Geo-Agriculture: Reviewing Opportunities through Which the Geosphere Can Help Address Emerging Crop Production Challenges
Abstract
:1. Introduction
2. Methods
2.1. Literature Review
2.2. Modelling Moisture Retention Capacity for Zeolite-Amended Soils
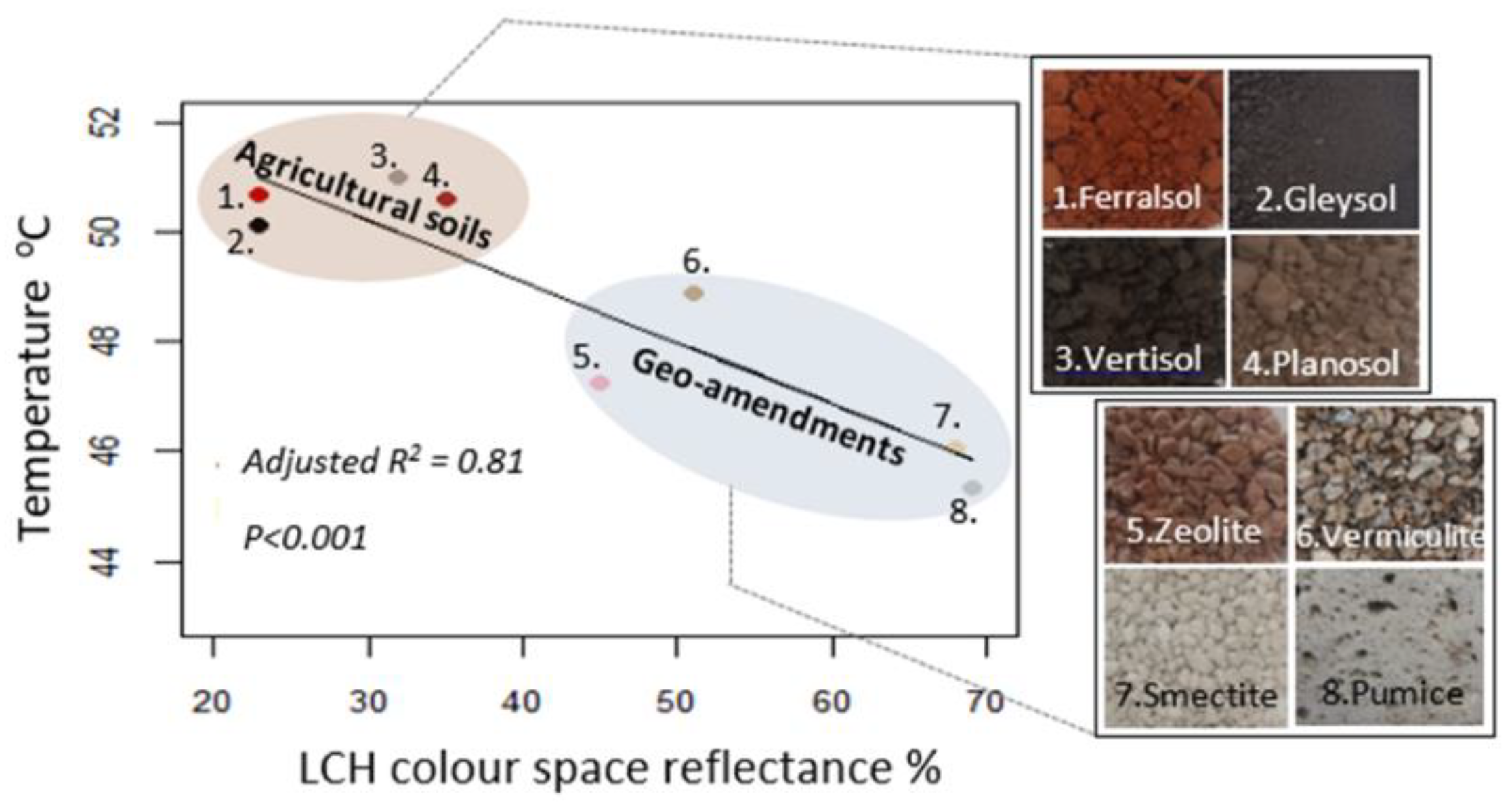
2.3. Preliminary Reflectance and Surface Temperature Experiment
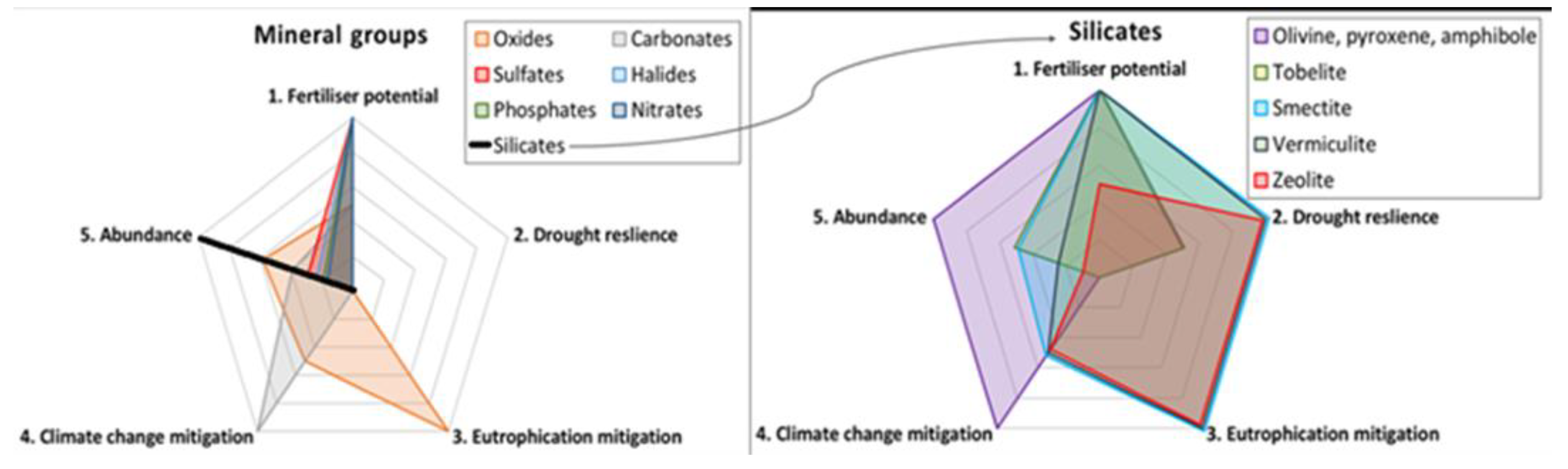
2.4. Semi-Quantitative Analysis of Beneficial Mineral Attributes
3. New Opportunities for Harnessing Geosphere Fertiliser Reserves
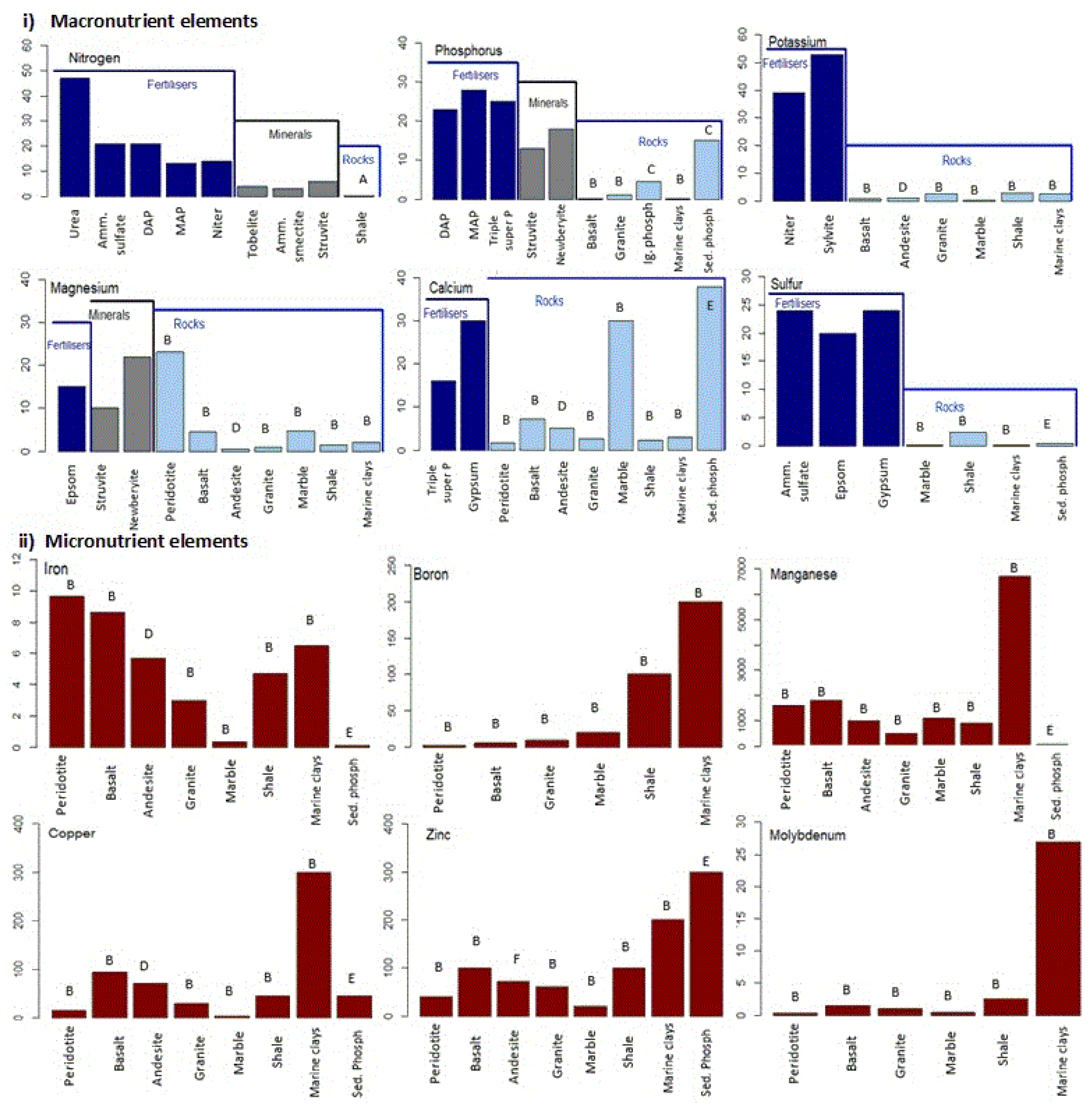
3.1. Geological Fertiliser Prospects: Overview
3.2. Geological N Prospects
3.3. Geological P Prospects
3.4. Geological Fertiliser Prospects: Best Contenders
4. Environmental Contamination Prevention
5. Embedding Drought Resilience into Agricultural Soils
6. Climate Change Mitigation
6.1. Carbon Dioxide
6.2. Methane
6.3. Nitrous Oxide
6.4. Integrating Geo-Based Climate Change Mitigation with Established Practices
7. Risks and Environmental Concerns
8. Outlook
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zimov, S.A.; Schuur, E.A.G.; Chapin, F.S. Permafrost and the Global Carbon Budget. Science 2006, 312, 1612–1613. [Google Scholar] [CrossRef] [PubMed]
- Tahir, S.; Marschner, P. Clay amendment to sandy soil—Effect of clay concentration and ped size on nutrient dynamics after residue addition. J. Soils Sediments 2016, 16, 2072–2080. [Google Scholar] [CrossRef]
- Abdu, H.; Robinson, D.A.; Seyfried, M.; Jones, S.B. Geophysical imaging of watershed subsurface patterns and prediction of soil texture and water holding capacity. Water Resour. Res. 2008, 44. [Google Scholar] [CrossRef] [Green Version]
- Brennan, R.F.; Bolland, M.D.A.; Jeffery, R.C.; Allen, D.G. Phosphorus adsorption by a range of western Australian soils related to soil properties. Commun. Soil Sci. Plant Anal. 1994, 25, 2785–2795. [Google Scholar] [CrossRef]
