Soybean Fertilized by P-Phases from Bagasse-Based Materials: P-Extraction Procedures, Diffusive Gradients in Thin Films (DGT), and X-ray Diffraction Analysis (XRD)
Abstract
1. Introduction
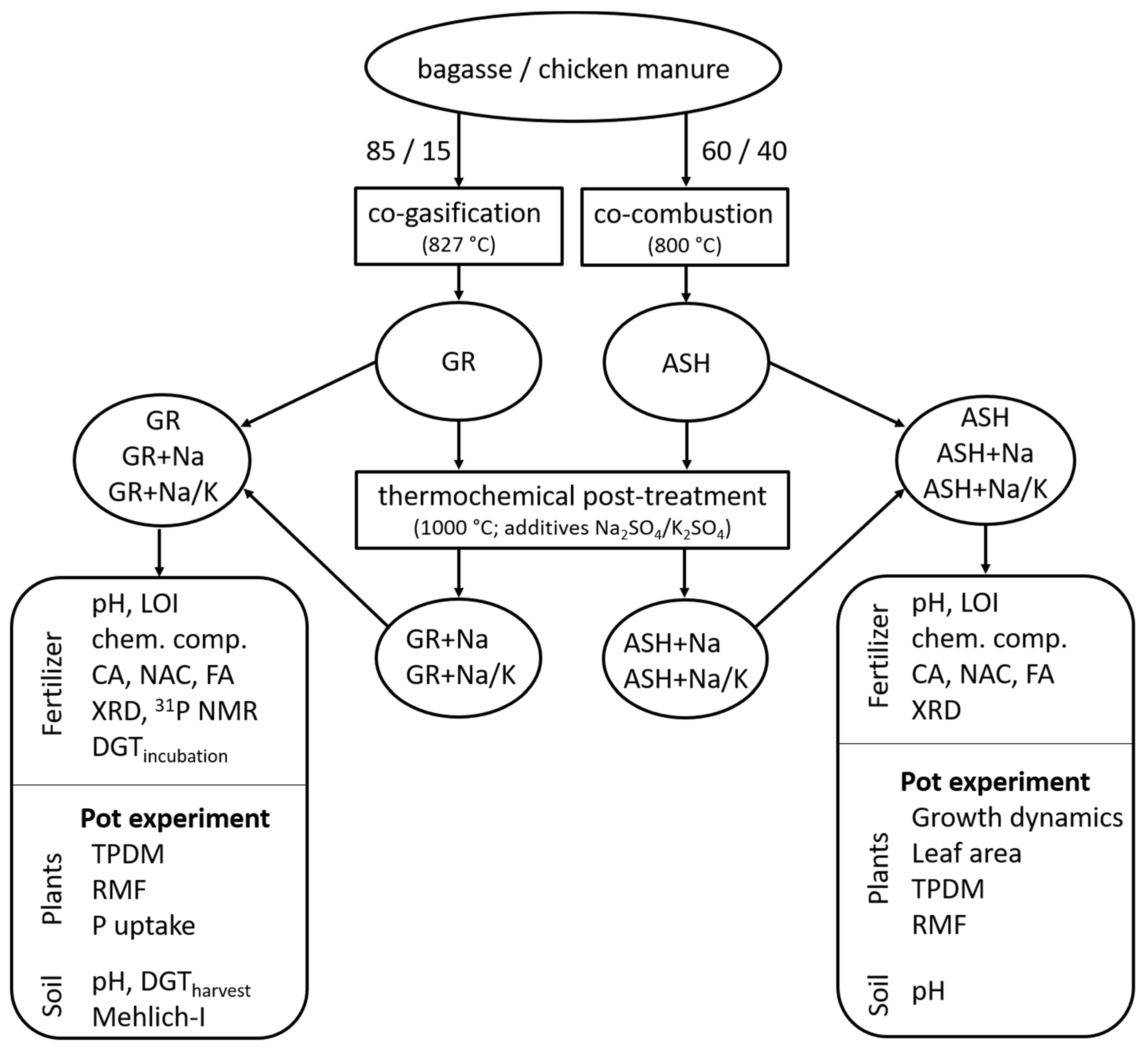
2. Materials and Methods
2.1. P-Fertilizer Preparation
2.2. Greenhouse Pot Experiments
2.2.1. GR-Based P-Fertilizer (First Experiment)
2.2.2. ASH-Based P-Fertilizer (Second Experiment)
2.3. Sample Preparation and Analysis
2.3.1. Non-Invasive Measurements
2.3.2. Harvest
2.3.3. Chemical Analysis of P for Calculation of P Uptake
2.3.4. Determination of pH Value and LOI
2.3.5. Chemical Extraction of Soils
2.3.6. Chemical Extraction of Fertilizers
2.3.7. DGT Experiments with Soils and Fertilizers
2.3.8. Crystalline Phase Analysis of Fertilizers by XRD
2.3.9. Identification and Quantification of P-Species with Liquid 31P-NMR
2.4. Statistics
3. Results
3.1. Gasification Residue and Its Thermochemical Products
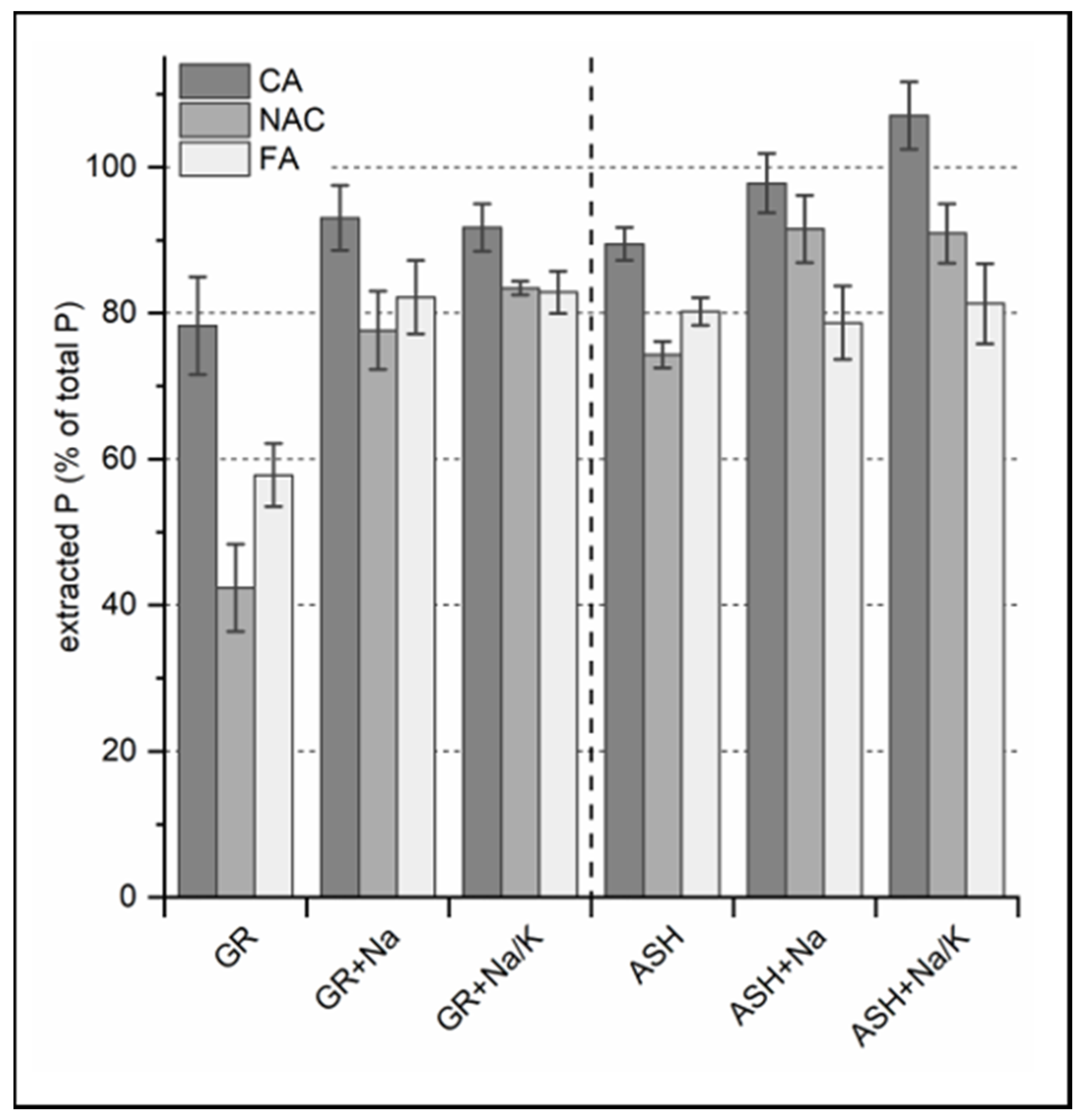
3.1.1. Chemical Extraction and P-Phase Analysis
3.1.2. P-Fertilization Effects
3.1.3. Correlations of Soil Tests (Mehlich-I and DGT) to P Uptake by Soybeans
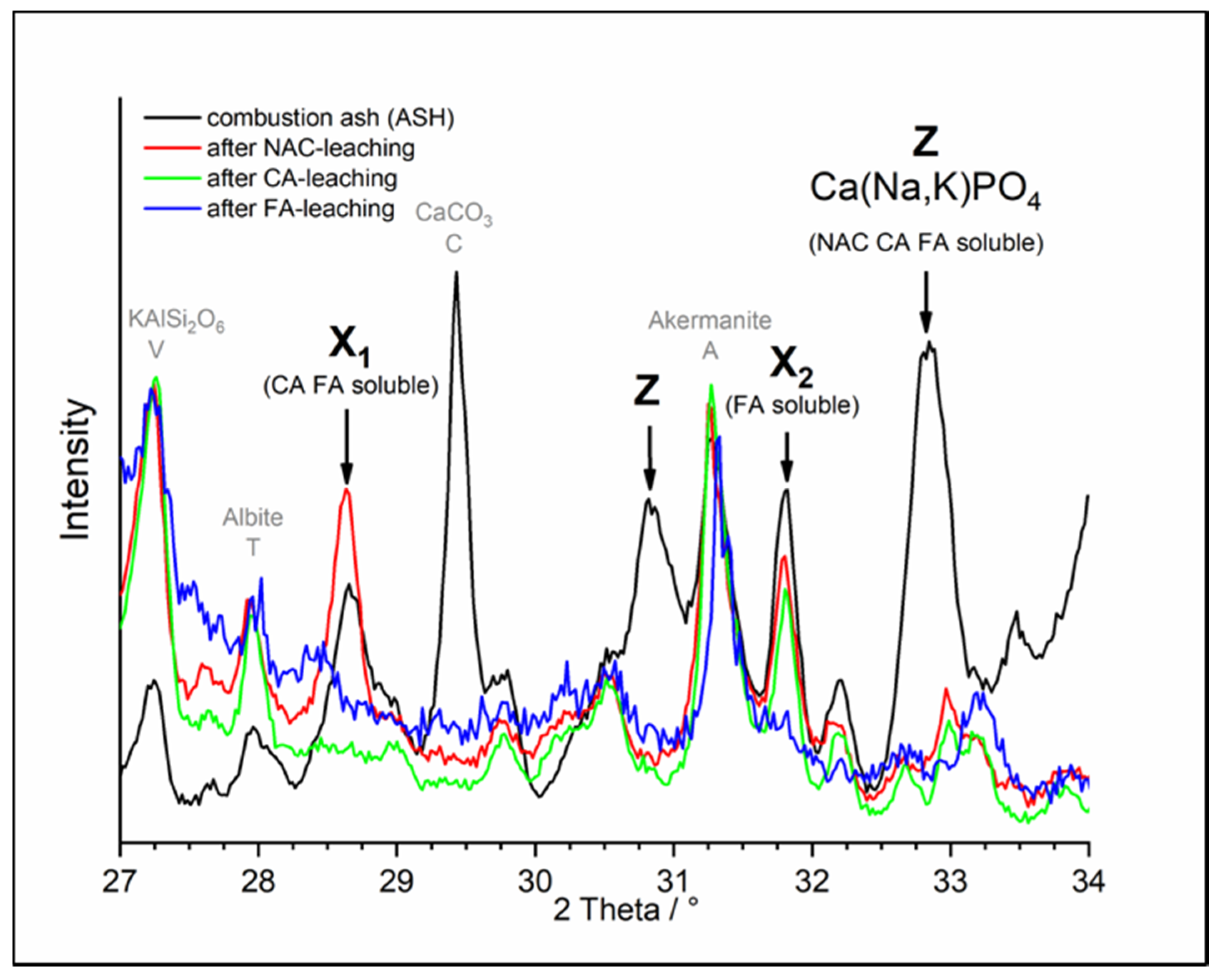
3.2. Combustion Ash and Its Thermochemical Products
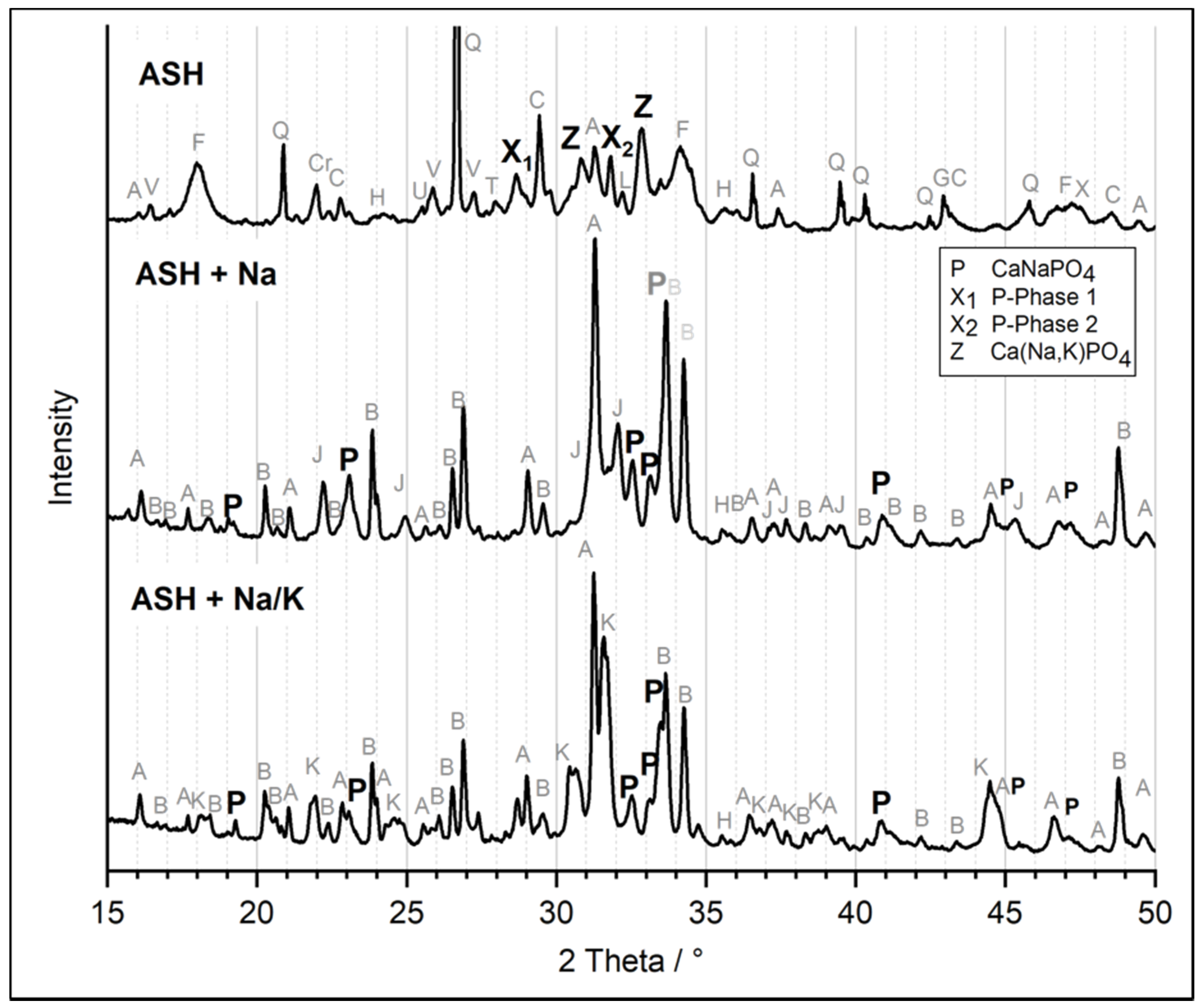
3.2.1. Chemical Extraction and P-Phase Analysis
3.2.2. P-Fertilization Effects of ASH-Based Fertilizers
4. Discussion
4.1. Gasification Residue (GR) and Its Thermochemical Products
4.1.1. Effect of Thermochemical Treatment on Plant Growth
4.1.2. Extraction Methods and Crystalline Phase Composition
4.1.3. Soil Tests
4.2. Different Phase Composition between GR and ASH
4.3. Thermochemical Treatment of ASH
5. Conclusions
6. Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| GR | ASH | Chicken Manure | Na2SO4 | K2SO4 | |
|---|---|---|---|---|---|
| GR+Na | 300 | 0 | 0 | 75 | 0 |
| GR+Na/K | 300 | 0 | 0 | 60 | 25 |
| ASH+Na | 0 | 300 | 90 | 135 | 0 |
| ASH+Na/K | 0 | 300 | 90 | 110 | 45 |

Appendix A.1. Description of Phases Which Contain No P
Appendix A.1.1. GR-Based Fertilizers
