Assessing Nitrogen Cycling in Corncob Biochar Amended Soil Columns for Application in Agricultural Treatment Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Charracteristics
2.2. Biochar Production and Properites
2.3. Experimental Design
2.4. Soil and Biochar Analysis
2.5. Data Analysis
3. Results
3.1. Organic Nitrogen Application (Treatment Groups 1 and 2)
3.2. Ammonium Applications (Treatment Groups 3 and 4)
3.3. Nitrate Application (Treament Group 5)
3.4. Changes in Biochar Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chia, C.; Downie, A.; Munroe, P. Characteristics of biochar: Physical and structural properties. In Biochar for Environmental Management: Science, Technology, and Implementation; Lehmann, J., Joseph, S., Eds.; Routledge: New York, NY, USA, 2015; pp. 89–109. [Google Scholar]
- Ippolito, J.A.; Spoka, K.A.; Novak, J.M.; Lentz, R.D.; Cantrell, K.B. Biochar elemental composition and factors influencing nutrient retention. In Biochar for Environmental Management: Science, Technology, and Implementation; Lehmann, J., Joseph, S., Eds.; Routledge: New York, NY, USA, 2015; pp. 139–163. [Google Scholar]
- Lehmann, J.; Joseph, S. Biochar for environmental management: An introduction. In Biochar for Environmental Management: Science, Technology, and Implementation; Lehmann, J., Joseph, S., Eds.; Routledge: New York, NY, USA, 2015; pp. 1–14. [Google Scholar]
- Ahmed, R.; Li, Y.; Mao, L.; Xu, C.; Lin, W.; Ahmed, S.; Ahmed, W. Biochar Effects on Mineral Nitrogen Leaching, Moisture Content, and Evapotranspiration after 15N Urea Fertilization for Vegetable Crop. Agronomy 2019, 9, 331. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.-X.; Wu, W.; Shi, D.; Yang, M.; Zhong, Z.-K. Evaluation of Biochar Effects on Nitrogen Retention and Leaching in Multi-Layered Soil Columns. Water Air Soil Pollut. 2010, 213, 47–55. [Google Scholar] [CrossRef]
- Kameyama, K.; Miyamoto, T.; Shiono, T.; Shinogi, Y. Influence of Sugarcane Bagasse-derived Biochar Application on Nitrate Leaching in Calcaric Dark Red Soil. J. Environ. Qual. 2012, 41, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, Y.; Yan, W.; Shangguan, Z. Effect of biochar application method on nitrogen leaching and hydraulic conductivity in a silty clay soil. Soil Tillage Res. 2018, 183, 100–108. [Google Scholar] [CrossRef]
