Role of Trichoderma aggressivum f. europaeum as Plant-Growth Promoter in Horticulture
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Isolates
2.2. Analysis of Plant Growth-Promoting Attributes
2.3. Mass Production of TA and TS on Solid Substrates
2.4. Analysis of Effects of TA and TS on Seed Germination under Laboratory Conditions
2.5. Analysis of Promoter Effects of TA and TS on Pepper and Tomato Seedlings: Experiment 1
2.6. Analysis of Effects of Applying Different Doses of TA and TS to Tomatoes: Experiment 2
2.7. Statistical Analysis
3. Results
3.1. Mass Production of Trichoderma Isolates on Solid Substrates
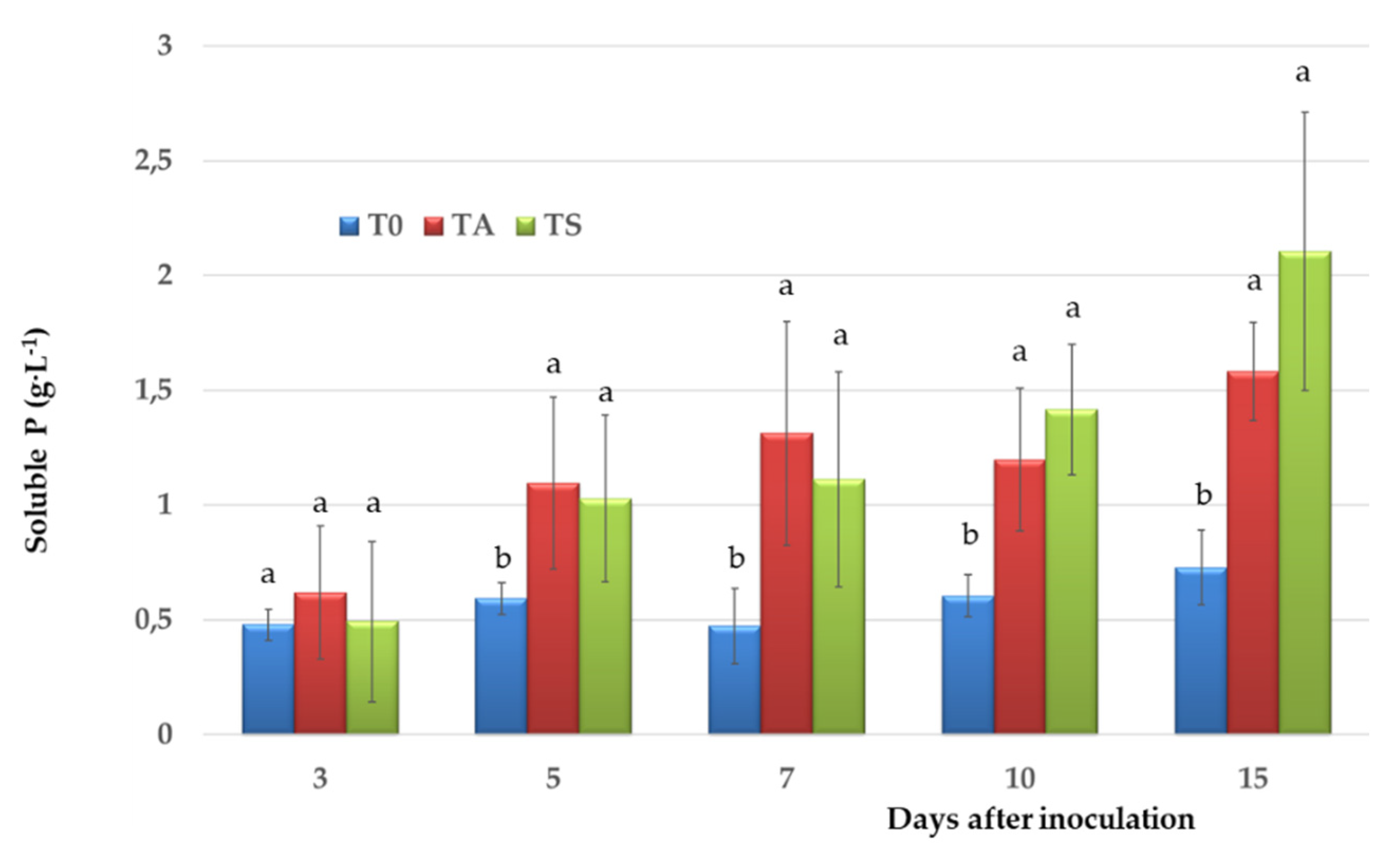
3.2. Siderophore Production, IAA and P Solubilisation
3.3. Effects of TA and TS Treatment on Germination and Vigour Index
3.4. Effects of Trichoderma Inoculation on Tomato and Pepper Seedlings
3.5. Effects of Dose of Application of T. aggressivum f. europaeum and T. saturnisporum
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sánchez-Montesinos, B.; Diánez, F.; Moreno-Gavíra, A.; Gea, F.J.; Santos, M. Plant growth promotion and biocontrol of Pythium ultimum by saline tolerant Trichoderma isolates under salinity stress. Int. J. Environ. Res. Public Health 2019, 16, 2053. [Google Scholar] [CrossRef] [PubMed]
- Khoshmanzar, E.; Aliasgharzad, N.; Neyshabouri, M.R.; Khoshru, B.; Arzanlou, M.; Asgari Lajayer, B. Effects of Trichoderma isolates on tomato growth and inducing its tolerance to water-deficit stress. Int. J. Environ. Sci. Technol. 2020, 17, 869–878. [Google Scholar] [CrossRef]
- Adnan, M.; Islam, W.; Shabbir, A.; Khan, K.A.; Ghramh, H.A.; Huang, Z.; Chen, H.Y.H.; Lu, G. Plant defense against fungal pathogens by antagonistic fungi with Trichoderma in focus. Microb. Pathog. 2019, 129, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Halifu, S.; Deng, X.; Song, X.; Song, R. Efects of Two Trichoderma Strains on Plant Growth, Rhizosphere Soil Nutrients, and Fungal Community of Pinus sylvestris var. mongolica Annual Seedlings. Forests 2019, 10, 758. [Google Scholar]
- Vargas, W.A.; Crutcher, F.K.; Kenerley, C.M. Functional characterization of a plant-like sucrose transporter + from the beneficial fungus Trichoderma virens. Regulation of the symbiotic association with plants by sucrose metabolism inside the fungal cells. New Phytol. 2010, 189, 777–789. [Google Scholar] [CrossRef]
