The Diversification and Intensification of Crop Rotations under No-Till Promote Earthworm Abundance and Biomass
Abstract
1. Introduction
2. Material and Methods
2.1. Study Area
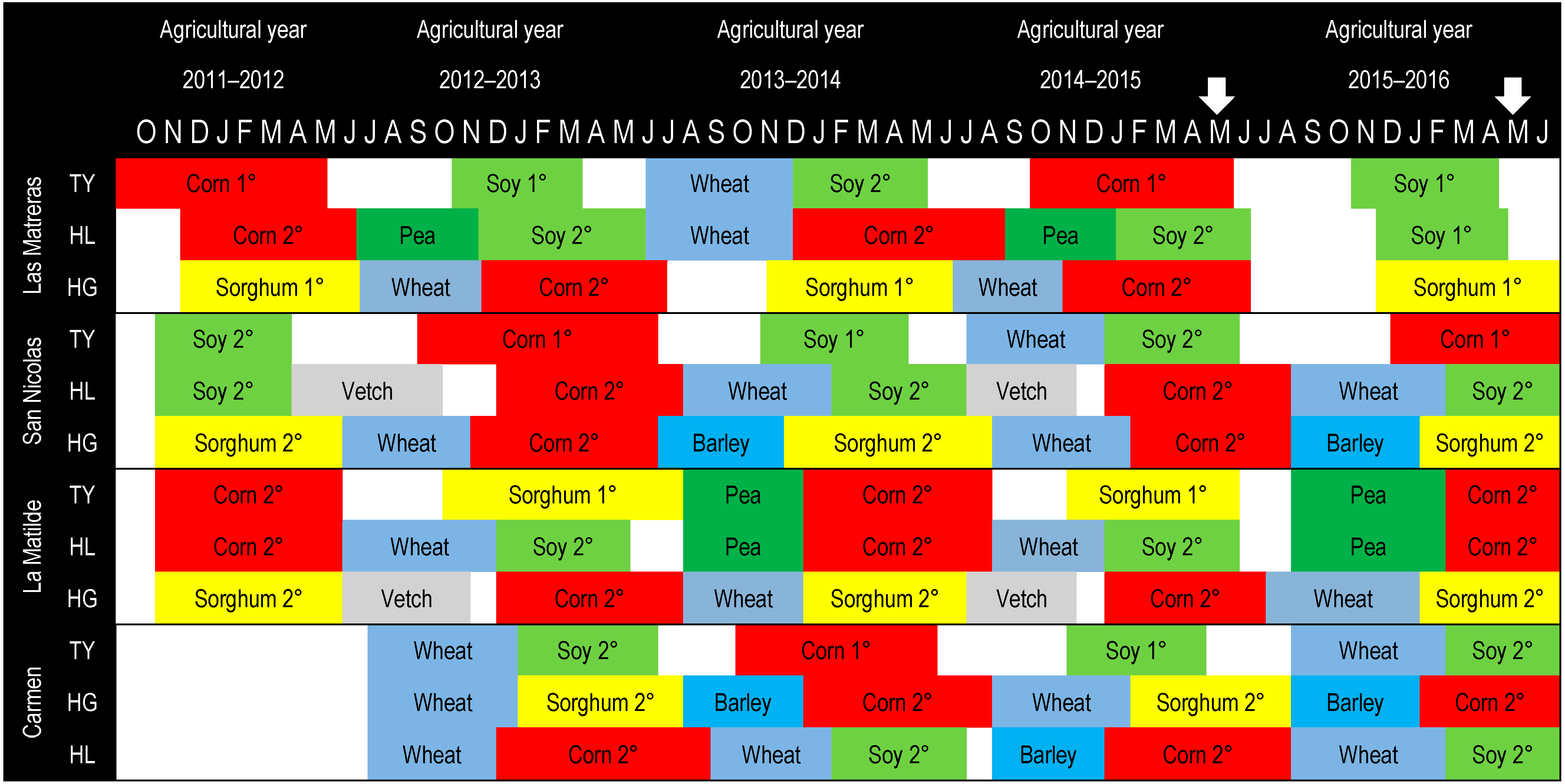
2.2. Experimental Design and Sampling
2.3. Earthworms
2.4. Soil Parameters
2.5. Statistical Analyses
3. Results
3.1. Earthworm Communities
3.2. Earthworm Relationships with Soil Properties and Management Parameters
4. Discussion
4.1. DICR Effects on Earthworm Communities
4.2. Earthworm Relationships with Soil Properties and Management Parameters
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pengue, W.A. Producción agroexportadora e (in) seguridad alimentaria: El caso de la soja en Argentina. Revibec 2004, 1, 46–55. [Google Scholar]
- Aizen, M.A.; Garibaldi, L.A.; Dondo, M. Expansión de la soja y diversidad de la agricultura argentina. Ecol. Austral 2009, 19, 45–54. [Google Scholar]
- Pengue, W.A. Cambios y Escenarios en la Agricultura Argentina del Siglo XXI. Available online: http://www.idaes.edu.ar/pdf_papeles/PENGUE_Agricultura%20Transformaciones%20Recursos%20y%20Escenarios%20en%20la%20Argentina%20FINAL%20ver%20SocialesBoll.pdf (accessed on 6 May 2020).