- Uzoma, K.C.; Inoue, M.; Andry, H.; Zahoor, A.; Nishihara, E. Influence of biochar application on sandy soil hydraulic properties and nutrient retention. J. Food Agric. Environ. 2011, 9, 1137–1143. [Google Scholar]
- Xu, N.; Tan, G.; Wang, H.; Gai, X. Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur. J. Soil Biol. 2016, 74, 1–8. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Zhang, M.; Inyang, M.; Zimmerman, A.R. Effect of biochar amendment on sorption and leaching of nitrate, ammonium, and phosphate in a sandy soil. Chemosphere 2012, 89, 1467–1471. [Google Scholar] [CrossRef]
- Bradley, A.; Larson, R.A.; Runge, T. Effect of Wood Biochar in Manure-Applied Sand Columns on Leachate Quality. J. Environ. Qual. 2015, 44, 1720–1728. [Google Scholar] [CrossRef]
- Laird, D.A.; Fleming, P.; Wang, B.; Horton, R.; Karlen, D. Biochar impact on nutrient leaching from a Midwestern agricultural soil. Geoderma 2010, 158, 436–442. [Google Scholar] [CrossRef]
- Harter, J.; Krause, H.-M.; Schuettler, S.; Ruser, R.; Fromme, M.; Scholten, T.; Kappler, A.; Behrens, S. Linking N2O emissions from biochar-amended soil to the structure and function of the N-cycling microbial community. ISME J. 2013, 8, 660–674. [Google Scholar] [CrossRef]
- Harter, J.; Elhadidi, M.; Huson, D.H.; Kappler, A.; Behrens, S. Soil biochar amendment affects the diversity of nosZ transcripts: Implications for N2O formation. Sci. Rep. 2017, 7, 3338. [Google Scholar] [CrossRef]
- Bai, S.H.; Reverchon, F.; Xu, C.-Y.; Xu, Z.; Blumfield, T.; Zhao, H.-T.; Van Zwieten, L.; Wallace, H. Wood biochar increases nitrogen retention in field settings mainly through abiotic processes. Soil Biol. Biochem. 2015, 90, 232–240. [Google Scholar] [CrossRef]
- Cayuela, M.L.; Sánchez-Monedero, M.A.; Roig, A.; Hanley, K.; Enders, A.; Lehmann, J. Biochar and denitrification in soils: When, how much and why does biochar reduce N2O emissions? Sci. Rep. 2013, 3, srep01732. [Google Scholar] [CrossRef]