- Garland, G.; Bünemann, E.K.; Six, J. New methodology for soil aggregate fractionation to investigate phosphorus transformations in iron oxide-rich tropical agricultural soil. Eur. J. Soil Sci. 2017, 68, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Montalvo, D.; McLaughlin, M.J.; Degryse, F. Efficacy of Hydroxyapatite Nanoparticles as Phosphorus Fertilizer in Andisols and Oxisols. Soil Sci. Soc. Am. J. 2015, 79, 551–558. [Google Scholar] [CrossRef]
- Frenkel, H.; Goertzen, J.O.; Rhoades, J.D. Effects of Clay Type and Content, Exchangeable Sodium Percentage, and Electrolyte Concentration on Clay Dispersion and Soil Hydraulic Conductivity1. Soil Sci. Soc. Am. J. 1978, 42, 32–39. [Google Scholar] [CrossRef]
- Sollins, P.; Robertson, G.P.; Uehara, G. Nutrient mobility in variable- and permanent-charge soils. Biogeochemistry 1988, 6, 181–199. [Google Scholar] [CrossRef]
- Basak, B.B.; Pal, S.; Datta, S.C. Use of modified clays for retention and supply of water and nutrients. Curr. Sci. 2012, 102, 1272–1278. [Google Scholar]
- van Straaten, P. Rocks for Crops: Agrominerals of Sub-Saharan Africa; ICRAF: Nairobi, Kenya, 2002. [Google Scholar]
- Edwards, D.P.; Lim, F.; James, R.H.; Pearce, C.R.; Scholes, J.; Freckleton, R.P.; Beerling, D.J. Climate change mitigation: Potential benefits and pitfalls of enhanced rock weathering in tropical agriculture. Biol. Lett. 2017, 13, 20160715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beerling, D.J.; Leake, J.R.; Long, S.P.; Scholes, J.D.; Ton, J.; Nelson, P.N.; Bird, M.; Kantzas, E.; Taylor, L.L.; Sarkar, B.; et al. Farming with crops and rocks to address global climate, food and soil security. Nat. Plants 2018, 4, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Kingston, K. Developing Drought-Protection Technologies for the Industry—Industry Report; Cotton Research and Development Corporation: Narrabri, NSW, Australia, 2019.