Appendix A.1.2. ASH-Based Fertilizers

 low
low  medium
medium  high amount. This quantification is a crude semi-quantification with comparison of reflex intensity and chemical composition.
high amount. This quantification is a crude semi-quantification with comparison of reflex intensity and chemical composition.
 low
low  medium
medium  high amount. This quantification is a crude semi-quantification with comparison of reflex intensity and chemical composition.
high amount. This quantification is a crude semi-quantification with comparison of reflex intensity and chemical composition.| Quartz SiO2 | Sylvin KCl | Calcite CaCO3 | Akermanite Ca2MgSi2O7 | Nepheline (K,Na)AlSiO4 | Kalsilite KAlSiO4 | Na2SO4 | K3Na(SO4)2 | Anhydrate CaSO4 | Magnetite Fe3O4 | Hematite Fe2O3 | |
| Q | Cl | C | A | E | L | N | K | An | M | H | |
| GR |  |  |  | - | - | - | - | - | - |  | - |
| GR + Na | - | - | - |  |  | - |  |  |  | - |  |
| GR + Na/K | - | - | - |  |  |  |  |  |  | - |  |
| Quartz SiO2 | Cristob. SiO2 | Calcite CaCO3 | Akermanite Ca2MgSi2O7 | Free lime Ca(OH)2 | Na4Ca4Si6O18 | KNaSO4 | K3Na(SO4)2 | Periclase MgO | Further silicates | Hematite Fe2O3 | |
| Q | Cr | C | A | F | B | J | K | G | T/U/V | H | |
| ASH |  |  |  |  |  | - | - | - |  |  |  |
| ASH + Na | - | - | - |  | - |  |  | - | - | - |  |
| ASH + Na/K | - | - | - |  | - |  | - |  | - | - |  |
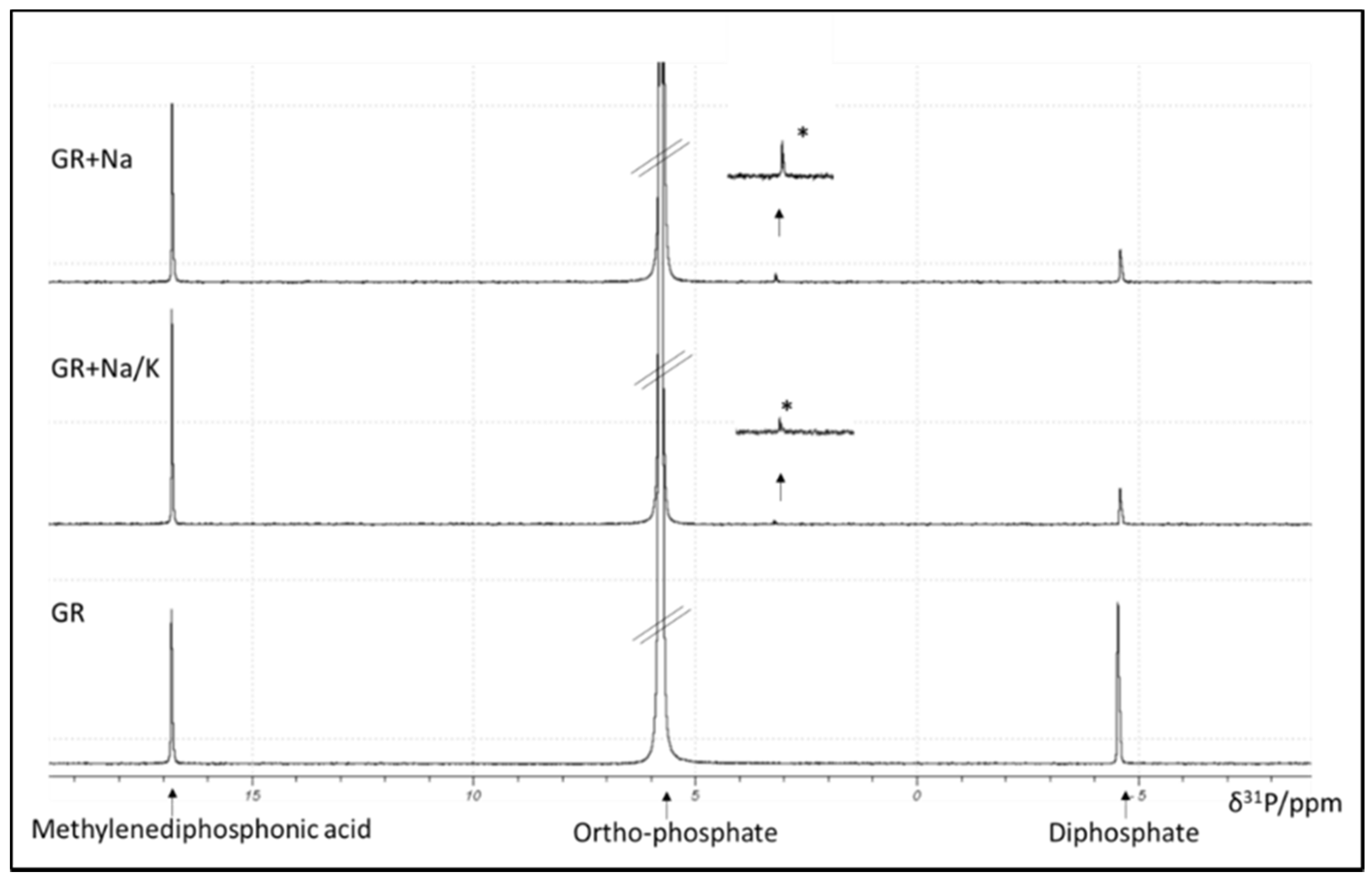
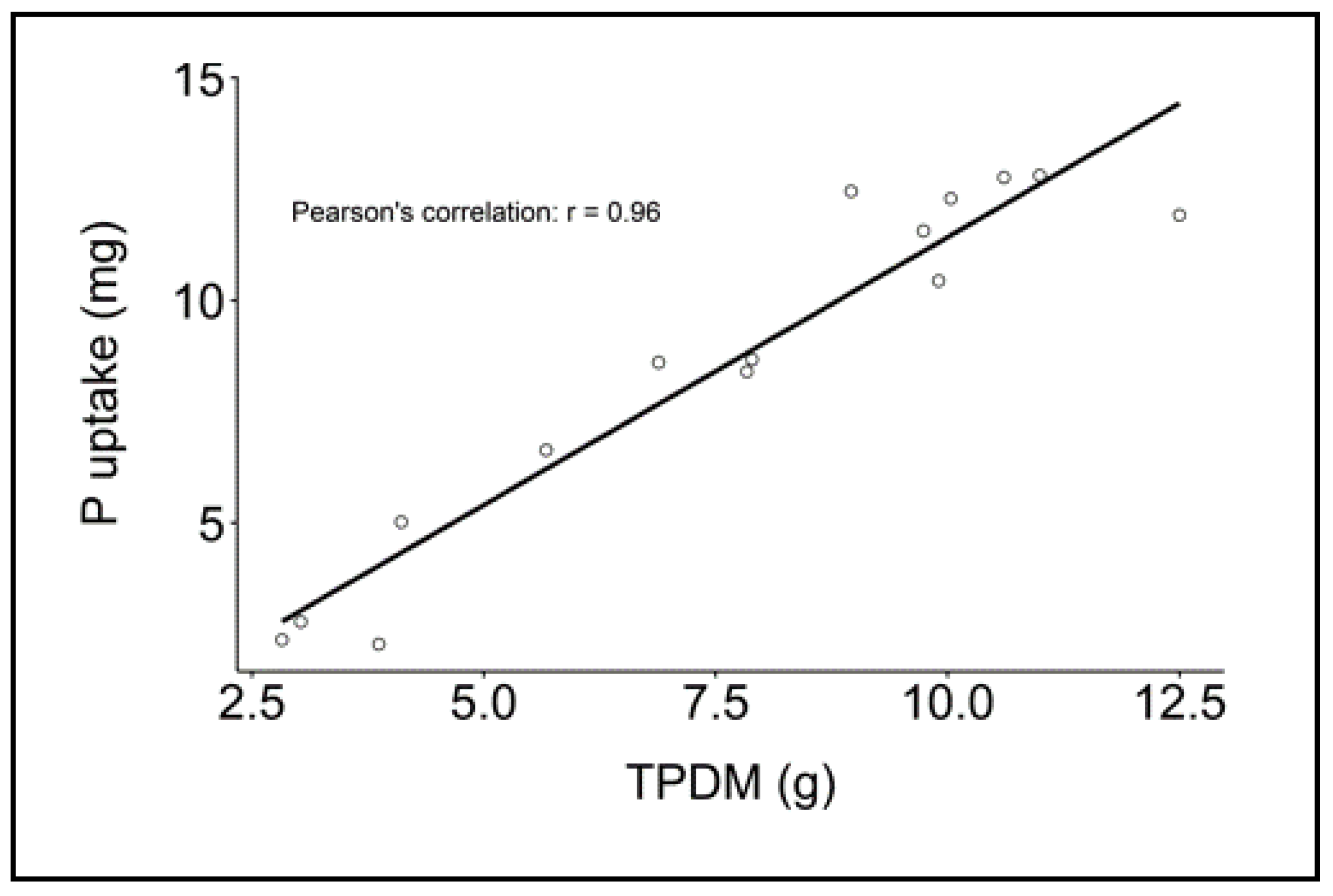
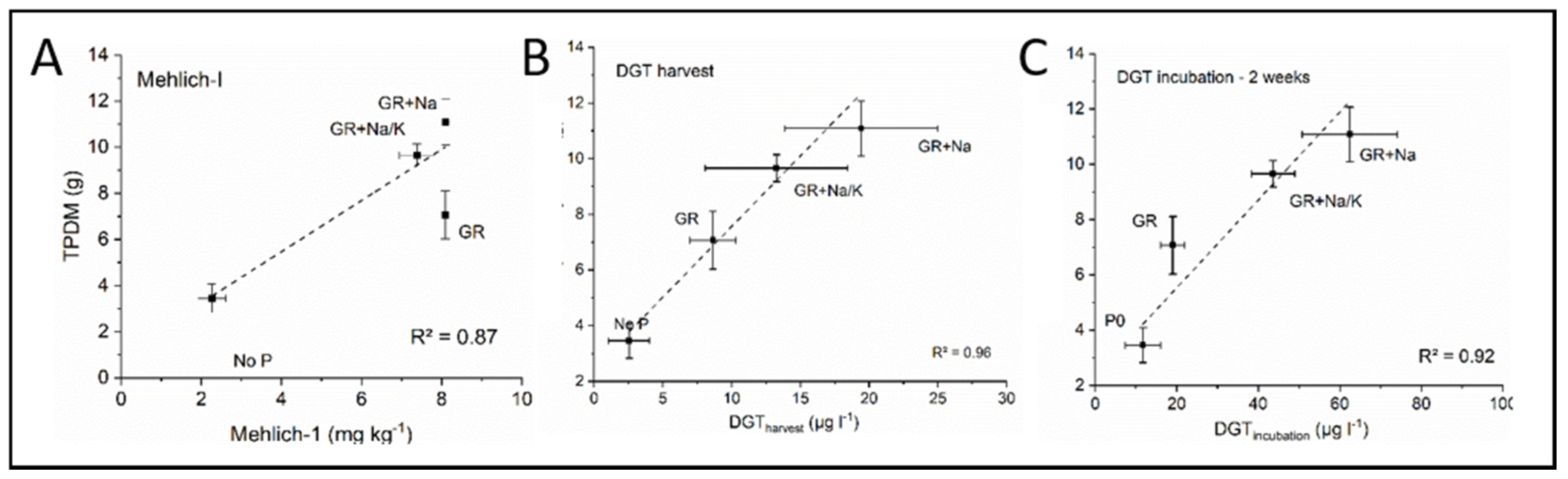
| Used Fertilizer | pH | Aqua Regia [mg/kg] | Mehlich-I [mg/kg] | DGTharvest [µg/l] | DGTincubation [µg/l] |
|---|---|---|---|---|---|
| No P | 5.4 | 447 ± 3 | 2.3 ± 0.5 | 2.5 ± 2.1 | 11.7 ± 6.1 |
| GR | 5.8 | 521 ± 15 | 8.1 ± < 0.1 | 8.6 ± 2.4 | 19.0 ± 4.2 |
| GR+Na | 5.7 | 530 ± 7 | 8.1 ± 0.1 | 19.4 ± 7.8 | 62.4 ± 16.5 |
| GR+Na/K | 5.7 | 529 ± 39 | 7.4 ± 0.6 | 13.2 ± 7.3 | 43.6 ± 7.6 |
References
- Almazan, O.; Gonzalez, L.; Galvez, L. The sugar cane, its by-products and co-products. In Proceedings of the AMAS, Réduit, Mauritius, 17–18 November 1998; pp. 13–25. [Google Scholar]