- Bedano, J.C.; Domínguez, A. Large-scale agricultural management and soil meso-and macrofauna conservation in the Argentine Pampas. Sustainability 2016, 8, 653. [Google Scholar] [CrossRef]
- Poisot, A.; Speedy, A.; Kueneman, E. Good Agricultural Practices—A working concept. In Proceedings of the FAO Internal Workshop on Good Agricultural Practices, Rome, Italy, 27–29 October 2004. [Google Scholar]
- Bedano, J.C.; Domínguez, A.; Arolfo, R.; Wall, L.G. Effect of Good Agricultural Practices under no-till on litter and soil invertebrates in areas with different soil types. Soil Till. Res. 2016, 158, 100–109. [Google Scholar] [CrossRef]
- Duval, M.E.; Galantini, J.A.; Iglesias, J.O.; Canelo, S.; Martinez, J.M.; Wall, L. Analysis of organic fractions as indicators of soil quality under natural and cultivated systems. Soil Till. Res. 2013, 131, 11–19. [Google Scholar] [CrossRef]
- Kraemer, F.B.; Soria, M.A.; Castiglioni, M.G.; Duval, M.; Galantini, J.; Morrás, H. Morpho-structural evaluation of various soils subjected to different use intensity under no-tillage. Soil Till. Res. 2017, 169, 124–137. [Google Scholar] [CrossRef]
- Caviglia, O.P.; Andrade, F.H. Sustainable intensification of agriculture in the Argentinean Pampas: Capture and use efficiency of environmental resources. Am. J. Plant Sci. Biotechnol. 2010, 3, 1–8. [Google Scholar]
- Caviglia, O.P.; Sadras, V.O.; Andrade, F.H. Intensification of agriculture in the south-eastern Pampas: I. Capture and efficiency in the use of water and radiation in double-cropped wheat–soybean. Field Crop. Res. 2004, 87, 117–129. [Google Scholar] [CrossRef]
- Andrade, J.F.; Poggio, S.L.; Ermacora, M.; Satorre, E.H. Land use intensification in the Rolling Pampa, Argentina: Diversifying crop sequences to increase yields and resource use. Eur. J. Agron. 2017, 82, 1–10. [Google Scholar] [CrossRef]
- Kladivko, E.J. Tillage systems and soil ecology. Soil Till. Res. 2001, 61, 61–76. [Google Scholar] [CrossRef]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Organisms as ecosystem engineers. In Ecosystem Management; Samson, F.B., Knopf, F.L., Eds.; Springer: New York, NY, USA, 1994; pp. 130–147. ISBN 978-1-4612-4018-1. [Google Scholar]
- Lavelle, P. Faunal activities and soil processes: Adaptive strategies that determine ecosystem function. Adv. Ecol. Res. 1997, 27, 93–132. [Google Scholar] [CrossRef]
- Lavelle, P.; Spain, A.V. Soil Ecology; Springer: Dordrecht, The Netherlands, 2001; p. 619, ISBN-13 978-1-4020-0490-2 (PB). [Google Scholar]
- Brown, G.G.; Doube, B.M. Functional interactions between earthworms, microorganisms, organic matter and plants. In Earthworm Ecology, 2nd ed.; Edwards, C.A., Ed.; CRC Press: Boca Raton, FL, USA, 2004; pp. 213–240. ISBN 0-8493-1819-X. [Google Scholar]