- Costantini, A.; Nester, M.; Podberscek, M. Site preparation for Pinus establishment in south-eastern Queensland. 1. Temporal changes in bulk density. Aust. J. Exp. Agric. 1995, 35, 1151–1158. [Google Scholar] [CrossRef]
- Silburn, D.M.; Tolmie, P.E.; Biggs, A.J.W.; Whish, J.P.M.; French, V. Deep drainage rates of Grey Vertosols depend on land use in semi-arid subtropical regions of Queensland, Australia. Soil Res. 2011, 49, 424–438. [Google Scholar] [CrossRef]
- Kiipli, E.; Kiipli, T. Nitrogen isotopes in kukersite and black shale implying Ordovician-Silurian seawater redox conditions. Oil Shale 2013, 30, 60–75. [Google Scholar] [CrossRef] [Green Version]
- Faure, G. Principles and Applications of Geochemistry, 2nd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1998. [Google Scholar]
- Pufahl, P.K.; Groat, L.A. Sedimentary and Igneous Phosphate Deposits: Formation and Exploration: An Invited Paper. Econ. Geol. 2017, 112, 483–516. [Google Scholar] [CrossRef]
- Kelemen, P.B. Genesis of high Mg andesites and the continental crust. Contrib. Mineral. Petrol. 1995, 120, 1–19. [Google Scholar] [CrossRef]
- Abed, A.M.; Fakhouri, K. On the chemical variability of phosphatic particles from Jordanian phosphorite deposits. Chem. Geol. 1996, 131, 1–13. [Google Scholar] [CrossRef]
- Cornell, D.H.; Schütte, S.S.; Eglington, B.L. The Ongeluk basaltic andesite formation in Griqualand West, South Africa: Submarine alteration in a 2222 Ma proterozoic sea. Precambrian Res. 1996, 79, 101–123. [Google Scholar] [CrossRef]
- Venterea, R.T.; Clough, T.J.; Coulter, J.A.; Breuillin-Sessoms, F.; Wang, P.; Sadowsky, M.J. Ammonium sorption and ammonia inhibition of nitrite-oxidizing bacteria explain contrasting soil N2O production. Sci. Rep. 2015, 5, 12153. [Google Scholar] [CrossRef]
- Pratt, C.; Redding, M.; Hill, J.; Brown, G.; Westermann, M. Clays Can Decrease Gaseous Nutrient Losses from Soil-Applied Livestock Manures. J. Environ. Qual. 2016, 45, 638–645. [Google Scholar] [CrossRef]
- Phillips, I.R. Nutrient leaching losses from undisturbed soil cores following applications of piggery wastewater. Soil Res. 2002, 40, 515–532. [Google Scholar] [CrossRef]
- Antonetti, E.; Iaquaniello, G.; Salladini, A.; Spadaccini, L.; Perathoner, S.; Centi, G. Waste-to-Chemicals for a Circular Economy: The Case of Urea Production (Waste-to-Urea). ChemSusChem 2017, 10, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Kramer, K.J.; Moll, H.C.; Nonhebel, S. Total greenhouse gas emissions related to the Dutch crop production system. Agric. Ecosyst. Environ. 1999, 72, 9–16. [Google Scholar] [CrossRef]
- Tallaksen, J.; Bauer, F.; Hulteberg, C.; Reese, M.; Ahlgren, S. Nitrogen fertilizers manufactured using wind power: Greenhouse gas and energy balance of community-scale ammonia production. J. Clean. Prod. 2015, 107, 626–635. [Google Scholar] [CrossRef]
- Lee, S.-I.; Lim, S.-S.; Lee, K.-S.; Kwak, J.-H.; Jung, J.-W.; Ro, H.-M.; Choi, W.-J. Kinetic Responses of Soil Carbon Dioxide Emission to Increasing Urea Application Rate. Korean J. Environ. Agric. 2011, 30, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Morford, S.L.; Houlton, B.Z.; Dahlgren, R.A. Increased forest ecosystem carbon and nitrogen storage from nitrogen rich bedrock. Nature 2011, 477, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Holloway, J.M.; Dahlgren, R.A. Nitrogen in rock: Occurrences and biogeochemical implications. Glob. Biogeochem. Cycles 2002, 16, 65-1–65-17. [Google Scholar] [CrossRef]
- Urbansky, E.T.; Brown, S.K.; Magnuson, M.L.; Kelty, C.A. Perchlorate levels in samples of sodium nitrate fertilizer derived from Chilean caliche. Environ. Pollut. 2001, 112, 299–302. [Google Scholar] [CrossRef] [Green Version]
- Higashi, S. Ammonium-bearing mica and mica/smectite of several pottery stone and pyrophyllite deposits in Japan: Their mineralogical properties and utilization. Appl. Clay Sci. 2000, 16, 171–184. [Google Scholar] [CrossRef]
- Drits, V.A.; Lindgreen, H.; Sakharov, B.A.; Jakobsen, H.J.; Salyn, A.L.; Dainyak, L.G. Tobelitization of smectite during oil generation in oil-source shales. Application to North Sea illite-tobelite-smectite-vermiculite. Clays Clay Miner. 2002, 50, 82–98. [Google Scholar] [CrossRef]
- Mesto, E.; Scordari, F.; Lacalamita, M.; Schingaro, E. Tobelite and NH4+-rich muscovite single crystals from Ordovician Armorican sandstones (Brittany, France, Structure and crystal chemistry. Am. Miner. 2012, 97, 1460–1468. [Google Scholar] [CrossRef]
- Redding, M.R. Bentonite can decrease ammonia volatilisation losses from poultry litter: Laboratory studies. Anim. Prod. Sci. 2013, 53, 1115–1118. [Google Scholar] [CrossRef] [Green Version]
- Velthof, G.L.; Kuikman, P.J.; Oenema, O. Nitrous oxide emission from animal manures applied to soil under controlled conditions. Biol. Fertil. Soils 2003, 37, 221–230. [Google Scholar] [CrossRef]
- Chu, H.; Fujii, T.; Morimoto, S.; Lin, X.; Yagi, K.; Hu, J.; Zhang, J. Community Structure of Ammonia-Oxidizing Bacteria under Long-Term Application of Mineral Fertilizer and Organic Manure in a Sandy Loam Soil. Appl. Environ. Microbiol. 2007, 73, 485–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordell, D.; Drangert, J.-O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Mar, S.S.; Okazaki, M. Investigation of Cd contents in several phosphate rocks used for the production of fertilizer. Microchem. J. 2012, 104, 17–21. [Google Scholar] [CrossRef]
- Gilbert, N. The disappearing nutrient: Phosphate-based fertilizers have helped spur agricultural gains in the past century, but the world may soon run out of them. Nature 2019, 461, 716. [Google Scholar] [CrossRef]
- Kahiluoto, H.; Kuisma, M.; Ketoja, E.; Salo, T.; Heikkinen, J. Phosphorus in Manure and Sewage Sludge More Recyclable than in Soluble Inorganic Fertilizer. Environ. Sci. Technol. 2015, 49, 2115–2122. [Google Scholar] [CrossRef] [Green Version]
- Zhou, K.; Barjenbruch, M.; Kabbe, C.; Inial, G.; Remy, C. Phosphorus recovery from municipal and fertilizer wastewater: China’s potential and perspective. J. Environ. Sci. 2017, 52, 151–159. [Google Scholar] [CrossRef] [Green Version]