- Garnica-Vergara, A.; Barrera-Ortiz, S.; Muñoz-Parra, E.; Raya-Gonzalez, J.; Mendez-Bravo, A.; Macias-Rodriguez, L.; Ruiz-Herrera, L.F.; Lopez-Bucio, J. The volatile 6-pentyl-2H-pyran-2-one from Trichoderma atroviride regulates Arabidopsis thaliana root morphogenesis via auxin signaling and ethylene insensitive 2 functioning. New Phytol. 2016, 209, 1496–1512. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species -opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Shoresh, M.; Harman, G.E.; Mastouri, F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu. Rev. Phytopathol. 2010, 48, 21–43. [Google Scholar] [CrossRef]
- Hermosa, R.; Viterbo, A.; Chet, I.; Monte, E. Plant-beneficial effects of Trichoderma and of its genes. Microbiology 2012, 158, 17–25. [Google Scholar] [CrossRef]
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrella, A. Trichoderma as biostimulant: Exploiting the multilevel properties of a plant beneficial fungus. Sci. Hortic. 2015, 196, 109–123. [Google Scholar] [CrossRef]
- Diánez, F.; Santos, M.; Carretero, F.; Marín, F. Biostimulant activity of Trichoderma saturnisporum in melon (Cucumis melo). Hortscience 2018, 53, 810–815. [Google Scholar]
- Bononi, L.; Chiaramonte, J.B.; Pansa, C.C.; Moitinho, M.A.; Melo, I.S. Phosphorus-solubilizing Trichoderma pp. from Amazon soils improve soybean plant growth. Sci. Rep. 2020, 10, 2858. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Huang, Y.; Ge, W.; Jia, Z.; Song, S.; Zhang, L.; Huang, Y. Involvement of jasmonic acid, ethylene and salicylic acid signaling pathways behind the systemic resistance induced by Trichoderma longibrachiatum H9 in cucumber. BMC Genom. 2019, 20, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Sanjeev, K.; Manibhushan, T.; Archana, R. Trichoderma: Mass production, formulation, quality control, delivery and its scope in commercialization in India for the management of plant diseases. Afr. J. Agric. Res. 2014, 9, 3838–3852. [Google Scholar]
- Khan, M.R.; Mohiddin, F.A. Trichoderma: Its Multifarious Utility in Crop Improvement. In Crop Improvement through Microbial Biotechnology. New and Future Developments in Microbial Biotechnology and Bioengineering; Aligarh Muslim University: Aligarh, India; Srinagar, India, 2018; Volume 13, pp. 263–291. [Google Scholar]
- Diánez, F.; Santos, M.; Carretero, F.; Marín, F. Trichoderma saturnisporum, a new biological control agent. J. Sci. Food Agric. 2016, 96, 1934–1944. [Google Scholar] [CrossRef]
- Rocha, I.; Ma, Y.; Souza-Alonso, P.; Vosátka, M.; Freitas, H.; Oliveira, R.S. Seed coating: A tool for delivering beneficial microbes to agricultural crops. Front. Plant Sci. 2019, 10, 1357. [Google Scholar] [CrossRef]
- Azarmi, R.; Hajieghrari, B.; Giglou, A. Effect of Trichoderma isolates on tomato seedling growth response and nutrient uptake. Afr. J. Biotechnol. 2011, 10, 5850–5855. [Google Scholar]