- Güereña, D.; Lehmann, J.; Hanley, K.; Enders, A.; Hyland, C.; Riha, S. Nitrogen dynamics following field application of biochar in a temperate North American maize-based production system. Plant Soil 2012, 365, 239–254. [Google Scholar] [CrossRef]
- Martin, S.L.; Clarke, M.L.; Othman, M.; Ramsden, S.J.; West, H.M. Biochar-mediated reductions in greenhouse gas emissions from soil amended with anaerobic digestates. Biomass Bioenergy 2015, 79, 39–49. [Google Scholar] [CrossRef]
- Sanford, J.; Larson, R.A. Treatment of horizontal silage bunker runoff using biochar amended vegetative filter strips. J. Environ. Manag. 2019, 253, 109746. [Google Scholar] [CrossRef]
- Van Zwieten, L.; Kimber, S.; Morris, S.; Chan, K.Y.; Downie, A.; Rust, J.; Joseph, S.; Cowie, A. Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 2009, 327, 235–246. [Google Scholar] [CrossRef]
- Yadav, V.; Karak, T.; Singh, S.; Singh, A.K.; Khare, P. Benefits of biochar over other organic amendments: Responses for plant productivity (Pelargonium graveolens L.) and nitrogen and phosphorus losses. Ind. Crop. Prod. 2019, 131, 96–105. [Google Scholar] [CrossRef]
- Rashti, M.R.; Esfandbod, M.; Phillips, I.R.; Chen, C. Rhizosphere management by biochar and leaching improved plant performance in fresh bauxite residue sand. J. Clean. Prod. 2019, 219, 66–74. [Google Scholar] [CrossRef]
- Lin, Y.; Munroe, P.; Joseph, S.; Henderson, R.K.; Ziolkowski, A. Water extractable organic carbon in untreated and chemical treated biochars. Chemosphere 2012, 87, 151–157. [Google Scholar] [CrossRef]
- Nelissen, V.; Rütting, T.; Huygens, D.; Staelens, J.; Ruysschaert, G.; Boeckx, P. Maize biochars accelerate short-term soil nitrogen dynamics in a loamy sand soil. Soil Biol. Biochem. 2012, 55, 20–27. [Google Scholar] [CrossRef]
- Hale, S.E.; Alling, V.; Martinsen, V.; Mulder, J.; Breedveld, G.D.; Cornélissen, G. The sorption and desorption of phosphate-P, ammonium-N and nitrate-N in cacao shell and corn cob biochars. Chemosphere 2013, 91, 1612–1619. [Google Scholar] [CrossRef]