- Mierzwa-Hersztek, M.; Gondek, K.; Klimkowicz-Pawlas, A.; Baran, A.; Bajda, T. Sewage sludge biochars management—Ecotoxicity, mobility of heavy metals, and soil microbial biomass. Environ. Toxicol. Chem. 2018, 37, 1197–1207. [Google Scholar] [CrossRef]
- Venkatesan, A.K.; Halden, R.U. National inventory of perfluoroalkyl substances in archived U.S. biosolids from the 2001 EPA National Sewage Sludge Survey. J. Hazard. Mater. 2013, 252–253, 413–418. [Google Scholar] [CrossRef] [Green Version]
- Frýda, J.; Breiter, K. Alkali feldspars as a main phosphorus reservoirs in rare-metal granites: Three examples from the Bohemian Massif (Czech Republic). Terra Nova 1995, 7, 315–320. [Google Scholar] [CrossRef]
- Fan, Q.; Hooper, P.R. The Cenozoic Basaltic Rocks of Eastern China: Petrology and Chemical Composition. J. Petrol. 1991, 32, 765–810. [Google Scholar] [CrossRef]
- Smyth, T.J.; Sanchez, P.A. Phosphate Rock Dissolution and Availability in Cerrado Soils as Affected by Phosphorus Sorption Capacity1. Soil Sci. Soc. Am. J. 1982, 46, 339–345. [Google Scholar] [CrossRef]
- Bolland, M.D.A.; Gilkes, R.J. Rock phosphates are not effective fertilizers in Western Australian soils: A review of one hundred years of research. Fertil. Res. 1990, 22, 79–95. [Google Scholar] [CrossRef]
- Azcon, R.; Barea, J.M.; Hayman, D.S. Utilization of rock phosphate in alkaline soils by plants inoculated with mycorrhizal fungi and phosphate-solubilizing bacteria. Soil Biol. Biochem. 1976, 8, 135–138. [Google Scholar] [CrossRef]
- Omar, S.A. The role of rock-phosphate-solubilizing fungi and vesicular–arbusular-mycorrhiza (VAM) in growth of wheat plants fertilized with rock phosphate. World J. Microbiol. Biotechnol. 1997, 14, 211–218. [Google Scholar] [CrossRef]
- Xiao, C.; Chi, R.; Li, X.; Xia, M.; Xia, Z. Biosolubilization of Rock Phosphate by Three Stress-Tolerant Fungal Strains. Appl. Biochem. Biotechnol. 2011, 165, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Berg, S.; Dennis, P.G.; Paungfoo-Lonhienne, C.; Anderson, J.; Robinson, N.; Brackin, R.; Royle, A.; DiBella, L.; Schmidt, S. Effects of commercial microbial biostimulants on soil and root microbial communities and sugarcane yield. Biol. Fertil. Soils 2019, 56, 565–580. [Google Scholar] [CrossRef]
- Paungfoo-Lonhienne, C.; Redding, M.; Pratt, C.; Wang, W. Plant growth promoting rhizobacteria increase the efficiency of fertilisers while reducing nitrogen loss. J. Environ. Manag. 2019, 233, 337–341. [Google Scholar] [CrossRef]
- Pi, T.; Lozano-García, S.; Caballero-Miranda, M.; Ortega-Guerrero, B.; Roy, P. Discovery and characterization of a struvite layer in the Chalco paleolake, Mexico. Rev. Mex. Cienc. Geol. 2010, 27, 573–580. [Google Scholar]
- Boistelle, R.; Abbona, F.; Lundager Madsen, H.E. On the transformation of struvite into newberyite in aqueous systems. Phys. Chem. Miner. 1983, 9, 216–222. [Google Scholar] [CrossRef]
- Donovan, J.J.; Grimm, E.C. Episodic struvite deposits in a Northern Great Plains flyway lake: Indicators of mid-Holocene drought? Holocene 2007, 17, 1155–1169. [Google Scholar] [CrossRef]
- Mihelcic, J.R.; Fry, L.M.; Shaw, R. Global potential of phosphorus recovery from human urine and feces. Chemosphere 2011, 84, 832–839. [Google Scholar] [CrossRef]
- Yuan, Z.; Pratt, S.; Batstone, D.J. Phosphorus recovery from wastewater through microbial processes. Curr. Opin. Biotechnol. 2012, 23, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Pratt, C.; Parsons, S.A.; Soares, A.; Martin, B.D. Biologically and chemically mediated adsorption and precipitation of phosphorus from wastewater. Curr. Opin. Biotechnol. 2012, 23, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Ueno, Y.; Fujii, M. Three Years Experience of Operating and Selling Recovered Struvite from Full-Scale Plant. Environ. Technology 2001, 22, 1373–1381. [Google Scholar]
- Booker, N.A.; Priestley, A.J.; Fraser, I.H. Struvite Formation in Wastewater Treatment Plants: Opportunities for Nutrient Recovery. Environ. Technol. 1999, 20, 777–782. [Google Scholar] [CrossRef]
- Stolzenburg, P.; Capdevielle, A.; Teychené, S.; Biscans, B. Struvite precipitation with MgO as a precursor: Application to wastewater treatment. Chem. Eng. Sci. 2015, 133, 9–15. [Google Scholar] [CrossRef]
- Aguado, D.; Barat, R.; Bouzas, A.; Seco, A.; Ferrer, J. P-recovery in a pilot-scale struvite crystallisation reactor for source separated urine systems using seawater and magnesium chloride as magnesium sources. Sci. Total Environ. 2019, 672, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.M.; Pearson, R.G.; Brodie, J.E.; Butler, B. Review and conceptual models of agricultural impacts and water quality in waterways of the Great Barrier Reef catchment area. Mar. Freshw. Res. 2017, 68, 1–19. [Google Scholar] [CrossRef]
- Wilson, R.S.; Schlea, D.A.; Boles, C.M.W.; Redder, T.M. Using models of farmer behavior to inform eutrophication policy in the Great Lakes. Water Res. 2018, 139, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, J.; Li, S.; Zheng, X. A study on removing nitrogen from paddy field rainfall runoff by an ecological ditch–zeolite barrier system. Environ. Sci. Pollut. Res. 2017, 24, 27090–27103. [Google Scholar] [CrossRef] [PubMed]
- Cruz, H.; Law, Y.Y.; Guest, J.S.; Rabaey, K.; Batstone, D.; Laycock, B.; Verstraete, W.; Pikaar, I. Mainstream Ammonium Recovery to Advance Sustainable Urban Wastewater Management. Environ. Sci. Technol. 2019, 53, 11066–11079. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Kumar, E.; Sillanpää, M. Nitrate removal from water by nano-alumina: Characterization and sorption studies. Chem. Eng. J. 2010, 163, 317–323. [Google Scholar] [CrossRef]
- Hua, G.; Salo, M.W.; Schmit, C.G.; Hay, C.H. Nitrate and phosphate removal from agricultural subsurface drainage using laboratory woodchip bioreactors and recycled steel byproduct filters. Water Res. 2016, 102, 180–189. [Google Scholar] [CrossRef]
- Dayton, E.A.; Basta, N.T.; Jakober, C.A.; Hattey, J.A. Using treatment residuals to reduce phosphorus in agricultural runoff. J. AWWA 2003, 95, 151–158. [Google Scholar] [CrossRef]