- Druzhinina, I.S.; Seidl-Seiboth, V.; Herrera-Estrella, A.; Horwitz, B.A.; Kenerley, C.M.; Monte, E.; Mukherjee, P.K.; Zeilinger, S.; Grigoriev, I.V.; Kubicek, C.P. Trichoderma: The genomics of opportunistic success. Nat. Rev. Microbiol. 2011, 9, 749–759. [Google Scholar] [CrossRef]
- Bimenya, G.S.; Kaviri, D.; Mbona, N.; Byarugaba, W. Monitoring the severity of iodine deficiency disorders in Uganda. Afr. Health Sci. 2002, 2, 63–68. [Google Scholar]
- Berg, G. Plant–microbe interactions promoting plant growth and health: Perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biot. 2009, 84, 11–18. [Google Scholar] [CrossRef]
- Samuels, G.J.; Dodd, S.L.; Gams, W.; Castlebury, L.A.; Petrini, O. Trichoderma species associated with the green mold epidemic of commercially grown Agaricus bisporus. Mycologia 2002, 94, 147–170. [Google Scholar] [CrossRef]
- Largeteau, M.L.; Savoie, J.M. Microbially induced diseases of Agaricus bisporus: Biochemical mechanisms and impact on commercial mushroom production. App. Microbiol. Biotechnol. 2010, 86, 63–73. [Google Scholar] [CrossRef]
- Krupke, O.A.; Castle, A.J.; Rinker, D.L. The North American mushroom competitor, Trichoderma aggressivum f. aggressivum, produces antifungal compounds in mushroom compost that inhibit mycelial growth of the commercial mushroom Agaricus bisporus. Mycol. Res. 2003, 102, 1467–1475. [Google Scholar]
- Guthrie, J.L.; Castle, A.J. Chitinase production during interaction of Trichoderma aggressivum and Agaricus bisporus. Can. J. Microbiol. 2006, 96, 961–967. [Google Scholar] [CrossRef]
- Hatvani, L.; Antal, Z.; Manczinger, L.; Szekeres, A.; Druzhinina, I.S.; Kubicek, C.P.; Nagy, A.; Nagy, E.; Vagvolgyi, C.; Kredics, L. Green mold diseases of Agaricus and Pleurotus spp. are caused by related but phylogenetically different Trichoderma species. Phytopathology 2007, 97, 532–537. [Google Scholar] [CrossRef]
- Sharma, V.; Shanmugam, V. Unraveling the multilevel aspects of least explored plant beneficial Trichoderma saturnisporum isolate GITX-Panog. Eur. J. Plant Pathol. 2018, 152, 169–183. [Google Scholar] [CrossRef]
- Louden, B.C.; Harmann, D.; Lynne, A.M. Use of Blue Agar CAS Assay for Siderophore Detection. J. Microbiol. Biol. Educ. 2011, 12, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Lima-Rivera, D.L.; Lopez-Lima, D.; Desgarennes, D.; Velázquez-Rodríguez, A.S.; Carrion, G. Phosphate solubilization by fungi with nematicidal potential. J. Soil Sci. Plant Nutr. 2016, 16, 507–524. [Google Scholar] [CrossRef]
- Fiske, C.H.; Subbarow, Y. The colorimetry determination of phosphorous. J. Biol. Chem. 1925, 66, 375–400. [Google Scholar]