- Takaya, C.; Fletcher, L.; Singh, S.; Anyikude, K.; Ross, A. Phosphate and ammonium sorption capacity of biochar and hydrochar from different wastes. Chemosphere 2016, 145, 518–527. [Google Scholar] [CrossRef]
- Wang, T.; Arbestain, M.C.; Hedley, M.; Bishop, P. Chemical and bioassay characterisation of nitrogen availability in biochar produced from dairy manure and biosolids. Org. Geochem. 2012, 51, 45–54. [Google Scholar] [CrossRef]
- Taghizadeh-Toosi, A.; Clough, T.J.; Sherlock, R.R.; Condron, L.M. Biochar adsorbed ammonia is bioavailable. Plant Soil 2011, 350, 57–69. [Google Scholar] [CrossRef]
- Joseph, S.; Kammann, C.; Shepherd, J.; Conte, P.; Schmidt, H.-P.; Hagemann, N.; Rich, A.M.; Marjo, C.E.; Allen, J.; Munroe, P.; et al. Microstructural and associated chemical changes during the composting of a high temperature biochar: Mechanisms for nitrate, phosphate and other nutrient retention and release. Sci. Total. Environ. 2018, 618, 1210–1223. [Google Scholar] [CrossRef]
- Kammann, C.; Schmidt, H.-P.; Messerschmidt, N.; Linsel, S.; Steffens, D.; Müller, C.; Koyro, H.-W.; Conte, P.; Joseph, S.; Stephen, J. Plant growth improvement mediated by nitrate capture in co-composted biochar. Sci. Rep. 2015, 5, 11080. [Google Scholar] [CrossRef] [PubMed]
- Dempster, D.N.; Jones, D.L.; Murphy, D.V. Clay and biochar amendments decreased inorganic but not dissolved organic nitrogen leaching in soil. Soil Res. 2012, 50, 216–221. [Google Scholar] [CrossRef]
- Hollister, C.C.; Bisogni, J.J.; Lehmann, J. Ammonium, Nitrate, and Phosphate Sorption to and Solute Leaching from Biochars Prepared from Corn Stover (Zea mays L.) and Oak Wood (Quercus spp.). J. Environ. Qual. 2013, 42, 137–144. [Google Scholar] [CrossRef]
- Ohe, K.; Nagae, Y.; Nakamura, S.; Baba, Y. Removal of Nitrate Anion by Carbonaceous Materials Prepared from Bamboo and Coconut Shell. J. Chem. Eng. Jpn. 2003, 36, 511–515. [Google Scholar] [CrossRef]
- Yang, J.; Li, H.; Zhang, D.; Wu, M.; Pan, B. Limited role of biochars in nitrogen fixation through nitrate adsorption. Sci. Total Environ. 2017, 592, 758–765. [Google Scholar] [CrossRef]