- Gillman, G.P. Charged clays: An environmental solution. Appl. Clay Sci. 2011, 53, 361–365. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Meng, Q. Removal of nitrate by zero-valent iron and pillared bentonite. J. Hazard. Mater. 2010, 174, 188–193. [Google Scholar] [CrossRef]
- Borie, F.; Zunino, H. Organic matter-phosphorus associations as a sink in P-fixation processes in allophanic soils of Chile. Soil Biol. Biochem. 1983, 15, 599–603. [Google Scholar] [CrossRef]
- Nguyen, M.L.; Tanner, C.C. Ammonium removal from wastewaters using natural New Zealand zeolites. N. Z. J. Agric. Res. 1998, 41, 427–446. [Google Scholar] [CrossRef] [Green Version]
- de Luna, M.D.G.; Futalan, C.M.; Jurado, C.A., Jr.; Colades, J.I.; Wan, M.-W. Removal of ammonium-nitrogen from aqueous solution using chitosan-coated bentonite: Mechanism and effect of operating parameters. J. Appl. Polym. Sci. 2018, 135, 45924. [Google Scholar] [CrossRef]
- Terry, P.A. Removal of Nitrates and Phosphates by Ion Exchange with Hydrotalcite. Environ. Eng. Sci. 2009, 26, 691–696. [Google Scholar] [CrossRef]
- McDowell, R.W.; Hawke, M.; McIntosh, J.J. Assessment of a technique to remove phosphorus from streamflow. N. Z. J. Agric. Res. 2007, 50, 503–510. [Google Scholar] [CrossRef]
- Hanly, J.A.; Hedley, M.J.; Horne, D.J. Evaluation of tephra for removing phosphorus from dairy farm drainage waters. Soil Res. 2008, 46, 542–551. [Google Scholar] [CrossRef]
- Chin, A.; Schmidt, S.; Buckley, S.; Pirie, R.; Redding, M.; Laycock, B.; Luckman, P.; Batstone, D.J.; Robinson, N.; Brackin, R. Sorbents can tailor nitrogen release from organic wastes to match the uptake capacity of crops. Sci. Total Environ. 2018, 645, 1474–1483. [Google Scholar] [CrossRef]
- Rowlings, D.W.; Grace, P.R.; Scheer, C.; Kiese, R. Influence of nitrogen fertiliser application and timing on greenhouse gas emissions from a lychee (Litchi chinensis) orchard in humid subtropical Australia. Agric. Ecosyst. Environ. 2013, 179, 168–178. [Google Scholar] [CrossRef]
- Faithful, J.W.; Brodie, J.; Hooper, A.; Leahy, P.; Henry, G.; Finlayson, W.; Green, D. Plot-Scale Runoff of Nutrients and Sediment under Varying Management Regimes on a Banana and Cane Farm in the Wet Tropics, Queensland—ACTFR Report No. 05/03; Australian Centre for Tropical Freshwater Research: Townsville, Australia, 2007. [Google Scholar]
- Leng, G.; Tang, Q.; Rayburg, S. Climate change impacts on meteorological, agricultural and hydrological droughts in China. Glob. Planet. Chang. 2015, 126, 23–34. [Google Scholar] [CrossRef]
- Cheal, A.J.; MacNeil, M.A.; Emslie, M.J.; Sweatman, H. The threat to coral reefs from more intense cyclones under climate change. Glob. Chang. Biol. 2017, 23, 1511–1524. [Google Scholar] [CrossRef]
- Hallett, C.S.; Hobday, A.J.; Tweedley, J.R.; Thompson, P.A.; McMahon, K.; Valesini, F.J. Observed and predicted impacts of climate change on the estuaries of south-western Australia, a Mediterranean climate region. Reg. Environ. Chang. 2018, 18, 1357–1373. [Google Scholar] [CrossRef]
- Jenkins, K.; Warren, R. Quantifying the impact of climate change on drought regimes using the Standardised Precipitation Index. Theor. Appl. Climatol. 2015, 120, 41–54. [Google Scholar] [CrossRef] [Green Version]
- Farrell, C.; Ang, X.Q.; Rayner, J.P. Water-retention additives increase plant available water in green roof substrates. Ecol. Eng. 2013, 52, 112–118. [Google Scholar] [CrossRef]
- Bhardwaj, A.K.; Shainberg, I.; Goldstein, D.; Warrington, D.N.; J.Levy, G. Water Retention and Hydraulic Conductivity of Cross-Linked Polyacrylamides in Sandy Soils Contributions from the ARO, The Volcani Center, Bet-Dagan 50-250, Israel, no. 606/2006 series. The use of trade names does not imply endorsement by the authors or ARO. Soil Sci. Soc. Am. J. 2007, 71, 406–412. [Google Scholar] [CrossRef]
- Siyamak, S.; Luckman, P.; Laycock, B. Rapid and solvent-free synthesis of pH-responsive graft-copolymers based on wheat starch and their properties as potential ammonium sorbents. Int. J. Biol. Macromol. 2020, 149, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.R.; Zou, J.L.; Li, G.B. Stabilization of heavy metals in sludge ceramsite. Water Res. 2010, 44, 2930–2938. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, G.-L.; Yang, J.-L.; Li, D.-C.; Zhao, Y.-G.; Liu, F.; Yang, R.-M.; Yang, F. Organic matter controls of soil water retention in an alpine grassland and its significance for hydrological processes. J. Hydrol. 2014, 519, 3086–3093. [Google Scholar] [CrossRef]
- Oyonarte, C.; Mingorance, M.D.; Durante, P.; Piñero, G.; Barahona, E. Indicators of change in the organic matter in arid soils. Sci. Total Environ. 2007, 378, 133–137. [Google Scholar] [CrossRef]
- Pennell, K.D.; Abriola, L.M.; Boyd, S.A. Surface Area of Soil Organic Matter Reexamined. Soil Sci. Soc. Am. J. 1995, 59, 1012–1018. [Google Scholar] [CrossRef]
- Xiubin, H.; Zhanbin, H. Zeolite application for enhancing water infiltration and retention in loess soil. Resour. Conserv. Recycl. 2001, 34, 45–52. [Google Scholar] [CrossRef]
- Du, P.; Yuan, P.; Liu, D.; Wang, S.; Song, H.; Guo, H. Calcination-induced changes in structure, morphology, and porosity of allophane. Appl. Clay Sci. 2018, 158, 211–218. [Google Scholar] [CrossRef]
- Midttomme, K.; Roaldset, E. The effect of grain size on thermal conductivity of quartz sands and silts. Pet. Geosci. 1998, 4, 165–172. [Google Scholar] [CrossRef]
- Maqueda, C.; Perez-Rodriguez, J.L.; Šubrt, J.; Murafa, N. Study of ground and unground leached vermiculite. Appl. Clay Sci. 2009, 44, 178–184. [Google Scholar] [CrossRef]
- Jiao, L.; Lin, F.; Cao, S.; Wang, C.; Wu, H.; Shu, M.; Hu, C. Preparation, characterization, antimicrobial and cytotoxicity studies of copper/zinc- loaded montmorillonite. J. Anim. Sci. Biotechnol. 2017, 8, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Favvas, E.P.; Tsanaktsidis, C.G.; Sapalidis, A.A.; Tzilantonis, G.T.; Papageorgiou, S.K.; Mitropoulos, A.C. Clinoptilolite, a natural zeolite material: Structural characterization and performance evaluation on its dehydration properties of hydrocarbon-based fuels. Microporous Mesoporous Mater. 2016, 225, 385–391. [Google Scholar] [CrossRef]