- Moreno-Gavíra, A.; Diánez, F.; Sánchez-Montesinos, B.; Santos, M. Paecilomyces variotii as a plant-growth promoter in horticulture. Agronomy 2020, 10, 597. [Google Scholar] [CrossRef]
- Murali, M.; Amruthesh, K.N.; Sudisha, J.S.; Niranjana, R.; Shetty, H.S. Screening for plant growth promoting fungi and their ability for growth promotion and induction of resistance in pearl millet against downy mildew disease. J. Phytol. 2012, 4, 30–36. [Google Scholar]
- Dickson, A.; Leaf, A.L.; Hosner, J.F. Quality appraisal of white spruce and white pine seedling stock in nurseries. Forest Chron. 1960, 36, 10–13. [Google Scholar] [CrossRef]
- Stewart, A.; Hill, R. Applications of Trichoderma in Plant growth promotion. In Biology and Applications of Trichoderma; Mukherjee, P.K., Horwitz, B.A., Singh, U.S., Mukherjee, M., Schmoll, M., Eds.; CABI: Wallingford, CT, USA, 2013; pp. 415–425. [Google Scholar]
- Allaga, H.; Bóka, B.; Poór, P.; Nagy, V.D.; Szuzs, A.; Stankovics, I.; Takó, M.; Manczinger, L.; Vágvölgyi, C.; Kredics, L.; et al. Composite Bioinoculant Based on the Combined Application of Beneficial Bacteria and Fungi. Agronomy 2020, 10, 220. [Google Scholar] [CrossRef]
- Wang, G.; Cao, X.; Ma, X.; Guo, M.; Liu, C.; Yan, L.; Bian, Y. Diversity and effect of Trichoderma spp. associated with green mold disease on Lentinula edodes in China. Microbiologyopen 2016, 5, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lu, M.Z.; Zhang, C.L.; Xu, J.Z. First Report of Trichoderma longibrachiatum Causing Green Mold Disease on Ganoderma lingzhi. Plant Dis. 2019, 103, 156. [Google Scholar] [CrossRef]
- Lane, B.S.; Trinci, A.P.J.; Gillespie, A.T. Influence of culture conditions on the virulence of conidia and blastospores of Beauveria bassiana to the green leafhopper, Nephotettix virescens. Mycol. Res. 1991, 95, 829–833. [Google Scholar] [CrossRef]
- Serna-Díaz, M.G.; Mercado-Flores, Y.; Jiménez-González, A.; Anducho-Reyes, M.A.; Medina-Marín, J.; Seck Tuoh-Mora, J.C.; Téllez-Jurado, A. Use of barley straw as a support for the production of conidiospores of Trichoderma harzianum. Biotechnol. Rep. 2020, 26, e00445. [Google Scholar] [CrossRef]
- Flodman, H.R.; Noureddini, H. Effects of intermittent mechanical mixing on solid-state fermentation of wet corn distiller grain with Trichoderma reesei. Biochem. Eng. J. 2013, 81, 24–28. [Google Scholar] [CrossRef]
- Deschamps, F.; Giuliano, C.; Asther, M.; Huet, M.C.; Roussos, S. Cellulase production by Trichoderma harzianum in static and mixed solid-state fermentation reactors under nonaseptic conditions. Biotechnol. Bioeng. 1985, 27, 1385–1388. [Google Scholar] [CrossRef]