- Haider, G.; Steffens, D.; Müller, C.; Kammann, C.I. Standard Extraction Methods May Underestimate Nitrate Stocks Captured by Field-Aged Biochar. J. Environ. Qual. 2016, 45, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Sanford, J.; Larson, R.A.; Runge, T. Nitrate sorption to biochar following chemical oxidation. Sci. Total Environ. 2019, 669, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Chen, Z.; Müller, C.; Zaman, M.M.; Kim, D.; Yu, H.; Ding, W. Yield-scaled N 2 O emissions were effectively reduced by biochar amendment of sandy loam soil under maize—Wheat rotation in the North China Plain. Atmos. Environ. 2017, 170, 58–70. [Google Scholar] [CrossRef]
- Senbayram, M.; Saygan, E.P.; Chen, R.; Aydemir, S.; Kaya, C.; Wu, D.; Bladogatskaya, E. Effect of biochar origin and soil type on the greenhouse gas emission and the bacterial community structure in N fertilised acidic sandy and alkaline clay soil. Sci. Total Environ. 2019, 660, 69–79. [Google Scholar] [CrossRef]
- Arnold, J.; Knapp, J.; Johnson, C. The use of yeasts to reduce the polluting potential of silage effluent. Water Res. 2000, 34, 3699–3708. [Google Scholar] [CrossRef]
- Gebrehanna, M.; Gordon, R.J.; Madani, A.; Vanderzaag, A.; Wood, J.D. Silage effluent management: A review. J. Environ. Manag. 2014, 143, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Holly, M.A.; Larson, R.A.; Cooley, E.T.; Wunderlin, A.M. Silage storage runoff characterization: Annual nutrient loading rate and first flush analysis of bunker silos. Agric. Ecosyst. Environ. 2018, 264, 85–93. [Google Scholar] [CrossRef]
- Bhattarai, R.; Kalita, P.K.; Patel, M.K. Nutrient transport through a Vegetative Filter Strip with subsurface drainage. J. Environ. Manag. 2009, 90, 1868–1876. [Google Scholar] [CrossRef]
- Holly, M.A.; Larson, R.A. Treatment of Silage Runoff Using Vegetated Filter Strips. Trans. ASABE 2016, 59, 1645–1650. [Google Scholar] [CrossRef]
- Faulkner, J.W.; Zhang, W.; Geohring, L.D.; Steenhuis, T.S. Nutrient transport within three vegetative treatment areas receiving silage bunker runoff. J. Environ. Manag. 2011, 92, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Larson, R.A.; Safferman, S.I. Field Application of Farmstead Runoff to Vegetated Filter Strips: Surface and Subsurface Water Quality Assessment. J. Environ. Qual. 2012, 41, 592–603. [Google Scholar] [CrossRef]