- Rocha, G.J.d.M.; Nascimento, V.M.; Gonçalves, A.R.; Silva, V.F.N.; Martín, C. Influence of mixed sugarcane bagasse samples evaluated by elemental and physical–chemical composition. Ind. Crops Prod. 2015, 64, 52–58. [Google Scholar] [CrossRef]
- Ochoa George, P.A.; Eras, J.J.C.; Gutierrez, A.S.; Hens, L.; Vandecasteele, C. Residue from Sugarcane Juice Filtration (Filter Cake): Energy Use at the Sugar Factory. Waste Biomass Valorization 2010, 1, 407–413. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#compare (accessed on 3 March 2020).
- Ferreira, E.P.d.B.; Fageriae, N.K.; Didonet, A.D. Chemical properties of an oxisol under organic management as influenced by application of sugarcane bagasse ash. Revista Ciência Agronômica 2012, 43, 228–236. [Google Scholar] [CrossRef]
- Pita, V.; Vasconcelos, E.; Cabral, F.; Ribeiro, H.M. Effect of ash from sugarcane bagasse and wood co-combustion on corn growth and soil properties. Arch. Agron. Soil Sci. 2012, 58, S206–S212. [Google Scholar] [CrossRef]
- Thind, H.S.; Singh, Y.; Sharma, S.; Singh, V.; Sran, H.S.; Singh, B. Phosphorus fertilizing potential of bagasse ash and rice husk ash in wheat–rice system on alkaline loamy sand soil. J. Agric. Sci. 2017, 155, 465–474. [Google Scholar] [CrossRef]
- Gonfa, A.; Bedadi, B.; Argaw, A. Effect of bagasse ash and filter cake amendments on wheat (Triticum turgidum L.var. durum) yield and yield components in nitisol. Int. J. Recycl. Org. Waste Agric. 2018, 7, 231–240. [Google Scholar] [CrossRef]
- Li, X.; Rubæk, G.H.; Sørensen, P. Availability of potassium in biomass combustion ashes and gasification biochars after application to soils with variable pH and clay content. Arch. Agron. Soil Sci. 2017, 64, 1119–1130. [Google Scholar] [CrossRef]
- Tran, Q.T.; Maeda, M.; Oshita, K.; Takaoka, M.; Saito, K. Phosphorus and potassium availability from cattle manure ash in relation to their extractability and grass tetany hazard. Soil Sci. Plant Nutr. 2018, 64, 415–422. [Google Scholar] [CrossRef]