- Michel-Aceves, A.C.; Otero-Sánchez, M.A.; Martínez-Rojero, R.D.; Rodríguez-Morán, N.L.; Ariza-Flores, R.; Barrios-Ayala, A. Producción masiva de Trichoderma harzianum Rifai en diferentes sustratos orgánicos. Rev. Chapingo Ser. Hortic. 2008, 14, 185–191. [Google Scholar] [CrossRef]
- Zhang, F.G.; Yuan, J.; Yang, X.M.; Cui, Y.Q.; Chen, L.H.; Ran, W.; Shen, Q.R. Putative Trichoderma harzianum mutant promotes cucumber growth by enhanced production of indoleacetic acid and plant colonization. Plant Soil 2013, 368, 433–444. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Cortés-Penagos, C.; López-Bucio, J. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Phys. 2009, 149, 1579–1592. [Google Scholar] [CrossRef] [PubMed]
- Hoyos-Carvajal, L.; Orduz, S.; Bissett, J. Growth stimulation in bean (Phaseolus vulgaris L.) by Trichoderma. Biol. Control. 2009, 51, 409–416. [Google Scholar] [CrossRef]
- Vinale, V.; Nigro, M.; Sivasithamparam, K.; Flematti, G.; Ghisalberti, E.L.; Ruocco, M.; Varlese, R.; Marra, R.; Lanzuise, S.; Eid, A.; et al. Harzianic acid: A novel siderophore from Trichoderma harzianum. FEMS Microbiol. Lett. 2013, 347, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Zhao, L. Study of the siderophore-producing Trichoderma asperellum Q1 on cucumber growth promotion under salt stress. J. Basic Microbiol. 2013, 53, 355–364. [Google Scholar] [CrossRef]
- Saber, W.I.A.; Ghoneem, K.; Rashad, Y.M.; AlAskar, A.A. Trichoderma Harzianum WKY1: An indole acetic acid producer for growth improvement and anthracnose disease control in sorghum. Biocontrol. Sci. Technol. 2017, 27, 654–676. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Woo, S.L.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Ruocco, M.; Lanzuise, S.; et al. Trichoderma secondary metabolites active on plants and fungal pathogens. Open Mycol. J. 2014, 8, 127–139. [Google Scholar] [CrossRef]
- Gravel, V.; Antoun, V.; Tweddell, R.J. Growth stimulation and fruit yield improvement of greenhouse tomato plants by inoculation with Pseudomonas putida or Trichoderma atroviride: Possible role ofindoleacetic acid (IAA). Soil Biol. Biochem. 2007, 39, 1968–1977. [Google Scholar] [CrossRef]
- Bader, A.N.; Salerno, G.L.; Covacevich, F.; Consolo, V.F. Native Trichoderma harzianum strains from Argentina produce indole-3 acetic acid and phosphorus solubilization, promote growth and control wilt disease on tomato (Solanum lycopersicum L.). J. King Saud Univ.-Sci. 2020, 32, 867–873. [Google Scholar] [CrossRef]
- Altomare, C.; Norvell, W.A.; Bjorkman, T.G.; Harman, E. Solubilization of phosphates and micronutrients by the plant-growth-promoting and biocontrol fungus Trichoderma harzianum Rifai. Appl. Environ. Microbiol. 1999, 65, 2926–2933. [Google Scholar] [CrossRef]