- Bouyoucos, G.J. Hydrometer method improved for making particle size analysis of soils. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Olk, D.; Fortuna, A.-M.; Honeycutt, C.W. Using Anion Chromatography-Pulsed Amperometry to Measure Amino Compounds in Dairy Manure-Amended Soils. Soil Sci. Soc. Am. J. 2008, 72, 1711–1720. [Google Scholar] [CrossRef][Green Version]
- King, K.; Williams, M.R.; Fausey, N. Effect of crop type and season on nutrient leaching to tile drainage under a corn-soybean rotation. J. Soil Water Conserv. 2016, 71, 56–68. [Google Scholar] [CrossRef]
- Snoeyink, V.L.; Jenkins, D. Water Chemistry, 1st ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1980; ISBN 978-0471051961. [Google Scholar]
- Aneja, V.; Blunden, J.; Claiborn, C.; Rogers, H. Dynamic Chamber System to Measure Gaseous Compounds Emissions and Atmospheric-Biospheric Interactions. In Environmental Simulation Chambers: Application to Atmospheric Chemical Processes; Barnes, I., Rudzinski, K., Eds.; Springer: Dordrecht, The Netherlands, 2006; Volume 62, ISBN 978-1-4020-4230-0. [Google Scholar]
- Parkin, T.B.; Venterea, R.T. USDA-ARS GRACEnet Project Protocols Chapter 3. Chamber-Based Trace Gas Flux Measurements. In Sampling Protocols; USDA: Washington, DC, USA, 2010; pp. 1–39. [Google Scholar]
- Bremner, J.M. Inorganic Forms of Nitrogen. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; American Society of Agronomy, Inc.: Madison, WI, USA, 1965; pp. 1179–1237. [Google Scholar]
- Ventura, M.; Sorrenti, G.; Panzacchi, P.; George, E.; Tonon, G. Biochar Reduces Short-Term Nitrate Leaching from A Horizon in an Apple Orchard. J. Environ. Qual. 2013, 42, 76–82. [Google Scholar] [CrossRef]
- Hagemann, N.; Kammann, C.I.; Schmidt, H.-P.; Kappler, A.; Behrens, S. Nitrate capture and slow release in biochar amended compost and soil. PLoS ONE 2017, 12, e0171214. [Google Scholar] [CrossRef]
