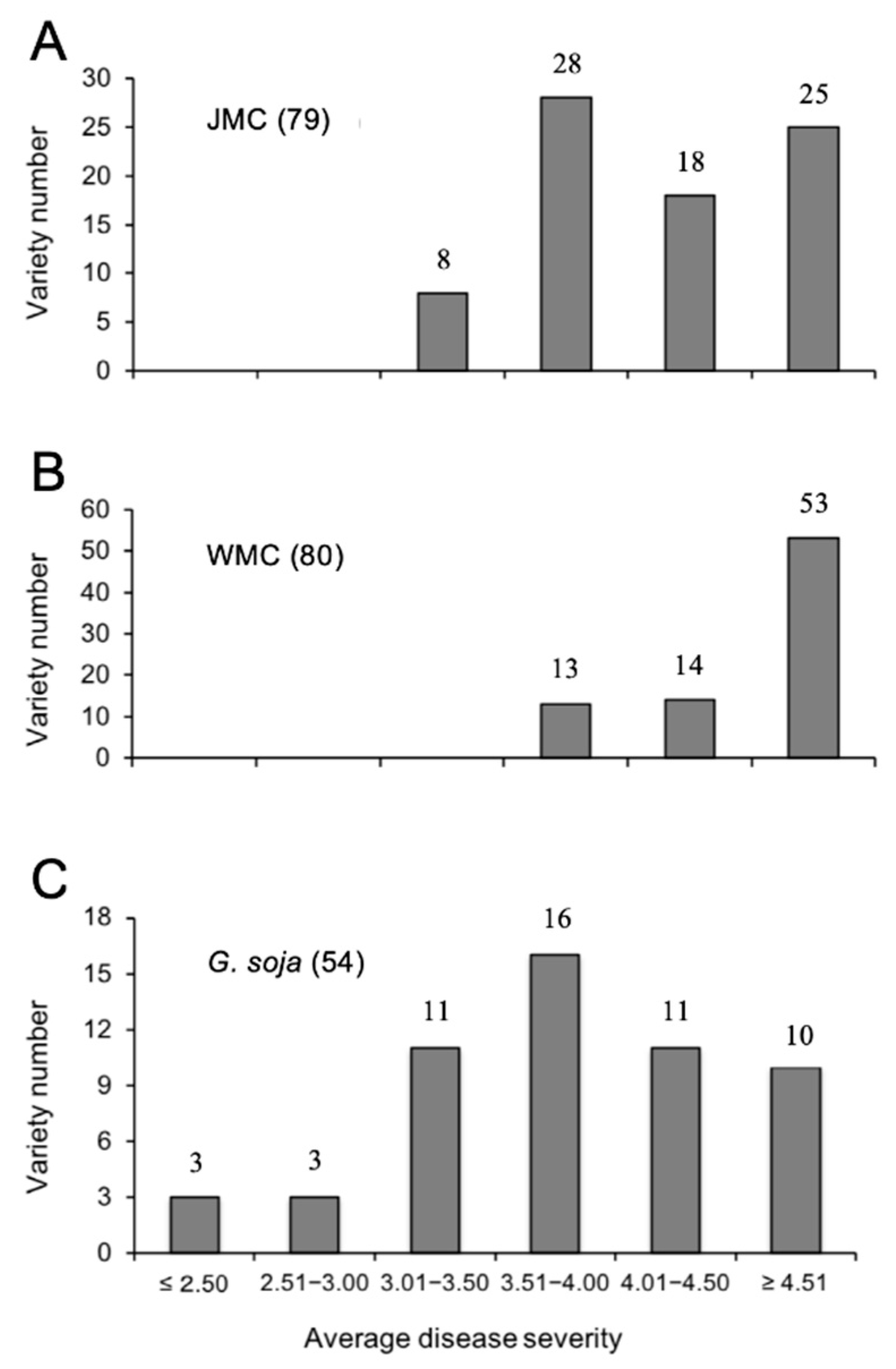
3.1. Evaluation of C. ilicicola-Resistance in Soybean Mini Core Collections
3.2. Identification of C. ilicicola-Resistance in Wild Soybeans (G. soja)
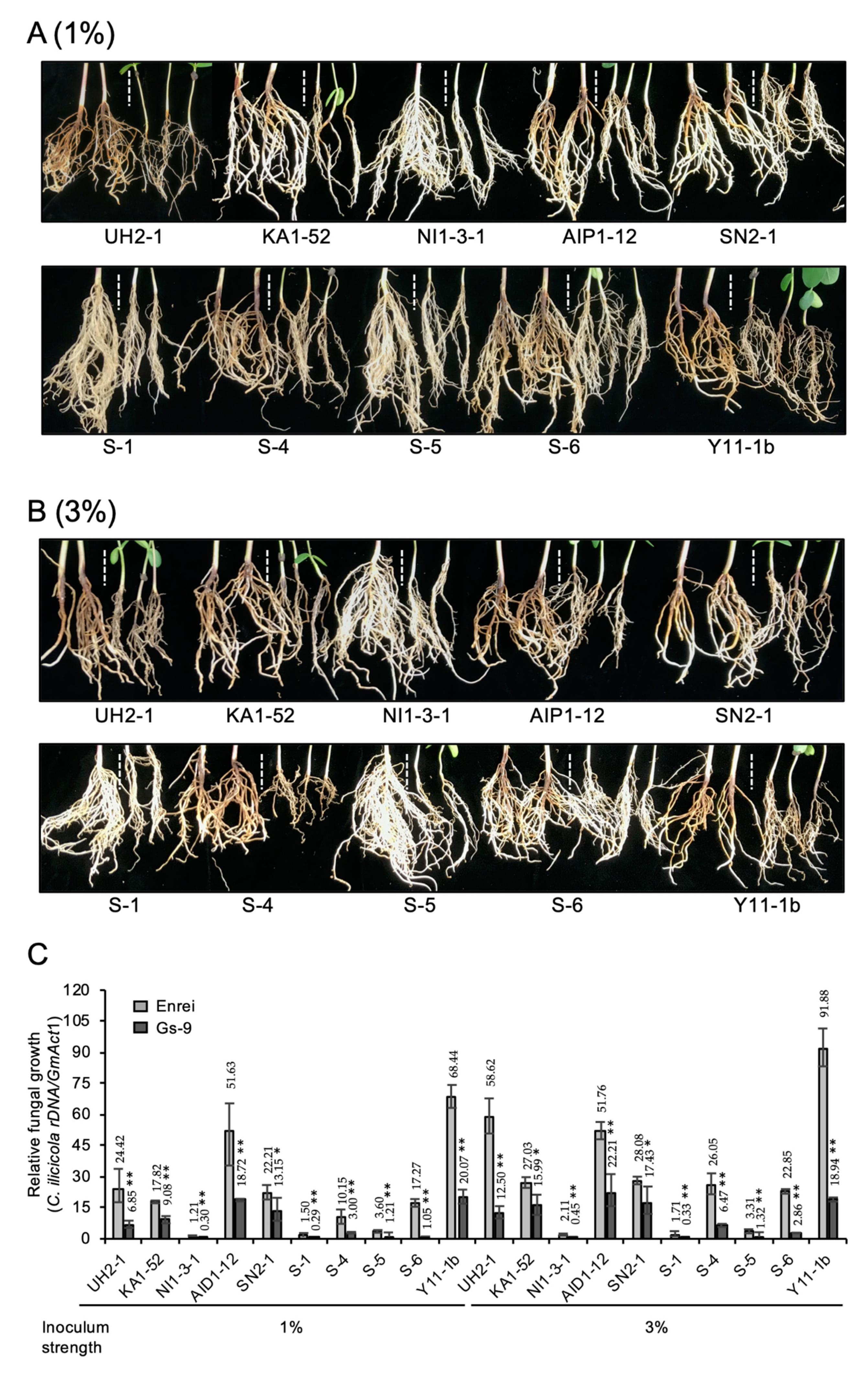
3.3. Evaluation of the Resistance Spectrum of Wild Soybean Gs-9 to C. ilicicola
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh, B.P.; Yadav, D.; Vij, S. Soybean bioactive molecules. In Current Trend and Future Prospective; Bioact. Mol. Food; Mérillon, J.M., Ramawat, K.G., Eds.; Springer: Basel, Switzerland, 2019; pp. 267–294. [Google Scholar]
- FAOSTAT. Crop Production. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 22 June 2019).