- Fu, H.; Quan, X. Complexes of fulvic acid on the surface of hematite, goethite, and akaganeite: FTIR observation. Chemosphere 2006, 63, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Kitis, M.; Kaplan, S.S.; Karakaya, E.; Yigit, N.O.; Civelekoglu, G. Adsorption of natural organic matter from waters by iron coated pumice. Chemosphere 2007, 66, 130–138. [Google Scholar] [CrossRef]
- Vossoughi, S.; Bartlett, G.W.; Willhite, P.G. Prediction of In-Situ Combustion Process Variables By Use of TGA/DSC Techniques and the Effect of Sand-Grain Specific Surface Area on the Process. Soc. Pet. Eng. J. 1985, 25, 656–664. [Google Scholar] [CrossRef]
- Mi, J.; Gregorich, E.G.; Xu, S.; McLaughlin, N.B.; Ma, B.; Liu, J. Effect of bentonite amendment on soil hydraulic parameters and millet crop performance in a semi-arid region. Field Crops Res. 2017, 212, 107–114. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Tarkalson, D.D.; Lehrsch, G.A. Zeolite Soil Application Method Affects Inorganic Nitrogen, Moisture, and Corn Growth. Soil Sci. 2011, 176, 136–142. [Google Scholar] [CrossRef]
- Anderson, W.K.; Hamza, M.A.; Sharma, D.L.; D′Antuono, M.F.; Hoyle, F.C.; Hill, N.; Shackley, B.J.; Amjad, M.; Zaicou-Kunesch, C. The role of management in yield improvement of the wheat cropa review with special emphasis on Western Australia. Aust. J. Agric. Res. 2005, 56, 1137–1149. [Google Scholar] [CrossRef]
- Turner, N.C. Measurement of plant water status by the pressure chamber technique. Irrig. Sci. 1988, 9, 289–308. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Huynh, W.Q.; Kronzucker, H.J. The Role of Silicon in Higher Plants under Salinity and Drought Stress. Front. Plant Sci. 2016, 7, 1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaller, J.; Cramer, A.; Carminati, A.; Zarebanadkouki, M. Biogenic amorphous silica as main driver for plant available water in soils. Sci. Rep. 2020, 10, 2424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raven, J.A. The Transport and Function Of Silicon In Plants. Biol. Rev. 1983, 58, 179–207. [Google Scholar] [CrossRef]
- He, C.; Wang, L.; Liu, J.; Liu, X.; Li, X.; Ma, J.; Lin, Y.; Xu, F. Evidence for ‘silicon’ within the cell walls of suspension-cultured rice cells. New Phytol. 2013, 200, 700–709. [Google Scholar] [CrossRef]
- Tayyab, M.; Islam, W.; Zhang, H. Promising role of silicon to enhance drought resistance in wheat. Commun. Soil Sci. Plant Anal. 2018, 49, 2932–2941. [Google Scholar] [CrossRef]
- Gong, H.; Zhu, X.; Chen, K.; Wang, S.; Zhang, C. Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci. 2005, 169, 313–321. [Google Scholar] [CrossRef]
- Chen, W.; Yao, X.; Cai, K.; Chen, J. Silicon Alleviates Drought Stress of Rice Plants by Improving Plant Water Status, Photosynthesis and Mineral Nutrient Absorption. Biol. Trace Elem. Res. 2011, 142, 67–76. [Google Scholar] [CrossRef]
- Ma, J.; Takahashi, E. Release of silicon from rice straw under flooded conditions. Soil Sci. Plant Nutr. 1989, 35, 663–667. [Google Scholar] [CrossRef] [Green Version]
- Haynes, R.J. A contemporary overview of silicon availability in agricultural soils. J. Plant Nutr. Soil Sci. 2014, 177, 831–844. [Google Scholar] [CrossRef]
- Nanayakkara, U.N.; Uddin, W.; Datnoff, L.E. Application of silicon sources increases silicon accumulation in perennial ryegrass turf on two soil types. Plant Soil 2008, 303, 83–94. [Google Scholar] [CrossRef]
- Schwieger, W.; Pohl, K.; Brenn, U.; Fyfe, C.A.; Grondey, H.; Fu, G.; Kokotailo, G.T. Isomorphous substitution of silicon by boron or aluminum in layered silicates. In Studies in Surface Science and Catalysis; Beyer, H.K., Ed.; Elsevier: Amsterdam, The Netherlands, 1995; pp. 47–54. [Google Scholar]
- Carlson, T.N.; Perry, E.M.; Schmugge, T.J. Remote estimation of soil moisture availability and fractional vegetation cover for agricultural fields. Agric. For. Meteorol. 1990, 52, 45–69. [Google Scholar] [CrossRef]
- Mott, J.J. Germination Studies on some Annual Species from an Arid Region of Western Australia. J. Ecol. 1972, 60, 293–304. [Google Scholar] [CrossRef]
- Greer, L.; Dole, J.M. Aluminum Foil, Aluminium-painted, Plastic, and Degradable Mulches Increase Yields and Decrease Insectvectored Viral Diseases of Vegetables. HortTechnology 2003, 13, 276–284. [Google Scholar] [CrossRef] [Green Version]
- Ham, J.M.; Kluitenberg, G.J. Modeling the effect of mulch optical properties and mulch-soil contact resistance on soil heating under plastic mulch culture. Agric. For. Meteorol. 1994, 71, 403–424. [Google Scholar] [CrossRef]
- Steinmetz, Z.; Wollmann, C.; Schaefer, M.; Buchmann, C.; David, J.; Tröger, J.; Muñoz, K.; Frör, O.; Schaumann, G.E. Plastic mulching in agriculture. Trading short-term agronomic benefits for long-term soil degradation? Sci. Total Environ. 2016, 550, 690–705. [Google Scholar] [CrossRef]
- Berner, R.A.; Lasaga, A.C. Modeling the Geochemical Carbon Cycle. Sci. Am. 1989, 260, 74–81. [Google Scholar] [CrossRef]
- Schuiling, R.D.; Krijgsman, P. Enhanced Weathering: An Effective and Cheap Tool to Sequester CO2. Clim. Chang. 2006, 74, 349–354. [Google Scholar] [CrossRef]
- Eggleton, R.A.; Foudoulis, C.; Varkevisser, D. Weathering of basalt: Changes in rock chemistry and mineralogy. Clays Clay Miner. 1987, 35, 161–169. [Google Scholar] [CrossRef]
- Pratt, C.; Tate, K. Mitigating Methane: Emerging Technologies To Combat Climate Change’s Second Leading Contributor. Environ. Sci. Technol. 2018, 52, 6084–6097. [Google Scholar] [CrossRef] [Green Version]
- Kantola, I.B.; Masters, M.D.; Beerling, D.J.; Long, S.P.; DeLucia, E.H. Potential of global croplands and bioenergy crops for climate change mitigation through deployment for enhanced weathering. Biol. Lett. 2017, 13, 20160714. [Google Scholar] [CrossRef] [Green Version]
- Strefler, J.; Amann, T.; Bauer, N.; Kriegler, E.; Hartmann, J. Potential and costs of carbon dioxide removal by enhanced weathering of rocks. Environ. Res. Lett. 2018, 13, 034010. [Google Scholar] [CrossRef]
- Casey, W.H.; Banfield, J.F.; Westrich, H.R.; McLaughlin, L. What do dissolution experiments tell us about natural weathering? Chem. Geol. 1993, 105, 1–15. [Google Scholar] [CrossRef]