- Walsh, J.; Sanford, J.; Larson, R.A. Evaluation of Biochar Nitrate Extraction Methods. Appl. Sci. 2019, 9, 3514. [Google Scholar] [CrossRef]
- Bremner, J.M. Total Nitrogen. In Methods for Soil Analysis, Part 2; Norman, A.G., Ed.; American Society of Agronomy: Madison, WI, USA, 1965; pp. 1149–1178. [Google Scholar]
- Cheng, C.-H.; Lehmann, J.; Thies, J.E.; Burton, S.D.; Engelhard, M. Oxidation of black carbon by biotic and abiotic processes. Org. Geochem. 2006, 37, 1477–1488. [Google Scholar] [CrossRef]
- SAS Base. SAS® 9.4 Procedures Guide: Statistical Procedures, 2nd ed.; SAS Institute Inc.: Cary, NC, USA, 2013. [Google Scholar]
- Hina, K.; Bishop, P.; Arbestain, M.C.; Pereira, R.C.; Maciá-Agulló, J.A.; Hindmarsh, J.; Hanly, J.A.; Macias, F.; Hedley, M.J. Producing biochars with enhanced surface activity through alkaline pretreatment of feedstocks. Soil Res. 2010, 48, 606–617. [Google Scholar] [CrossRef]
- Sharma, R.; Wooten, J.B.; Baliga, V.L.; Lin, X.; Chan, W.G.; Hajaligol, M.R. Characterization of chars from pyrolysis of lignin. Fuel 2004, 83, 1469–1482. [Google Scholar] [CrossRef]
- Wang, S.; Wang, K.; Liu, Q.; Gu, Y.; Luo, Z.; Cen, K.; Fransson, T. Comparison of the pyrolysis behavior of lignins from different tree species. Biotechnol. Adv. 2009, 27, 562–567. [Google Scholar] [CrossRef]
- Chun, Y.; Sheng, G.; Chiou, C.T.; Xing, B. Compositions and Sorptive Properties of Crop Residue-Derived Chars. Environ. Sci. Technol. 2004, 38, 4649–4655. [Google Scholar] [CrossRef] [PubMed]
- Kloss, S.; Zehetner, F.; Dellantonio, A.; Hamid, R.; Ottner, F.; Liedtke, V.; Schwanninger, M.; Gerzabek, M.H.; Soja, G. Characterization of Slow Pyrolysis Biochars: Effects of Feedstocks and Pyrolysis Temperature on Biochar Properties. J. Environ. Qual. 2012, 41, 990–1000. [Google Scholar] [CrossRef]
- Tang, X.; Liu, T.; Li, H.; Yang, D.; Chen, L.; Tang, X. Notably enhanced thermoelectric properties of lamellar polypyrrole by doping with-naphthalene sulfonic acid. RSC Adv. 2017, 7, 20192–20200. [Google Scholar] [CrossRef]
- Wang, L.; Feng, S.; Zhao, J.; Zheng, J.; Wang, Z.; Li, L.; Zhu, Z. A facile method to modify carbon nanotubes with nitro/amino groups. Appl. Surf. Sci. 2010, 256, 6060–6064. [Google Scholar] [CrossRef]