- MAFF. Current Situation and Issues of Soybean Production in Japan. Available online: http://www.maff.go.jp/j/kokusai/kokkyo/yosan/attach/pdf/h28_jigyo_report-61.pdf (accessed on 22 June 2014).
- Guan, M.; Pan, R.; Gao, X.; Xu, D.; Deng, Q.; Deng, M. First report of red crown rot caused by Cylindrocladium parasiticum on soybean in Guangdong, Southern China. Plant Dis. 2010, 94, 485. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhang, Z.; Wang, Y.; Yang, X. Cylindrocladium crotalariae causing red crown rot of soybean in China. Plant Pathol. 2004, 53, 357. [Google Scholar] [CrossRef]
- Kuruppu, P.; Schneider, R.; Russin, J. Factors affecting soybean root colonization by Calonectria ilicicola and development of red crown rot following delayed planting. Plant Dis. 2004, 88, 613–619. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kuruppu, P.; Schneider, R.; Russin, J. Effects of soil temperature on microsclerotia of Calonectria ilicicola and soybean root colonization by this fungus. Plant Dis. 2004, 88, 620–624. [Google Scholar] [CrossRef][Green Version]
- Tazawa, J. Occurrence and control of red crown rot of soybean. Plant Prot. 2013, 67, 46–49. [Google Scholar]
- Nishi, K. Calonectria ilicicola, the causal pathogen of soybean red crown rot. MAFF Microorg. Genet. Resour. Man. No.21 (Jpn.) 2007, 21, 1–13. [Google Scholar]
- Sung, J.M. An investigation of undescribed black root rot disease of soybean caused by Cylindrocladium (Calonectria) crotalariae in Korea. Korean J. Mycol. 1980, 8, 53–57. [Google Scholar]
- Ochi, S. Studies on the epidemiology and control of red crown rot of soybean. J. Gen. Plant Pathol. 2017, 83, 427–428. [Google Scholar] [CrossRef]
- Padgett, G.B.; Kuruppu, P.U.; Russin, J.S. Red crown rot. In Compendium of Soybean Diseases and Pest; Artman, G.L., Rupe, J.C., Sikora, E.J., Domier, L.L., Davis, J.A., Steffey, K.L., Eds.; The American Phytopathological Society Press: St. Paul, MN, USA, 2015; pp. 79–80. [Google Scholar]
- Nakagawa, A.; Shimata, S.; Yamaguchi, T. Studies on soybean root necrosis caused by Calonectoria crotalariae 3. Varietal differences of growth and yield component of soybean under pathogen inoculated condition. Proc. Kansai Pl.Prot. 1990, 32, 1–8. [Google Scholar]
- Bel, D.; Sobers, E.K. A peg, pod, and root necrosis of peanuts caused by a species of Calonectria. Phytopathology 1966, 56, 1361–1364. [Google Scholar]
- Nishi, K.; Sato, T. Changes in inoculum potential of Calonectria crotalariae in artificially infected soil kept in natural condition. Proc. Kanto-Tosan Plant Prot. Soc. 1994, 41, 45–46. [Google Scholar] [CrossRef]
- Kim, K.D.; Russin, J.S.; Snow, J.P. Effect of plant age on infection of soybean by Calonectria ilicicola. Kor. J. Plant Pathol. 1998, 14, 247–252. [Google Scholar]
- Nishi, K.; Sato, F.; Karasawa, T.; Sato, T.; Takahashi, H.; Nishi, K. Ecology and control of root necrosis of soybean caused by Calonectria crotalariae. Bull. Nat. Agric. Res. Cent. 1999, 30, 11–109. [Google Scholar]