- Demeyer, A.; Voundi Nkana, J.C.; Verloo, M.G. Characteristics of wood ash and influence on soil properties and nutrient uptake: An overview. Bioresour. Technol. 2001, 77, 287–295. [Google Scholar] [CrossRef]
- Freire, M.; Lopes, H.; Tarelho, L.A.C. Critical aspects of biomass ashes utilization in soils: Composition, leachability, PAH and PCDD/F. Waste Manag. 2015, 46, 304–315. [Google Scholar] [CrossRef]
- Schiemenz, K.; Eichler-Löbermann, B. Biomass ashes and their phosphorus fertilizing effect on different crops. Nutr. Cycl. Agroecosyst. 2010, 87, 471–482. [Google Scholar] [CrossRef]
- Müller-Stöver, D.S.; Jakobsen, I.; Grønlund, M.; Rolsted, M.M.M.; Magid, J.; Hauggaard-Nielsen, H.; Goss, M. Phosphorus bioavailability in ash from straw and sewage sludge processed by low-temperature biomass gasification. Soil Use Manag. 2018, 34, 9–17. [Google Scholar] [CrossRef]
- Basso, C.J.; Muraro, D.S.; Girotto, E.; Silva, D.R.O.d.; Silva, A.N.d. Poultry litter and swine compost as nutrients sources in millet. Biosci. J. 2017, 288–296. [Google Scholar] [CrossRef]
- Abdala, D.B.; Ghosh, A.K.; da Silva, I.R.; de Novais, R.F.; Alvarez Venegas, V.H. Phosphorus saturation of a tropical soil and related P leaching caused by poultry litter addition. Agric. Ecosyst. Environ. 2012, 162, 15–23. [Google Scholar] [CrossRef]
- Christel, W.; Bruun, S.; Magid, J.; Jensen, L.S. Phosphorus availability from the solid fraction of pig slurry is altered by composting or thermal treatment. Bioresour. Technol. 2014, 169, 543–551. [Google Scholar] [CrossRef]
- Bergfeldt, B.; Tomasi Morgano, M.; Leibold, H.; Richter, F.; Stapf, D. Recovery of Phosphorus and other Nutrients during Pyrolysis of Chicken Manure. Agriculture 2018, 8, 187. [Google Scholar] [CrossRef]
- Kratz, S.; Vogel, C.; Adam, C. Agronomic performance of P recycling fertilizers and methods to predict it: A review. Nutr. Cycl. Agroecosyst. 2019, 115, 1–39. [Google Scholar] [CrossRef]
- Severin, M.; Breuer, J.; Rex, M.; Stemann, J.; Adam, C.; Van den Weghe, H.; Kücke, M. Phosphate fertiliser value of heat treated sewage sludge ash. Plant Soil Environ. 2014, 60, 555–561. [Google Scholar] [CrossRef]