- Brussaard, L. Ecosystem services provided by the soil biota. In Soil Ecology and Ecosystem Services; Wall, D.H., Ed.; Oxford University Press: Oxford, UK, 2012; pp. 45–58. ISBN 978-0-19-957592-3 (hbk.). [Google Scholar]
- Bertrand, M.; Barot, S.; Blouin, M.; Whalen, J.; de Oliveira, T.; Roger-Estrade, J. Earthworm services for cropping systems. A review. Agron. Sustain. Dev. 2015, 35, 553–567. [Google Scholar] [CrossRef]
- Van Groenigen, J.W.; Lubbers, I.M.; Vos, H.M.; Brown, G.G.; De Deyn, G.B.; Van Groenigen, K.J. Earthworms increase plant production: A meta-analysis. Sci. Rep. 2014, 4, 6365. [Google Scholar] [CrossRef]
- Bedano, J.C.; Vaquero, F.; Domínguez, A.; Rodríguez, M.P.; Wall, L.; Lavelle, P. Earthworms contribute to ecosystem process in no-till systems with high crop rotation intensity in Argentina. Acta Oecol. 2019, 98, 14–24. [Google Scholar] [CrossRef]
- Brown, G.G.; Benito, N.P.; Pasini, A.; Sautter, K.D.; de F Guimarães, M.; Torres, E. No-tillage greatly increases earthworm populations in Paraná state, Brazil: The 7th international symposium on earthworm ecology·Cardiff·Wales 2002. Pedobiologia 2003, 47, 764–771. [Google Scholar] [CrossRef]
- Pelosi, C.; Pey, B.; Hedde, M.; Caro, G.; Capowiez, Y.; Guernion, M.; Peigné, J.; Piron, D.; Bertrand, M.; Cluzeau, D. Reducing tillage in cultivated fields increases earthworm functional diversity. Appl. Soil Ecol. 2014, 83, 79–87. [Google Scholar] [CrossRef]
- Crittenden, S.J.; Huerta, E.; De Goede, R.G.M.; Pulleman, M.M. Earthworm assemblages as affected by field margin strips and tillage intensity: An on-farm approach. Eur. J. Soil Biol. 2015, 66, 49–56. [Google Scholar] [CrossRef]
- Roarty, S.; Hackett, R.A.; Schmidt, O. Earthworm populations in twelve cover crop and weed management combinations. Appl. Soil Ecol. 2017, 114, 142–151. [Google Scholar] [CrossRef]
- Domínguez, A.; Bedano, J.C.; Becker, A.R. Negative effects of no-till on soil macrofauna and litter decomposition in Argentina as compared with natural grasslands. Soil Till. Res. 2010, 110, 51–59. [Google Scholar] [CrossRef]
- Falco, L.B.; Sandler, R.; Momo, F.; Di Ciocco, C.; Saravia, L.; Coviella, C. Earthworm assemblages in different intensity of agricultural uses and their relation to edaphic variables. PeerJ 2015, 3, e979. [Google Scholar] [CrossRef]
- Domínguez, A.; Bedano, J.C. Earthworm and enchytraeid co-occurrence pattern in organic and conventional farming: Consequences for ecosystem engineering. Soil Sci. 2016, 181, 148–156. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2014; p. 372.