- Ochi, S. Studies on Soybean Red Crown Rot Disease. Ph.D. Thesis, Hokkaido University, Sapporo, Japan, 2014. [Google Scholar]
- Nakajima, T.; Sakai, S.; Gomi, T.; Kikuchi, A. Development of methods for assessing resistance to black root rot caused by Calonectria crotalariae in soybean [Glycine max] and screening for resistant germplasm. Bull. Tohoku Natl. Agric. Exp. Stn. (Japan) 1994. [Google Scholar]
- Kim, K.D. Susceptibility in Soybean to Red Crown Rot and Characteristics of Virulence in Calonectria Crotalariae. Ph.D. Thesis, LSU Historical Dissertations and Theses. 5805. Louisiana State University and Agricultural & Mechanical College, Baton Rouge, LA, USA, 1994. [Google Scholar]
- Muñoz, N.; Liu, A.; Kan, L.; Li, M.-W.; Lam, H.-M. Potential uses of wild germplasms of grain legumes for crop improvement. Int. J. Mol. Sci. 2017, 18, 328. [Google Scholar]
- Mammadov, J.; Buyyarapu, R.; Guttikonda, S.K.; Parliament, K.; Abdurakhmonov, I.; Kumpatla, S.P. Wild relatives of maize, rice, cotton, and soybean: Treasure troves for tolerance to biotic and abiotic stresses. Front. Plant Sci. 2018, 9, 886. [Google Scholar] [CrossRef]
- Li, Y.-H.; Zhou, G.; Ma, J.; Jiang, W.; Jin, L.-G.; Zhang, Z.; Guo, Y.; Zhang, J.; Sui, Y.; Zheng, L. De novo assembly of soybean wild relatives for pan-genome analysis of diversity and agronomic traits. Nat. Biotechnol. 2014, 32, 1045. [Google Scholar] [CrossRef]
- Oki, N.; Kaga, A.; Shimizu, T.; Takahashi, M.; Kono, Y.; Takahashi, M. QTL mapping of antixenosis resistance to common cutworm (Spodoptera litura Fabricius) in wild soybean (Glycine soja). PLoS ONE 2017, 12, e0189440. [Google Scholar] [CrossRef]
- Wang, D.; Diers, B.; Arelli, P.; Shoemaker, R. Loci underlying resistance to race 3 of soybean cyst nematode in Glycine soja plant introduction 468916. Theor.Appl. Genet. 2001, 103, 561–566. [Google Scholar] [CrossRef]
- Winter, S.M.J.; Shelp, B.J.; Anderson, T.R.; Welacky, T.W.; Rajcan, I. QTL associated with horizontal resistance to soybean cyst nematode in Glycine soja PI464925B. Theor. Appl. Genet. 2007, 114, 461–472. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C.; Davis, E.L.; Wang, J.; Griffin, J.D.; Kofsky, J.; Song, B.-H. Genome-wide association study of resistance to soybean cyst nematode (Heterodera glycines) HG Type 2.5. 7 in wild soybean (Glycine soja). Front. Plant Sci. 2016, 7, 1214. [Google Scholar] [PubMed]
- Qi, X.; Li, M.-W.; Xie, M.; Liu, X.; Ni, M.; Shao, G.; Song, C.; Yim, A.K.-Y.; Tao, Y.; Wong, F.-L. Identification of a novel salt tolerance gene in wild soybean by whole-genome sequencing. Nat. Commu. 2014, 5, 4340. [Google Scholar] [CrossRef]