- Steckenmesser, D.; Vogel, C.; Adam, C.; Steffens, D. Effect of various types of thermochemical processing of sewage sludges on phosphorus speciation, solubility, and fertilization performance. Waste Manag. 2017, 62, 194–203. [Google Scholar] [CrossRef]
- Vogel, C.; Rivard, C.; Wilken, V.; Muskolus, A.; Adam, C. Performance of secondary P-fertilizers in pot experiments analyzed by phosphorus X-ray absorption near-edge structure (XANES) spectroscopy. Ambio 2018, 47, 62–72. [Google Scholar] [CrossRef]
- Herzel, H.; Krüger, O.; Hermann, L.; Adam, C. Sewage sludge ash—A promising secondary phosphorus source for fertilizer production. Sci. Total Environ. 2016, 542, 1136–1143. [Google Scholar] [CrossRef]
- Brod, E.; Øgaard, A.F.; Haraldsen, T.K.; Krogstad, T. Waste products as alternative phosphorus fertilisers part II: Predicting P fertilisation effects by chemical extraction. Nutr. Cycl. Agroecosys. 2015, 103, 187–199. [Google Scholar] [CrossRef]
- Empresa Brasileira de Pesquisa Agropecuária (EMBRAPA). Manual for Methods of Soil Analysis, 2nd ed.; National Service for Soil Survey and Soil Conservation: Rio de Janeiro, Brazil, 1997.
- Six, L.; Smolders, E.; Merckx, R. The performance of DGT versus conventional soil phosphorus tests in tropical soils—Maize and rice responses to P application. Plant Soil 2013, 366, 49–66. [Google Scholar] [CrossRef]
- Six, L.; Smolders, E.; Merckx, R. Testing phosphorus availability for maize with DGT in weathered soils amended with organic materials. Plant Soil 2014, 376, 177–192. [Google Scholar] [CrossRef]
- Hartmann, T.E.; Wollmann, I.; You, Y.; Müller, T. Sensitivity of Three Phosphate Extraction Methods to the Application of Phosphate Species Differing in Immediate Plant Availability. Agronomy 2019, 9, 29. [Google Scholar] [CrossRef]
- Duboc, O.; Santner, J.; Golestani Fard, A.; Zehetner, F.; Tacconi, J.; Wenzel, W.W. Predicting phosphorus availability from chemically diverse conventional and recycling fertilizers. Sci. Total Environ. 2017, 599–600, 1160–1170. [Google Scholar] [CrossRef]