- Kapri, A.; Tiwari, L. Phosphate solubilization potential and phosphatase activity of rhizospheric Trichoderma spp. Braz. J. Microbiol. 2010, 41, 787–795. [Google Scholar] [CrossRef]
- Tandon, A.; Fatima, T.; Gautam, A.; Yadav, U.; Srivastava, S.; Singh, P.C. Effect of Trichoderma koningiopsis on chickpea rhizosphere activities under different fertilization regimes. Open J. Soil Sci. 2018, 8, 261–275. [Google Scholar] [CrossRef]
- Tandon, A.; Fatima, T.; Shukla, D.; Tripathi, P.; Srivastava, S.; Singh, P.C. Phosphate solubilization by Trichoderma koningiopsis (NBRI-PR5) under abiotic stress conditions. J. King Saud Univ.-Sci. 2020, 32, 791–798. [Google Scholar] [CrossRef]
- Hajieghrari, B. Effects of some Iranian Trichoderma isolates on maize seed germination and seedling vigor. Afr. J. Biotechnol. 2010, 9, 4342–4347. [Google Scholar]
- You, J.; Zhang, J.; Wua, M.; Yang, L.; Chen, W.; Li, G. Multiple criteria-based screening of Trichoderma isolates for biological control of Botrytis cinerea on tomato. Biol. Control 2016, 101, 31–38. [Google Scholar] [CrossRef]
- Diniz, K.A.; Silva, P.A.; Oliveira, J.A.; Evangelista, J.R.E. Sweet pepper seed responses to inoculation with microorganisms and coating with micronutrients, aminoacids and plant growth regulators. Sci. Agric. 2009, 66, 293–297. [Google Scholar] [CrossRef]
- Liu, B.; Jib, S.; Zhang, H.; Wang, Y.; Liu, Z. Isolation of Trichoderma in the rhizosphere soil of Syringa oblata from Harbin and their biocontrol and growth promotion function. Microbiol. Res. 2020, 235, 126445. [Google Scholar] [CrossRef]



| Treatments | T. aggressivum f. europaeum | T. saturnisporum |
|---|---|---|
| 90% BH + 10% O | 6.65·108 ± 3.04·107 c | 6.48·108 ± 2.84·107 c |
| 80% BH + 20% O | 5.63·108 ± 3.20·107 d | 5.17·108 ± 6.60·107 d |
| 70% BH + 30% O | 1.04·109 ± 1.44·107 a | 9.98·108 ± 5.69·107 a |
| 90% BH + 10% R | 8.32·108 ± 1.61·107 b | 7.88·108 ± 6.45·107 b |
| 80% BH + 20% R | 1.04·109 ± 1.04·107 a | 1.02·108 ± 6.26·107 a |
| 70% BH + 30% R | 8.00·108 ± 5.00·107 b | 7.12·108 ± 4.25·107 bc |
| p-value | 0.0000 | 0.0000 |
| Radius of Siderophores Production (mm) | IAA (mg mL−1) | ||||
|---|---|---|---|---|---|
| Treatment | 24 h | 48 h | 72 h | +Trp | −Trp |
| p-value | 0.0000 | 0.0000 | - | 0.0068 | 0.0304 |
| T. aggressivum | 9.73 ± 0.89 | 18.50 ± 1.70 | - | 0.145 ± 0.011 | 0.085 ± 0.009 |
| T. saturnisporum | 5.45 ± 0.31 | 9.82 ± 0.56 | - | 0.199 ± 0.014 | 0.129 ± 0.021 |
| Treatment | % Germination | Root Length (cm) | Shoot Length (cm) | Seed Vigour Index |
|---|---|---|---|---|
| Pepper | ||||
| p-value | 0.5420 | 0.0126 | 0.0010 | 0.0030 |