- Agosti, M.B.; Madias, A.; Gil, R. Informe Anual de Resultados Campaña 2015–2016 Chacra Pergamino; INTA and Sistema Chacras-Aapresid: Pergamino, Argentina, 2016. [Google Scholar]
- Farahani, H.J.; Peterson, G.A.; Westfall, D.G. Dry land cropping intensification: A fundamental solution to efficient use of precipitation. Adv. Agron. 1998, 64, 197–223. [Google Scholar]
- Andriulo, A.; Mary, B.; Guerif, J. Modelling soil carbon dynamics with various cropping sequences on the rolling pampas. Agronomie 1999, 19, 365–377. [Google Scholar] [CrossRef]
- International Organization for Standardization (ISO). Soil Quality—Sampling of Soil Invertebrates—Part 1: Hand-sorting and Formalin Extraction of Earthworms. ISO 23611-1:2006; International Organization for Standardization: Geneva, Switzerland, 2006. [Google Scholar]
- Righi, G. Introducción al estudio de los Oligoquetos Megadrilos de la Provincia de Santa Fe. Rev. As. Cs. Nat. Litoral 1979, 10, 89–155. [Google Scholar]
- Mischis, C.; Moreno, A.G. Taxonomía de Oligoquetos: Criterios y Metodologías; Curso de Postgrado, Universidad Nacional de Córdoba: Córdoba, Argentina, 1999. [Google Scholar]
- Blakemore, R.J. Cosmopolitan Earthworms: An Eco-Taxonomic Guide to the Peregrine Species of the World; Verm Ecology: Kippax, ACT, Australia, 2002; p. 586. [Google Scholar]
- Momo, F.R.; Falco, L.B. Las lombrices de tierra. In Biología y Ecología de la Fauna del Suelo; Momo, F.R., Falco, L.B., Eds.; Imago Mundi: Longchamps, Argentina, 2010; pp. 141–160. ISBN 9789507930942. [Google Scholar]
- Blake, G.R.; Hartge, K.H. Bulk Density. In Methods of soil Analisys, 2nd ed.; Klute, A., Ed.; American Society of Agronomy Madison: Wisconsin, WI, USA, 1986; pp. 363–375. [Google Scholar]
- Galantini, J.A. Separación y análisis de las fracciones orgánicas. In Información y Tecnología en los Laboratorios de Suelos para el Desarrollo Agropecuario Sostenible; Marbán., L., Ratto, S.E., Eds.; Asociación Argentina de Ciencia del Suelo: Buenos Aires, Argentina, 2005; pp. 103–114. [Google Scholar]
- Jackson, M.L. Análisis Químico de Suelos, 1st ed.; Omega SA: Barcelona, España, 1976; p. 662, ISBN-13 9788428202619. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002; p. 485. ISBN 0-387-95364-7. [Google Scholar]
- Di Rienzo, J.A.; Macchiavelli, R.; Casanoves, F. Modelos lineales Generalizados Mixtos Aplicaciones en InfoStat, 1st special ed.; Julio Alejandro Di Rienzo: Córdoba, Argentina, 2017; p. 101. ISBN 978-987-42-4985-2. [Google Scholar]
- Di Rienzo, J.A.; Guzmán, A.W.; Casanoves, F. A multiple-comparisons method based on the distribution of the root node distance of a binary tree. J. Agric. Biol. Environ. Stat. 2002, 7, 129–142. [Google Scholar] [CrossRef]
- Zuur, A.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer Science & Business Media: New York, NY, USA, 2009; p. 573. ISBN 978-0-387-87458-6. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology, 2nd ed.; Elsevier Science: Amsterdam, The Netherlands; New York, NY, USA, 1998; p. 852. [Google Scholar]
- Breheny, P.; Burchett, W. Visualization of regression models using visreg. R J. 2017, 9, 56–71. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: https://www.R-project.org/ (accessed on 6 May 2020).
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat. Centro de Transferencia InfoStat, FCA, Ing Agr. Felix Aldo Marrone 746–Ciudad Universitaria, Universidad Nacional de Córdoba, Córdoba, Argentina: 2018. Available online: http://www.infostat.com.ar (accessed on 6 May 2020).