- Vogel, C.; Sekine, R.; Steckenmesser, D.; Lombi, E.; Steffens, D.; Adam, C. Phosphorus availability of sewage sludge-based fertilizers determined by the diffusive gradients in thin films (DGT) technique. J. Plant Nutr. Soil Sci. 2017, 180, 594–601. [Google Scholar] [CrossRef]
- Nanzer, S.; Oberson, A.; Huthwelker, T.; Eggenberger, U.; Frossard, E. The Molecular Environment of Phosphorus in Sewage Sludge Ash: Implications for Bioavailability. J. Environ. Qual. 2014, 43, 1050–1060. [Google Scholar] [CrossRef]
- Yusiharni, B.E.; Ziadi, H.; Gilkes, R.J. A laboratory and glasshouse evaluation of chicken litter ash, wood ash, and iron smelting slag as liming agents and P fertilisers. Soil Res. 2007, 45, 374–389. [Google Scholar] [CrossRef]
- Staroń, P.; Kowalski, Z.; Staroń, A.; Banach, M. Thermal treatment of waste from the meat industry in high scale rotary kiln. Int. J. Environ. Sci. Technol. 2017, 14, 1157–1168. [Google Scholar] [CrossRef]
- Bogush, A.A.; Stegemann, J.A.; Williams, R.; Wood, I.G. Element speciation in UK biomass power plant residues based on composition, mineralogy, microstructure and leaching. Fuel 2018, 211, 712–725. [Google Scholar] [CrossRef]
- Schettino, M.A.S.; Holanda, J.N.F. Characterization of Sugarcane Bagasse ash Waste for Its Use in Ceramic Floor Tile. Procedia Mater. Sci. 2015, 8, 190–196. [Google Scholar] [CrossRef]
- Govindarajan, D.; Jayalakshmi, G. XRD, FTIR and Microstructure Studies of Calcined Sugarcane Bagasse Ash. Adv. Appl. Sci. Res. 2011, 2, 544–549. [Google Scholar]
- Stemann, J.; Peplinski, B.; Adam, C. Thermochemical treatment of sewage sludge ash with sodium salt additives for phosphorus fertilizer production—Analysis of underlying chemical reactions. Waste Manag. 2015, 45, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Kuepper, B.; Steinweg, T.; Piotrowski, M. Feed and Livestock in Brazil, China, EU Consume Most Cerrado Soy. Available online: www.chainreactionresearch.com (accessed on 6 March 2020).