| T. aggressivum | 83 ± 6.83a | 0.64 ± 0.18b | 1.66 ± 0.55b | 138.14 ± 35.48b |
| T. saturnisporum | 80 ± 3.26a | 0.91 ± 0.11b | 1.29 ± 0.17b | 176.50 ± 18.14b |
| Control | 78 ± 4.61a | 2.16 ± 1.01a | 1.98 ± 0.56a | 320.32 ± 83.36a |
| Tomato | ||||
| p-value | 0.5268 | 0.0020 | 0.3154 | 0.1918 |
| T. aggressivum | 89 ± 6.83a | 4.53 ± 0.31a | 2.97 ± 0.47a | 671.80 ± 112.91a |
| T. saturnisporum | 92 ± 7.30a | 3.25 ± 0.37b | 2.55 ± 0.27a | 536.29 ± 76.26a |
| Control | 85 ± 8.32a | 3.95 ± 0.28b | 2.81 ± 0.29a | 580.13 ± 96.37a |
| Treatment | Length of Stem (cm) | Diameter (mm) | Number of Leaves | Aerial Dry Weight (g) | Root Dry Weight (g) | Leaf Area (mm2) | DQI |
|---|---|---|---|---|---|---|---|
| Pepper | |||||||
| p-value | 0.0000 | 0.0000 | 0.0092 | 0.0000 | 0.0000 | 0.0001 | 0.0000 |
| T. aggressivum | 29.18 ± 1.36a | 4.10 ± 0.24a | 7.22 ± 0.76a | 0.44 ± 0.06a | 0.15 ± 0.30b | 86.38 ± 12.58a | 0.06 ± 0.01a |
| T. saturnisporum | 29.31 ± 1.75a | 3.68 ± 0.22b | 7.07 ± 0.82a | 0.45 ± 0.06a | 0.18 ± 0.09a | 86.31 ± 13.22a | 0.06 ± 0.01a |
| Control | 27.01 ± 2.07b | 3.65 ± 0.23b | 6.70 ± 0.72b | 0.36 ± 0.04b | 0.11 ± 0.03c | 75.88 ± 11.15b | 0.04 ± 0.01b |
| Tomato | |||||||
| p-value | 0.0000 | 0.4387 | 0.0031 | 0.0232 | 0.0245 | 0.0295 | 0.0793 |
| T. aggressivum | 27.93 ± 1.99a | 3.86 ± 1.84a | 4.45 ± 0.50a | 0.63 ± 0.09a | 0.15 ± 0.02ab | 74.63 ± 10.99a | 0.06 ± 0.01a |
| T. saturnisporum | 27.17 ± 1.62a | 3.90 ± 0.24a | 4.57 ± 0.50a | 0.59 ± 0.10ab | 0.14 ± 0.02b | 75,52 ± 13.40a | 0.06 ± 0.01a |
| Control | 25.61 ± 2.07b | 3.84 ± 0.26a | 4.20 ± 0.46b | 0.56 ± 0.10b | 0.15 ± 0.02a | 69.12 ± 6.96b | 0.06 ± 0.00a |
| Tomato Seedling | ||||||
|---|---|---|---|---|---|---|
| Treatment | Length of Stem (mm) | Diameter (mm) | N° Leaves | Aerial Dry Weight (g) | Root Dry Weight (g) | DQI |
| p-value | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| TA D1 | 135.21 ± 28.79de | 3.91 ± 0.46ab | 4 ± 0.65b | 0.27 ± 0.09a | 0.03 ± 0.02cd | 0.025 ± 0.01de |
| TA D2 | 143.80 ± 32.58d | 3.81 ± 0.34b | 4 ± 0.51b | 0.28 ± 0.07a | 0.05 ± 0.018a | 0.034 ± 0.01a |
| TA D3 | 127.02 ± 25.41e | 3.76 ± 0.46b | 3.98 ± 0.64b | 0.27 ± 0.10a | 0.04 ± 0.02ab | 0.030 ± 0.01ab |
| TS D1 | 141.25 ± 19.83d | 3.55 ± 0.49c | 3.65 ± 0.60cd | 0.23 ± 0.10b | 0.03 ± 0.01cd | 0.024 ± 0.01de |
| TS D2 | 188.44 ± 30.29a | 4.07 ± 0.33a | 4.25 ± 0.73a | 0.28 ± 0.07a | 0.03 ± 0.01d | 0.021 ± 0.01e |
| TS D3 | 174.83 ± 27.78b | 4.01 ± 0.45a | 3.67 ± 0.63cd | 0.27 ± 0.09a | 0.04 ± 0.02a | 0.030 ± 0.01ab |
| M D1 | 157.25 ± 29.14c | 3.5 ± 0.39c | 3.56 ± 0.62de | 0.27 ± 0.061a | 0.04 ± 0.01bc | 0.026 ± 0.00bcd |