- Decaëns, T.; Jiménez, J.J. Earthworm communities under an agricultural intensification gradient in Colombia. Plant Soil 2002, 2401, 133–143. [Google Scholar] [CrossRef]
- Domínguez, A.; Bedano, J.C.; Becker, A.R.; Arolfo, R.V. Organic farming fosters agroecosystem functioning in Argentinian temperate soils: Evidence from litter decomposition and soil fauna. Appl. Soil Ecol. 2014, 83, 170–176. [Google Scholar] [CrossRef]
- Domínguez, A.; Bedano, J.C. The adoption of no-till instead of reduced tillage does not improve some soil quality parameters in Argentinean Pampas. Appl. Soil Ecol. 2016, 98, 166–176. [Google Scholar] [CrossRef]
- Curry, J.P. Factors affecting the abundance of earthworms in soils. In Earthworm Ecology, 2nd ed.; Edwards, C.A., Ed.; CRC Press: Boca Raton, FL, USA, 2004; pp. 91–108. ISBN 0-8493-1819-X. [Google Scholar]
- Fragoso, C.; Lavelle, P.; Blanchart, E.; Senapati, B.K.; Jimenez, J.J.; Martínez, M.A.; Decaens, T.; Tondoh, J. Earthworm communities of tropical agroecosystems: Origin, structure and influence of management practices. In Earthworm Management in Tropical Agroecosystems; Lavelle, P., Brussaard, L., Hendrix, P., Eds.; CABI: Wallingford Oxon, UK, 1999; pp. 27–55. [Google Scholar]
- Felten, D.; Emmerling, C. Effects of bioenergy crop cultivation on earthworm communities—A comparative study of perennial (Miscanthus) and annual crops with consideration of graded land-use intensity. Appl. Soil Ecol. 2011, 49, 167–177. [Google Scholar] [CrossRef]
- Díaz-Zorita, M.; Duarte, G.A.; Grove, J.H. A review of no-till systems and soil management for sustainable crop production in the subhumid and semiarid Pampas of Argentina. Soil Till. Res. 2002, 65, 1–18. [Google Scholar] [CrossRef]
- Schmidt, O.; Clements, R.O.; Donaldson, G. Why do cereal–legume intercrops support large earthworm populations? Appl. Soil Ecol. 2003, 22, 181–190. [Google Scholar] [CrossRef]
- Kautz, T.; Stumm, C.; Kösters, R.; Köpke, U. Effects of perennial fodder crops on soil structure in agricultural headlands. J. Plant Nutr. Soil Sci. 2010, 173, 490–501. [Google Scholar] [CrossRef]
- Van Eekeren, N.; van Liere, D.; de Vries, F.; Rutgers, M.; de Goede, R.; Brussaard, L. A mixture of grass and clover combines the positive effects of both plant species on selected soil biota. Appl. Soil Ecol. 2009, 42, 254–263. [Google Scholar] [CrossRef]
- Shipitalo, M.J.; Protz, R.; Tomlin, A.D. Effect of diet on the feeding and casting activity of Lumbricus terrestris and L. rubellus in laboratory culture. Soil Biol. Biochem. 1988, 20, 233–237. [Google Scholar] [CrossRef]
- Molina, Y.; Mora, A.; Ramos, M.; Parra, L. Evaluación de dos especies leguminosas como abono verde. Cuenca alta del Río Chama, Mérida, Venezuela. Rev. Forest. Venez. 2011, 55, 183–193. [Google Scholar]
- Vanzolini, J.I.; Galantini, J.; Agamennoni, R. Cultivos de cobertura de Vicia villosa Roth. en el valle bonaerense del Río Colorado. In Contribuciones de los Cultivos de Cobertura a la Sostenibilidad de los Sistemas de Producción; Álvarez, C., Quiroga, A., Santos, D., Bodrero, M., Eds.; INTA: La Pampa, Argentina, 2013; pp. 21–28. ISBN 978-987-679-177-9. [Google Scholar]
- Forján, H.; Manso, L. Los Cereales de Invierno en la Secuencia de Cultivos. Su Aporte a la Sustentabilidad del Sistema de Producción. Available online: http://rian.inta.gov.ar/Boletines/Articulos/Documentos/Cereales_de_invierno_en_lasecuencia_de_cultivos.pdf (accessed on 6 May 2020).