- Bordonal, R.d.O.; Carvalho, J.L.N.; Lal, R.; de Figueiredo, E.B.; de Oliveira, B.G.; La Scala, N. Sustainability of sugarcane production in Brazil. A review. Agron. Sustain. Dev. 2018, 38, 13. [Google Scholar] [CrossRef]
- Withers, P.J.A.; Rodrigues, M.; Soltangheisi, A.; de Carvalho, T.S.; Guilherme, L.R.G.; Benites, V.d.M.; Gatiboni, L.C.; de Sousa, D.M.G.; Nunes, R.d.S.; Rosolem, C.A.; et al. Transitions to sustainable management of phosphorus in Brazilian agriculture. Sci. Rep. 2018, 8, 2537. [Google Scholar] [CrossRef]
- Vodegel, S.; Müller, F. BioÖkonomie International: ASHES—Rückführung von Nährstoffen aus Aschen von Thermo-Chemischen Prozessen mit Bagasse bzw. Bagassestroh, Teilprojekt C.; CUTEC: Clausthal-Zellerfeld, Germany, 2019. [Google Scholar]
- Pettersson, A.; Åmand, L.-E.; Steenari, B.-M. Leaching of ashes from co-combustion of sewage sludge and wood—Part I: Recovery of phosphorus. Biomass Bioenergy 2008, 32, 224–235. [Google Scholar] [CrossRef]
- Pan, H.; Eberhardt, T. Characterization of fly ash from the gasification of wood and assessment for its application as a soil amendment. Bioresources 2011, 6. [Google Scholar] [CrossRef]
- FAO. Fertilizer Use by Crop in Brazil; Food and Agriculture Organization of the United Nation: Rome, Italiy, 2004. [Google Scholar]
- Nabel, M.; Schrey, S.D.; Poorter, H.; Koller, R.; Nagel, K.A.; Temperton, V.M.; Dietrich, C.C.; Briese, C.; Jablonowski, N.D. Coming Late for Dinner: Localized Digestate Depot Fertilization for Extensive Cultivation of Marginal Soil With Sida hermaphrodita. Front. Plant Sci. 2018, 9, 1095. [Google Scholar] [CrossRef]
- Nakhforoosh, A.; Bodewein, T.; Fiorani, F.; Bodner, G. Identification of Water Use Strategies at Early Growth Stages in Durum Wheat from Shoot Phenotyping and Physiological Measurements. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef]
- Scharr, H.; Briese, C.; Embgenbroich, P.; Fischbach, A.; Fiorani, F.; Müller-Linow, M. Fast High Resolution Volume Carving for 3D Plant Shoot Reconstruction. Front. Plant Sci. 2017, 8, 1680. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-boem, F.H.; Thomas, G.W. Phosphorus nutrition and water deficits in field-grown soybeans. Plant Soil 1999, 207, 87–96. [Google Scholar] [CrossRef]
- DIN EN 16174. Schlamm, behandelter Bioabfall und Boden—Aufschluss von mit Königswasser löslichen Anteilen von Elementen. Deutsches Institut für Normung 2012. [Google Scholar] [CrossRef]
- Mehlich, A. Determination of P, Ca, Mg, K, Na, and NH4. Soil Test. Div. 1953, 1–53, 23–89. [Google Scholar]
- EU. Regulation (EC) No 2003/2003 of the European Parliament and of the Council of 13 October 2003 Relating to Fertilizers; L304, O.J.o.t.E.U., Ed.; EU: Brussels, Belgium, 2003. [Google Scholar]
- Vestergren, J.; Vincent, A.G.; Jansson, M.; Persson, P.; Ilstedt, U.; Gröbner, G.; Giesler, R.; Schleucher, J. High-Resolution Characterization of Organic Phosphorus in Soil Extracts Using 2D 1H–31P NMR Correlation Spectroscopy. Environ. Sci. Technol. 2012, 46, 3950–3956. [Google Scholar] [CrossRef]
- Cade-Menun, B.; Liu, C.W. Solution Phosphorus-31 Nuclear Magnetic Resonance Spectroscopy of Soils from 2005 to 2013: A Review of Sample Preparation and Experimental Parameters. Soil Sci. Soc. Am. J. 2014, 78, 19–37. [Google Scholar] [CrossRef]
- Ando, J.; Matsuno, S. Ca3(PO4)2-CaNaPO4 System. B Chem. Soc. Jpn. 1968, 41, 342–347. [Google Scholar] [CrossRef]
- Zogli, P.; Pingault, L.; Libault, M. Physiological and Molecular Mechanisms and Adaptation Strategies in Soybean (Glycine max) Under Phosphate Deficiency. In Legume Nitrogen Fixation in Soils with Low Phosphorus Availability: Adaptation and Regulatory Implication; Sulieman, S., Tran, L.-S.P., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 219–242. [Google Scholar] [CrossRef]
- Lopez, R.; Padilla, E.; Bachmann, S.; Eichler-Löbermann, B. Effects of biomass ashes on plant nutrition in tropical and temperate regions. J. Agric. Rural Dev. Trop. Subtrop. 2009, 110, 49–58. [Google Scholar]
- Akkajit, P.; DeSutter, T.; Tongcumpou, C. Short-term effects of sugarcane waste products from ethanol production plant as soil amendments on sugarcane growth and metal stabilization. Environ. Sci. Process. Impacts 2013, 15, 947–954. [Google Scholar] [CrossRef]
- Webber, C.L., III; White, P.M., Jr.; Spaunhorst, D.J.; Petrie, E.C. Impact of Sugarcane Bagasse Ash as an Amendment on the Physical Properties, Nutrient Content and Seedling Growth of a Certified Organic Greenhouse Growing Media. J. Agric. Sci. 2017, 9, 1. [Google Scholar] [CrossRef]
- Camps-Arbestain, M.; Shen, Q.; Wang, T.; van Twieten, L.; Novak, J. Available nutrients in biochar. In Biochar: A Guide to Analytical Methods; Singh, B., Camps-Arbestain, M., Lehmann, J., Eds.; CSIRO Publishing: Clayton, VIC, Australia, 2017; pp. 109–125. [Google Scholar]
- Gunawardane, R.P.; Glasser, F.P. Reaction of chlorapatite, Ca5(PO4)3(Cl,F) with sodium carbonate and silica. J. Mater. Sci. 1979, 14, 2797–2810. [Google Scholar] [CrossRef]
- Vance, C.P.; Uhde-Stone, C.; Allan, D.L. Phosphorus acquisition and use: Critical adaptations by plants for securing a nonrenewable resource. New Phytol. 2003, 157, 423–447. [Google Scholar] [CrossRef]
- Tawaraya, K.; Horie, R.; Shinano, T.; Wagatsuma, T.; Saito, K.; Oikawa, A. Metabolite profiling of soybean root exudates under phosphorus deficiency. Soil Sci. Plant Nutr. 2014, 60, 679–694. [Google Scholar] [CrossRef]
- Haynes, R.J. Soil acidification induced by leguminous crops. Grass Forage Sci. 1983, 38, 1–11. [Google Scholar] [CrossRef]
- Hinsinger, P.; Plassard, C.; Tang, C.; Jaillard, B. Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: A review. Plant Soil 2003, 248, 43–59. [Google Scholar] [CrossRef]
- Hinsinger, P.; Herrmann, L.; Lesueur, D.; Robin, A.; Trap, J.; Waithaisong, K.; Plassard, C. Impact of roots, microorganisms and microfauna on the fate of soil phosphorus in the rhizosphere. In Annual Plant Reviews Volume 48; Plaxton, W.C., Lambers, H., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 375–407. [Google Scholar] [CrossRef]
- Rowe, J.J.; Morey, G.W.; Zen, C.S. The Quinary Reciprocal Salt System Na, K, Mg, Ca/Cl, SO4—A Review of the Literature with New Data; U.S. Government Printing Office Washington: Washington, DC, USA, 1972.