- Hangx, S.J.T.; Spiers, C.J. Coastal spreading of olivine to control atmospheric CO2 concentrations: A critical analysis of viability. Int. J. Greenh. Gas Control 2009, 3, 757–767. [Google Scholar] [CrossRef]
- Hausrath, E.M.; Treiman, A.H.; Vicenzi, E.; Bish, D.L.; Blake, D.; Sarrazin, P.; Hoehler, T.; Midtkandal, I.; Steele, A.; Brantley, S.L. Short- and Long-Term Olivine Weathering in Svalbard: Implications for Mars. Astrobiology 2008, 8, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- IPCC Climate Change 2014. Synthesis Report. In Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; 151p. [Google Scholar]
- Watanabe, A.; Kimura, M. Influence of chemical properties of soils on methane emission from rice paddies. Commun. Soil Sci. Plant Anal. 1999, 30, 2449–2463. [Google Scholar] [CrossRef]
- Jäckel, U.; Schnell, S. Suppression of methane emission from rice paddies by ferric iron fertilization. Soil Biol. Biochem. 2000, 32, 1811–1814. [Google Scholar] [CrossRef]
- Ali, M.A.; Oh, J.H.; Kim, P.J. Evaluation of silicate iron slag amendment on reducing methane emission from flood water rice farming. Agric. Ecosyst. Environ. 2008, 128, 21–26. [Google Scholar] [CrossRef]
- Wang, W.Q.; Li, P.F.; Zeng, C.S.; Tong, C. Evaluation of Silicate Iron Slag as a Potential Methane Mitigating Method. Adv. Mater. Res. 2012, 468–471, 1626–1630. [Google Scholar] [CrossRef]
- Denier van der Gon, H.A.; van Bodegom, P.M.; Wassmann, R.; Lantin, R.S.; Metra-Corton, T.M. Sulfate-containing amendments to reduce methane emissions from rice fields: Mechanisms, effectiveness and costs. Mitig. Adapt. Strateg. Glob. Chang. 2001, 6, 71–89. [Google Scholar] [CrossRef]
- Lindau, C.W.; Alford, D.P.; Bollich, P.K.; Linscombe, S.D. Inhibition of methane evolution by calcium sulfate addition to flooded rice. Plant Soil 1994, 158, 299–301. [Google Scholar] [CrossRef]
- Minamikawa, K.; Sakai, N.; Hayashi, H. The effects of ammonium sulfate application on methane emission and soil carbon content of a paddy field in Japan. Agric. Ecosyst. Environ. 2005, 107, 371–379. [Google Scholar] [CrossRef]
- Haruna Ahmed, O.; Husin, A.; Husni Mohd Hanif, A. Ammonia volatilization and ammonium accumulation from urea mixed with zeolite and triple superphosphate. Acta Agric. Scand. Sect. B Soil Plant Sci. 2008, 58, 182–186. [Google Scholar] [CrossRef]
- Bernal, M.P.; Lopez-Real, J.M. Natural zeolites and sepiolite as ammonium and ammonia adsorbent materials. Bioresour. Technol. 1993, 43, 27–33. [Google Scholar] [CrossRef]
- Ramesh, K.; Reddy, D.D. Chapter Four—Zeolites and Their Potential Uses in Agriculture. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2011; pp. 219–241. [Google Scholar]
- Zaman, M.; Nguyen, M.L. Effect of lime or zeolite on N2O and N2 emissions from a pastoral soil treated with urine or nitrate-N fertilizer under field conditions. Agric. Ecosyst. Environ. 2010, 136, 254–261. [Google Scholar] [CrossRef]
- Hill, J.; Redding, M.; Pratt, C. A novel and effective technology for mitigating nitrous oxide emissions from land-applied manures. Anim. Prod. Sci. 2016, 56, 362–369. [Google Scholar] [CrossRef] [Green Version]
- FAO Mitigation of Climate Change in Agriculture (MICCA) Programme. Available online: http://www.fao.org/in-action/micca/en/ (accessed on 29 June 2020).
- Lal, R.; Delgado, J.A.; Groffman, P.M.; Millar, N.; Dell, C.; Rotz, A. Management to mitigate and adapt to climate change. J. Soil Water Conserv. 2011, 66, 276–285. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.; Olesen, J.E. Synergies between the mitigation of, and adaptation to, climate change in agriculture. J. Agric. Sci. 2010, 148, 543–552. [Google Scholar] [CrossRef] [Green Version]
- Burt, R.; Fillmore, M.; Wilson, M.A.; Gross, E.R.; Langridge, R.W.; Lammers, D.A. Soil properties of selected pedons on ultramafic rocks in Klamath Mountains, Oregon. Commun. Soil Sci. Plant Anal. 2001, 32, 2145–2175. [Google Scholar] [CrossRef]
- Thornton, F.C.; Dev Joslin, J.; Bock, B.R.; Houston, A.; Green, T.H.; Schoenholtz, S.; Pettry, D.; Tyler, D.D. Environmental effects of growing woody crops on agricultural land: First year effects on erosion, and water quality. Biomass Bioenergy 1998, 15, 57–69. [Google Scholar] [CrossRef]
- Panagos, P.; Borrelli, P.; Meusburger, K.; Yu, B.; Klik, A.; Jae Lim, K.; Yang, J.E.; Ni, J.; Miao, C.; Chattopadhyay, N.; et al. Global rainfall erosivity assessment based on high-temporal resolution rainfall records. Sci. Rep. 2017, 7, 4175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, B.; Yu, K.; Gambrell, R.P. Effects of ferric iron reduction and regeneration on nitrous oxide and methane emissions in a rice soil. Chemosphere 2009, 74, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Chiavegatto, C.V.; Carneiro, A.P.S.; Dias, E.C.; Nascimento, M.S. Diagnosis of Severe Silicosis in Young Adults Working in Stone Polishing and Mining in Minas Gerais, Brazil. Int. J. Occup. Environ. Health 2010, 16, 139–142. [Google Scholar] [CrossRef]
- Drenovsky, R.E.; Steenwerth, K.L.; Jackson, L.E.; Scow, K.M. Land use and climatic factors structure regional patterns in soil microbial communities. Glob. Ecol. Biogeogr. 2010, 19, 27–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lepleux, C.; Turpault, M.P.; Oger, P.; Frey-Klett, P.; Uroz, S. Correlation of the Abundance of Betaproteobacteria on Mineral Surfaces with Mineral Weathering in Forest Soils. Appl. Environ. Microbiol. 2012, 78, 7114–7119. [Google Scholar] [CrossRef] [Green Version]
- López-Fernández, M.; Fernández-Sanfrancisco, O.; Moreno-García, A.; Martín-Sánchez, I.; Sánchez-Castro, I.; Merroun, M.L. Microbial communities in bentonite formations and their interactions with uranium. Appl. Geochem. 2014, 49, 77–86. [Google Scholar] [CrossRef]
- Quilliam, R.S.; Glanville, H.C.; Wade, S.C.; Jones, D.L. Life in the ‘charosphere’—Does biochar in agricultural soil provide a significant habitat for microorganisms? Soil Biol. Biochem. 2013, 65, 287–293. [Google Scholar] [CrossRef]
- Index-Mundi Commodity Price Indices—Urea. 2020. Available online: https://www.indexmundi.com/commodities/?commodity=urea&months=360¤cy=aud (accessed on 25 May 2020).