- Abail, Z.; Whalen, J.K. Corn residue inputs influence earthworm population dynamics in a no-till corn-soybean rotation. Appl. Soil Ecol. 2018, 127, 120–128. [Google Scholar] [CrossRef]
- Darmawan, A.; Atmowidi, T.; Manalu, W.; Suryobroto, B. Land-use change on Mount Gede, Indonesia, reduced native earthworm populations and diversity. Aust. J. Zool. 2018, 65, 217–225. [Google Scholar] [CrossRef]
- Domínguez, A.; Jiménez, J.J.; Ortíz, C.E.; Bedano, J.C. Soil macrofauna diversity as a key element for building sustainable agriculture in Argentine Pampas. Acta Oecol. 2018, 92, 102–116. [Google Scholar] [CrossRef]
- Fragoso, C. Diversidad y patrones biogeográficos de las lombrices de tierra de México (Oligochaeta, Annelida). In Minhocas na América Latina: Biodiversidade e Ecología; Brown, G.G., Fragoso, C., Eds.; Embrapa Soja: Londrina, Brazil, 2007; pp. 107–124. ISBN 978-85-7033-019-2. [Google Scholar]
- Eijsackers, H. Earthworms as colonizers of natural and cultivated soil environments. Appl. Soil Ecol. 2011, 50, 1–13. [Google Scholar] [CrossRef]
- Frazão, J.; de Goede, R.G.; Brussaard, L.; Faber, J.H.; Groot, J.C.; Pulleman, M.M. Earthworm communities in arable fields and restored field margins, as related to management practices and surrounding landscape diversity. Agric. Ecosyst. Environ. 2017, 248, 1–8. [Google Scholar] [CrossRef]
- Falco, L.B.; Momo, F.R.; Mischis, C.C. Ecología y biogeografía de las lombrices de tierra en la Argentina. In Minhocas na América Latina: Biodiversidade e Ecología; Brown, G.G., Fragoso, C., Eds.; Embrapa Soja: Londrina, Brazil, 2007; pp. 247–253. ISBN 978-85-7033-019-2. [Google Scholar]
- Fragoso, C.; Brown, G.G.; Patron, J.C.; Blanchart, E.; Lavelle, P.; Pashanasi, B.; Senapati, B.; Kumar, T. Agricultural intensification, soil biodiversity and agroecosystem function in the tropics: The role of earthworms. Appl. Soil Ecol. 1997, 6, 17–35. [Google Scholar] [CrossRef]
- Schmidt, O.; Curry, J.P.; Hackett, R.A.; Purvis, G.; Clements, R.O. Earthworm communities in conventional wheat monocropping and low-input wheat-clover intercropping systems. Ann. Appl. Biol. 2001, 138, 377–388. [Google Scholar] [CrossRef]
- Fragoso, C.; Barois, I.; Gonzalez, C.; Arteaga, C.; Patron, J.C. Relationship between earthworms and soil organic matter levels in natural and managed ecosystems in the Mexican tropics. In Soil Organic Matter Dynamics and Sustainability of Tropical Agriculture, 1st ed.; Mulongoy, K., Merckx, R., Eds.; Wiley-Sayce Co-Publication: Chichester, UK, 1993; pp. 231–239, ISBN-13 978-0471939153. [Google Scholar]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez, M.P.; Domínguez, A.; Moreira Ferroni, M.; Wall, L.G.; Bedano, J.C. The Diversification and Intensification of Crop Rotations under No-Till Promote Earthworm Abundance and Biomass. Agronomy 2020, 10, 919. https://doi.org/10.3390/agronomy10070919
Rodríguez MP, Domínguez A, Moreira Ferroni M, Wall LG, Bedano JC. The Diversification and Intensification of Crop Rotations under No-Till Promote Earthworm Abundance and Biomass. Agronomy. 2020; 10(7):919. https://doi.org/10.3390/agronomy10070919
Chicago/Turabian StyleRodríguez, María Pía, Anahí Domínguez, Melisa Moreira Ferroni, Luis Gabriel Wall, and José Camilo Bedano. 2020. "The Diversification and Intensification of Crop Rotations under No-Till Promote Earthworm Abundance and Biomass" Agronomy 10, no. 7: 919. https://doi.org/10.3390/agronomy10070919
APA StyleRodríguez, M. P., Domínguez, A., Moreira Ferroni, M., Wall, L. G., & Bedano, J. C. (2020). The Diversification and Intensification of Crop Rotations under No-Till Promote Earthworm Abundance and Biomass. Agronomy, 10(7), 919. https://doi.org/10.3390/agronomy10070919