| Fertilizer | GR | GR+Na | GR+Na/K | ASH | ASH+Na | ASH+Na/K |
|---|---|---|---|---|---|---|
| pH | 11.6 | 10.5 | 10.6 | 12.7 | 9.5 | 9.3 |
| LOI (550 °C) | 36.0 | 8.0 | 8.1 | 2.8 | 0 | 0 |
| Chemical Compositions | ||||||
| Al | 2.3 ± 0.1 | 2.5 ± <0.1 | 2.2 ± 0.1 | 1.6 ± <0.1 | 1.3 ± <0.1 | 1.2 ± <0.1 |
| Ca | 8.8 ± 0.3 | 8.8 ± 0.5 | 8.3 ± 0.3 | 15.2 ± 0.1 | 12.4 ± 0.1 | 11.8 ± 0.1 |
| Fe | 3.4 ± 0.1 | 3.3 ± <0.1 | 3.1 ± 0.1 | 2.0 ± 0.1 | 1.5 ± <0.1 | 1.4 ± <0.1 |
| K | 3.6 ± 0.1 | 4.0 ± 0.1 | 6.5 ± 0.3 | 5.2 ± 0.1 | 4.4 ± 0.1 | 6.8 ± 0.1 |
| Mg | 1.6 ± <0.1 | 1.6 ± 0.1 | 1.4 ± <0.1 | 1.8 ± <0.1 | 1.5 ± <0.1 | 1.4 ± <0.1 |
| Na | 0.1 ± <0.1 | 2.9 ± 0.1 | 2.2 ± 0.1 | 0.2 ± <0.1 | 3.1 ± 0.1 | 2.5 ± <0.1 |
| P | 3.3 ± 0.1 | 3.1 ± 0.2 | 2.9 ± <0.1 | 4.0 ± <0.1 | 3.2 ± <0.1 | 3.1 ± 0.1 |
| S | 0.1 ± <0.1 | 5.5 ± <0.1 | 5.4 ± 0.1 | 0.4 ± <0.1 | 4.6 ± 0.1 | 5.0 ± 0.1 |
| Si | 13.0 ± 0.2 | 12.4 ± <0.1 | 11.6 ± 0.4 | 17.0 ± 1.0 | 15.8 ± 0.5 | 14.9 ± 0.2 |
 low amount,
low amount,  high amount,
high amount,  only one P-phase identified. This quantification is a rough semi-quantification with comparison of reflex intensity and further information about extractable P and chemical composition. 1 two ‘undefined P-Phases’, extractable in FA and/or CA.
only one P-phase identified. This quantification is a rough semi-quantification with comparison of reflex intensity and further information about extractable P and chemical composition. 1 two ‘undefined P-Phases’, extractable in FA and/or CA.
 low amount,
low amount,  high amount,
high amount,  only one P-phase identified. This quantification is a rough semi-quantification with comparison of reflex intensity and further information about extractable P and chemical composition. 1 two ‘undefined P-Phases’, extractable in FA and/or CA.
only one P-phase identified. This quantification is a rough semi-quantification with comparison of reflex intensity and further information about extractable P and chemical composition. 1 two ‘undefined P-Phases’, extractable in FA and/or CA.| P-Extractability in CA and NAC | |||||
|---|---|---|---|---|---|
| Lower | Higher | ||||
| Whitlockite | Undefined 1 | Pyro-Phosp. | Ca(Na,K)PO4 | CaNaPO4 | |
| Ca9M(PO4)7 | CaK2P2O7 | ||||
| GR |  | - |  | - | - |
| GR+Na |  | - | - | - |  |
| GR+Na/K |  | - | - | - |  |
| ASH | - |  | - |  | - |
| ASH+Na | - | - | - | - |  |
| ASH+Na/K | - | - | - | - |  |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herzel, H.; Dombinov, V.; Vogel, C.; Willbold, S.; Vettorazzi Levandowski, G.; Meiller, M.; Müller, F.; Zang, J.W.; da Fonseca-Zang, W.A.; Jablonowski, N.D.; et al. Soybean Fertilized by P-Phases from Bagasse-Based Materials: P-Extraction Procedures, Diffusive Gradients in Thin Films (DGT), and X-ray Diffraction Analysis (XRD). Agronomy 2020, 10, 895. https://doi.org/10.3390/agronomy10060895
Herzel H, Dombinov V, Vogel C, Willbold S, Vettorazzi Levandowski G, Meiller M, Müller F, Zang JW, da Fonseca-Zang WA, Jablonowski ND, et al. Soybean Fertilized by P-Phases from Bagasse-Based Materials: P-Extraction Procedures, Diffusive Gradients in Thin Films (DGT), and X-ray Diffraction Analysis (XRD). Agronomy. 2020; 10(6):895. https://doi.org/10.3390/agronomy10060895
Chicago/Turabian StyleHerzel, Hannes, Vitalij Dombinov, Christian Vogel, Sabine Willbold, Gabriel Vettorazzi Levandowski, Martin Meiller, Felix Müller, Joachim Werner Zang, Warde Antonieta da Fonseca-Zang, Nicolai David Jablonowski, and et al. 2020. "Soybean Fertilized by P-Phases from Bagasse-Based Materials: P-Extraction Procedures, Diffusive Gradients in Thin Films (DGT), and X-ray Diffraction Analysis (XRD)" Agronomy 10, no. 6: 895. https://doi.org/10.3390/agronomy10060895
APA StyleHerzel, H., Dombinov, V., Vogel, C., Willbold, S., Vettorazzi Levandowski, G., Meiller, M., Müller, F., Zang, J. W., da Fonseca-Zang, W. A., Jablonowski, N. D., Schrey, S. D., & Adam, C. (2020). Soybean Fertilized by P-Phases from Bagasse-Based Materials: P-Extraction Procedures, Diffusive Gradients in Thin Films (DGT), and X-ray Diffraction Analysis (XRD). Agronomy, 10(6), 895. https://doi.org/10.3390/agronomy10060895