- USGS Vermiculite Statistics and Information. 2019. Available online: https://www.usgs.gov/centers/nmic/vermiculite-statistics-and-information (accessed on 10 February 2020).
- Borsatto, F.; Inglezakis, V.J. Natural zeolite markets and strategic considerations. In Handbook of Natural Zeolites; Inglezakis, V.J., Zorpas, A.A., Eds.; Bentham E Books: Potomac, MD, USA, 2012. [Google Scholar]
- USGS Clays Statistics and Information. 2020. Available online: https://www.usgs.gov/centers/nmic/clays-statistics-and-information (accessed on 10 February 2020).
- Statista Major Countries in Gypsum Mine Production from 2014 to 2018. 2018. Available online: https://www.statista.com/statistics/264936/global-gypsum-production-by-major-countries/ (accessed on 10 February 2020).
- USGS Iron Ore Statistics and Information. 2020. Available online: https://www.usgs.gov/centers/nmic/iron-ore-statistics-and-information (accessed on 10 February 2020).
- Hartmann, J.; Moosdorf, N. The new global lithological map database GLiM: A representation of rock properties at the Earth surface. Geochem. Geophys. Geosyst. 2012, 13. [Google Scholar] [CrossRef]
- Tromans, D.; Meech, J.A. Fracture toughness and surface energies of covalent minerals: Theoretical estimates. Miner. Eng. 2004, 17, 1–15. [Google Scholar] [CrossRef]
- Bennett, P.C.; Rogers, J.R.; Choi, W.J.; Hiebert, F.K. Silicates, Silicate Weathering, and Microbial Ecology. Geomicrobiol. J. 2001, 18, 3–19. [Google Scholar]
- Gouda, S.; Kerry, R.G.; Das, G.; Paramithiotis, S.; Shin, H.-S.; Patra, J.K. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol. Res. 2018, 206, 131–140. [Google Scholar]
- Liu, K.; McInroy, J.A.; Hu, C.-H.; Kloepper, J.W. Mixtures of Plant-Growth-Promoting Rhizobacteria Enhance Biological Control of Multiple Plant Diseases and Plant-Growth Promotion in the Presence of Pathogens. Plant Dis. 2018, 102, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Yuan-Yuan, L.; Zhan-Li, W.; Bing, W.; Jun-E, L.; Nian, J. Impacts of natural polymer derivative neutral polysaccharide Jag S and cationic hydroxypropyl polysaccharide Jag C162 on rainfall infiltration on an experimental loess hillslope. Soil Sci. Plant Nutr. 2018, 64, 244–252. [Google Scholar] [CrossRef]
- Saruchi; Kumar, V.; Mittal, H.; Alhassan, S.M. Biodegradable hydrogels of tragacanth gum polysaccharide to improve water retention capacity of soil and environment-friendly controlled release of agrochemicals. Int. J. Biol. Macromol. 2019, 132, 1252–1261. [Google Scholar] [CrossRef] [PubMed]



| Mineral | Nutrient Targeted | Stream Type | Solution Concentration | Maximum Sorption Capacity | Removal % | Reference |
|---|---|---|---|---|---|---|
| Zeolite (clinoptilolite) | Ammonium | Rice paddy runoff | 14 mg NH4+/L | 2 g NH4+-N/kg | 30–50 | [66] |
| Zeolite | Ammonium | Piggery and dairy effluent | 129 mg NH4+/L | 2 to 9 g NH4+-N/kg | - | [74] |
| Chitosan-coated smectite | Ammonium | Synthetic effluent | 33 mg NH4+/L | 4 g NH4+-N/kg | - | [75] |
| Vermiculite and Smectite | Ammonium | Piggery, poultry, and beef cattle effluent | - | - | 80 | [23] |
| Nano Al oxides | Nitrate | Synthetic effluent | 20 mg NO3−-N OR total N (unclear)/L | 4 g NO3−-N/kg | - | [68] |
| Hydrotalcite (hydroxide) | Nitrate | Eutrophied stream | >25 mg NO3−-N/L | 27 g NO3−-N/kg | >90 | [76] |
| Hydrotalcite (hydroxide) | Phosphate | Eutrophied stream | >25 mg PO43−-P/L | 9.8 g PO43−-P/kg | >90 | [76] |
| Amorphous Fe and Al oxides | Phosphate | Agricultural runoff | 1 to 10 mg PO43-P/L | 5 g PO43−-P/kg | - | [70] |
| Artificial Fe and Al oxides | Phosphate | Eutrophied stream | 120 mg PO43−-P/L | 4.5 g PO43−-P/kg | 35 | [77] |
| Allophane | Phosphate | Dairy farm runoff | 10 mg PO43−-P/L | 3 g PO43−-P/kg | - | [78] |
| Material | Mineralogy | Specific Surface Area m2/g | Reference |
|---|---|---|---|
| Organic matter | NA | 60–480 | [92] |
| Zeolite | Mordenite | 1150 | [93] |
| Allophane | Pure | 1000 | [94] |
| Smectite | Montmorillonite | 750 | [95] |
| Vermiculite | Pure | 504 | [96] |
| Smectite | Montmorillonite | 230 | [97] |
| Zeolite | Clinoptilolite | 200 | [98] |
| Iron oxide | Goethite | 31 | [99] |
| Pumice | Sodium feldspar, pyroxene, olivine | 5–15 | [100] |
| Sand | Quartz, feldspar | 7.6 | [101] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pratt, C.; Kingston, K.; Laycock, B.; Levett, I.; Pratt, S. Geo-Agriculture: Reviewing Opportunities through Which the Geosphere Can Help Address Emerging Crop Production Challenges. Agronomy 2020, 10, 971. https://doi.org/10.3390/agronomy10070971
Pratt C, Kingston K, Laycock B, Levett I, Pratt S. Geo-Agriculture: Reviewing Opportunities through Which the Geosphere Can Help Address Emerging Crop Production Challenges. Agronomy. 2020; 10(7):971. https://doi.org/10.3390/agronomy10070971
Chicago/Turabian StylePratt, Chris, Kate Kingston, Bronwyn Laycock, Ian Levett, and Steven Pratt. 2020. "Geo-Agriculture: Reviewing Opportunities through Which the Geosphere Can Help Address Emerging Crop Production Challenges" Agronomy 10, no. 7: 971. https://doi.org/10.3390/agronomy10070971
APA StylePratt, C., Kingston, K., Laycock, B., Levett, I., & Pratt, S. (2020). Geo-Agriculture: Reviewing Opportunities through Which the Geosphere Can Help Address Emerging Crop Production Challenges. Agronomy, 10(7), 971. https://doi.org/10.3390/agronomy10070971