- Lee, J.-D.; Shannon, J.G.; Vuong, T.D.; Nguyen, H.T. Inheritance of salt tolerance in wild soybean (Glycine soja Sieb. and Zucc.) accession PI483463. J. Hered. 2009, 100, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Tuyen, D.D.; Lal, S.K.; Xu, D.H. Identification of a major QTL allele from wild soybean (Glycine soja Sieb. & Zucc.) for increasing alkaline salt tolerance in soybean. Theor. Appl. Genet. 2010, 121, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Kaga, A.; Shimizu, T.; Watanabe, S.; Tsubokura, Y.; Katayose, Y.; Harada, K.; Vaughan, D.A.; Tomooka, N. Evaluation of soybean germplasm conserved in NIAS genebank and development of mini core collections. Breed Sci. 2012, 61, 566–592. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.-J.; Sugano, S.; Kaga, A.; Lee, S.S.; Sugimoto, T.; Takahashi, M.; Ishimoto, M. Evaluation of resistance to Phytophthora sojae in soybean mini core collections using an improved assay system. Phytopathology 2016, 107, 216–223. [Google Scholar] [CrossRef]
- Ochi, S.; Nakagawa, A. A simple method for long-term cryopreservation of Calonectria ilicicola on barley grains. J. Gen. Plant Pathol. 2010, 76, 112–115. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. Isolation of DNA from fresh plant tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Sugano, S.; Sugimoto, T.; Takatsuji, H.; Jiang, C.J. Induction of resistance to Phytophthora sojae in soyabean (Glycine max) by salicylic acid and ethylene. Plant Pathol. 2013, 62, 1048–1056. [Google Scholar] [CrossRef]
- Jiang, C.J.; Shimono, M.; Sugano, S.; Kojima, M.; Yazawa, K.; Yoshida, R.; Inoue, H.; Hayashi, N.; Sakakibara, H.; Takatsuji, H. Abscisic acid interacts antagonistically with salicylic acid signaling pathway in rice-Magnaporthe grisea interaction. Mol. Plant Microbe Interact. 2010, 23, 791–798. [Google Scholar] [CrossRef]
- Qi, M.; Yang, Y. Quantification of Magnaporthe grisea during infection of rice plants using real-time polymerase chain reaction and northern blot/phosphoimaging analyses. Phytopathology 2002, 92, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Gai, Y.; Deng, Q.; Chen, X.; Guan, M.; Xiao, X.; Xu, D.; Deng, M.; Pan, R. Phylogenetic diversity of Calonectria ilicicola causing Cylindrocladium black rot of peanut and red crown rot of soybean in southern China. J. Gen. Plant Pathol. 2017, 83, 273–282. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, C.-J.; Sugano, S.; Ochi, S.; Kaga, A.; Ishimoto, M. Evaluation of Glycine max and Glycine soja for Resistance to Calonectria ilicicola. Agronomy 2020, 10, 887. https://doi.org/10.3390/agronomy10060887
Jiang C-J, Sugano S, Ochi S, Kaga A, Ishimoto M. Evaluation of Glycine max and Glycine soja for Resistance to Calonectria ilicicola. Agronomy. 2020; 10(6):887. https://doi.org/10.3390/agronomy10060887
Chicago/Turabian StyleJiang, Chang-Jie, Shoji Sugano, Sunao Ochi, Akito Kaga, and Masao Ishimoto. 2020. "Evaluation of Glycine max and Glycine soja for Resistance to Calonectria ilicicola" Agronomy 10, no. 6: 887. https://doi.org/10.3390/agronomy10060887
APA StyleJiang, C.-J., Sugano, S., Ochi, S., Kaga, A., & Ishimoto, M. (2020). Evaluation of Glycine max and Glycine soja for Resistance to Calonectria ilicicola. Agronomy, 10(6), 887. https://doi.org/10.3390/agronomy10060887