| M D2 | 99.08 ± 21.02b | 3.13 ± 0.23b | 3.35 ± 0.39bc | 0.28 ± 0.08a | 0.04 ± 0.01bcd | 0.029 ± 0.00bcd |
| M D3 | 98.54 ± 21.37f | 3.11 ± 0.45d | 3.33 ± 0.63ef | 0.18 ± 0.08c | 0.04 ± 0.01ab | 0.029 ± 0.00abc |
| Control | 125.73 ± 22.3e | 3.48 ± 0.31c | 3.29 ± 0.62f | 0.24 ± 0.07b | 0.03 ± 0.01cd | 0.025 ± 0.01cde |
| Tomato Plants | ||||||
| Treatment | Length of Stem (cm) | Diameter (mm) | Internodes | Aerial Dry Weight (g) | Root Dry Weight (g) | DQI |
| p-value | 0.0000 | 0.5379 | 0.0159 | 0.0015 | 0.2769 | 0.5373 |
| TA D1 | 101.40 ± 15.06ab | 12.11 ± 0.90a | 15.00 ± 1.15ab | 61.19 ± 11.27a | 4.99 ± 0.68ab | 3.23 ± 0.24a |
| TA D2 | 102.80 ± 9.47a | 12.11 ± 1.24a | 14.10 ± 1.29ab | 52.49 ± 11.26 | 5.35 ± 0.57a | 3.20 ± 0.46a |
| TA D3 | 92.00 ± 9.82bc | 11.26 ± 1.17ab | 14.40 ± 0.84abc | 54.74 ± 13.09abc | 4.70 ± 1.10abc | 3.01 ± 0.58ab |
| TS D1 | 77.55 ± 7.76e | 11.74 ± 1.18ab | 13.90 ± 1.37bc | 49.26 ± 14.78bcde | 4.40 ± 1.04bc | 2.91 ± 0.56ab |
| TS D2 | 88.75 ± 10.63cd | 11.92 ± 1.57ab | 15.20 ± 1.03a | 55.37 ± 8.24ab | 5.05 ± 1.23ab | 3.19 ± 0.42a |
| TS D3 | 81.00 ± 12.39de | 11.58 ± 1.37ab | 13.20 ± 2.57c | 51.71 ± 7.97abcd | 4.34 ± 1.17bc | 3.01 ± 0.94ab |
| M D1 | 79.05 ± 13.20e | 10.87 ± 1.38b | 13.90 ± 1.29bc | 47.00 ± 11.38bcde | 4.14 ± 1.11bc | 2.73 ± 0.64ab |
| M D2 | 76.18 ± 11.91e | 11.61 ± 0.95ab | 13.20 ± 1.23c | 45.30 ± 9.79cde | 4.23 ± 0.99bc | 2.85 ± 0.59ab |
| M D3 | 78.45 ± 8.08e | 11.22 ± 1.18ab | 14.30 ± 1.16abc | 40.91 ± 11.56e | 4.28 ± 1.37bc | 2.72 ± 0.86ab |
| Control | 77.96 ± 7.29e | 11.52 ± 2.03ab | 14.25 ± 1.16abc | 42.73 ± 6.46de | 3.84 ± 0.62c | 2.60 ± 0.43b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Montesinos, B.; Diánez, F.; Moreno-Gavíra, A.; Gea, F.J.; Santos, M. Role of Trichoderma aggressivum f. europaeum as Plant-Growth Promoter in Horticulture. Agronomy 2020, 10, 1004. https://doi.org/10.3390/agronomy10071004
Sánchez-Montesinos B, Diánez F, Moreno-Gavíra A, Gea FJ, Santos M. Role of Trichoderma aggressivum f. europaeum as Plant-Growth Promoter in Horticulture. Agronomy. 2020; 10(7):1004. https://doi.org/10.3390/agronomy10071004
Chicago/Turabian StyleSánchez-Montesinos, Brenda, Fernando Diánez, Alejandro Moreno-Gavíra, Francisco J. Gea, and Mila Santos. 2020. "Role of Trichoderma aggressivum f. europaeum as Plant-Growth Promoter in Horticulture" Agronomy 10, no. 7: 1004. https://doi.org/10.3390/agronomy10071004
APA StyleSánchez-Montesinos, B., Diánez, F., Moreno-Gavíra, A., Gea, F. J., & Santos, M. (2020). Role of Trichoderma aggressivum f. europaeum as Plant-Growth Promoter in Horticulture. Agronomy, 10(7), 1004. https://doi.org/10.3390/agronomy10071004