- Yang, L.; May, P.W.; Yin, L.; Smith, J.; Rosser, K.N. Ultra fine carbon nitride nanocrystals synthesized by laser ablation in liquid solution. J. Nanopart. Res. 2007, 9, 1181–1185. [Google Scholar] [CrossRef]
- Mia, S.; Singh, B.; Dijkstra, F.A. Chemically oxidized biochar increases ammonium−15N recovery and phosphorus uptake in a grassland. Biol. Fertil. Soils 2019, 55, 577–588. [Google Scholar] [CrossRef]
- Li, S.; Harris, S.; Anandhi, A.; Chen, G. Predicting biochar properties and functions based on feedstock and pyrolysis temperature: A review and data syntheses. J. Clean. Prod. 2019, 215, 890–902. [Google Scholar] [CrossRef]

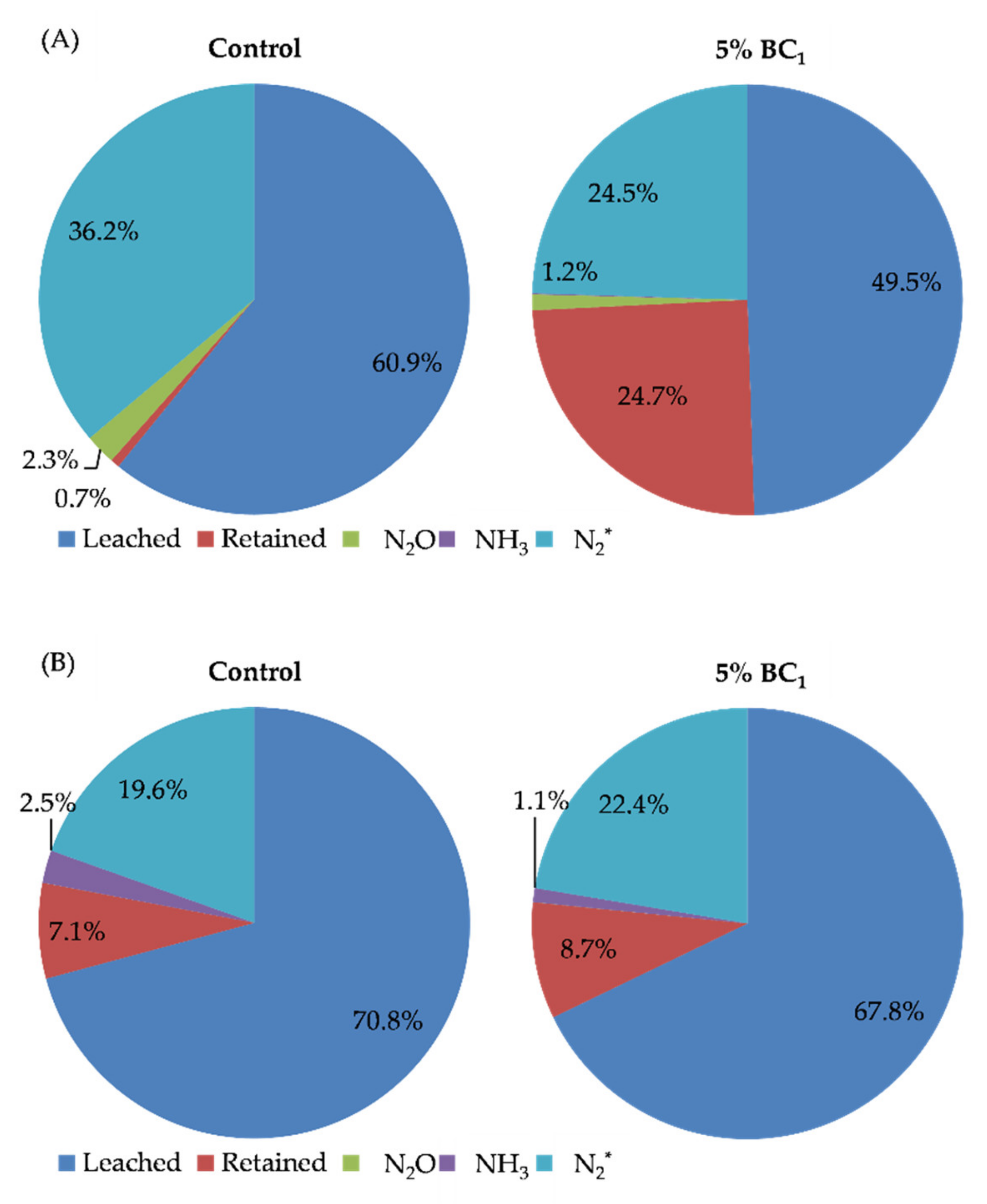
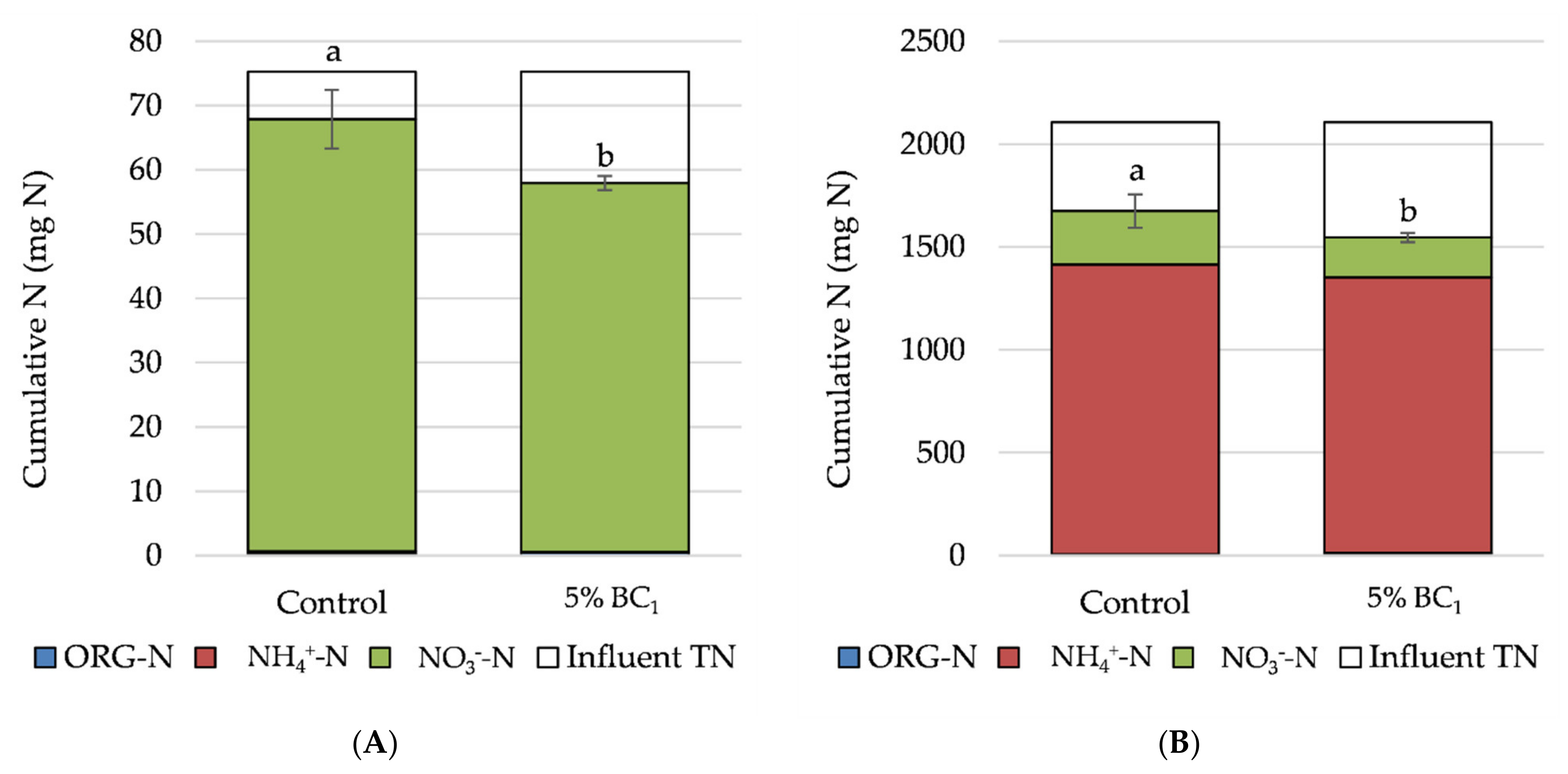

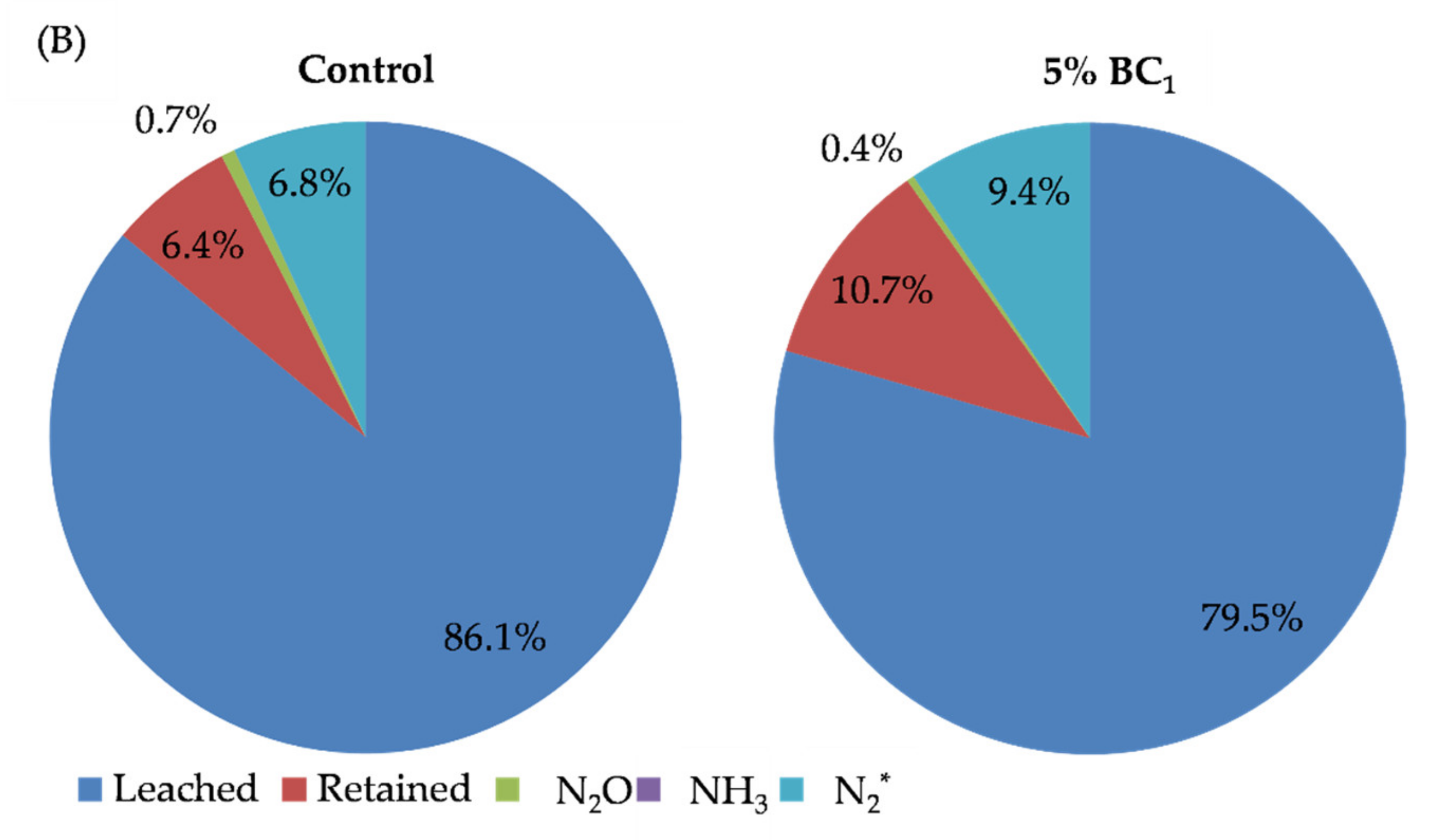


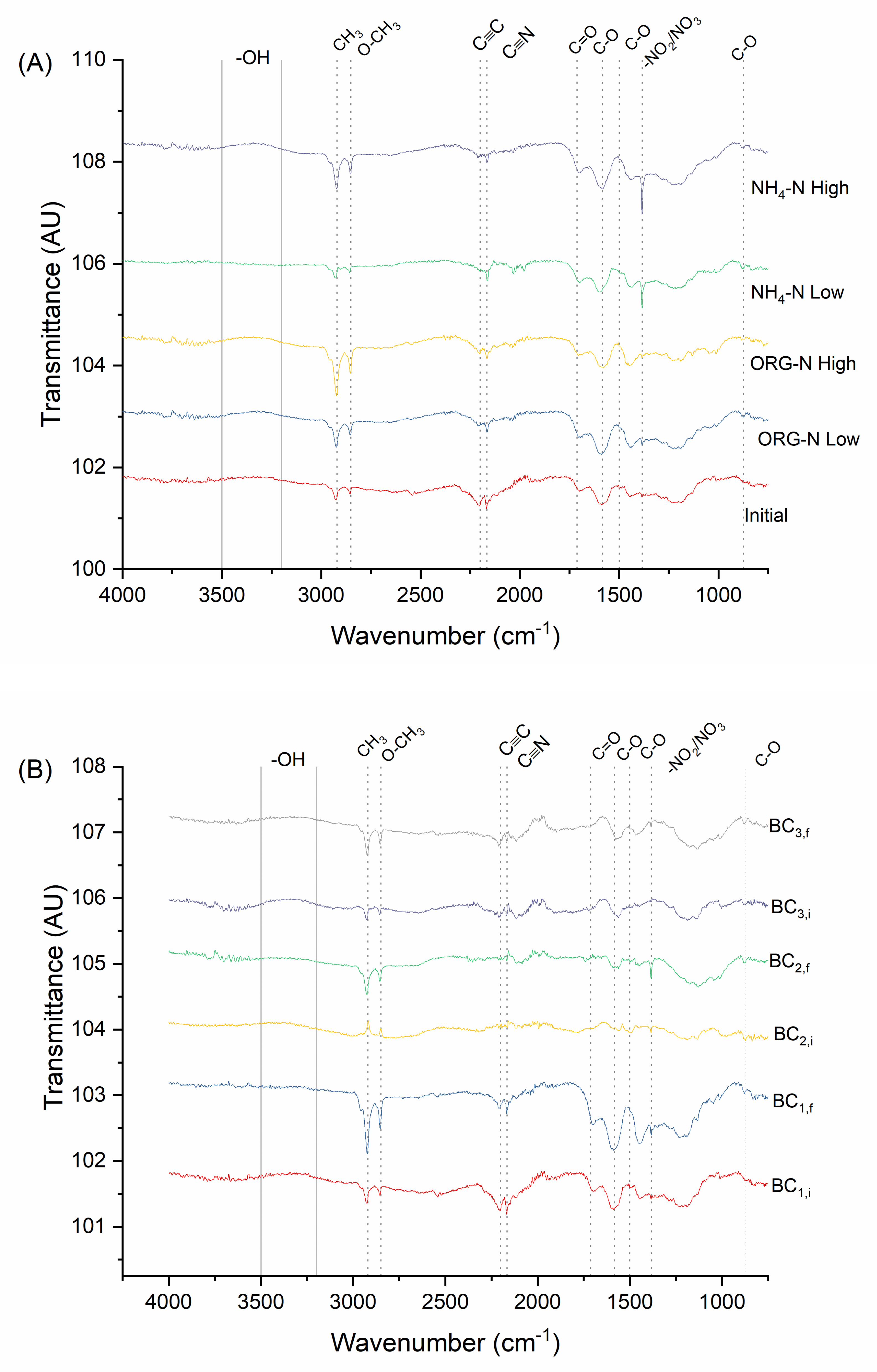
| Treatment Group | N Applied | Concentration (mg N L−1) | Rate (kg N ha−1) | Total Mass Loading (mg N) | Treatment | Bulk Density (g cm−3) |
|---|---|---|---|---|---|---|
| 1 | ORG-N Low | 150 | 198 | 225 | Control | 1.70 ± 0.01 |
| 2.5% BC1 | 1.39 ± 0.04 | |||||
| 2 | ORG-N High | 2500 | 3306 | 3750 | Control | 1.71 ± 0.03 |
| 2.5% BC1 | 1.39 ± 0.03 | |||||
| 3 | NH4+ Low | 50 | 66 | 75 | Control | 1.64 ± 0.08 |
| 2.5% BC1 | 1.42 ± 0.02 | |||||
| 4 | NH4+ High | 1250 | 1653 | 1875 | Control | 1.72 ± 0.05 |
| 2.5% BC1 | 1.41 ± 0.01 | |||||
| 5 | NO3− | 20 | 26 | 30 | Control | 1.69 ± 0.03 |
| 2.5% BC1 | 1.38 ± 0.01 | |||||
| 2.5% BC2 | 1.39 ± 0.01 | |||||
| 2.5% BC3 | 1.53 ± 0.11 |
| Application † | Treatment | NH4+-N (mg N) | NO3—N (mg N) | TKN (mg N) | ORG-N ‡ (mg N) | TN (mg N) |
|---|---|---|---|---|---|---|
| ORG-N Low | Control | 10.52 (1.28) a | 0.91 (1.10) a | 0.60 (8.41) a | −9.91 (7.75) a | 1.51 (9.50) a |
| BC1 | 10.52 (2.19) a | 6.76 (1.00) b | 63.64 (27.00) b | 53.13 (26.24) b | 55.21 (26.39) b | |
| ORG-N High | Control | 180.61 (9.60) a | −3.65 (0.07) a | 294.32 (3.59) a | 113.70 (6.20) a | 290.66 (3.54) a |
| BC1 | 217.15 (17.68) a | −4.17 (1.04) a | 360.04 (46.66) a | 142.89 (60.80) a | 326.47 (16.26) a | |
| NH4+-N Low | Control | 2.42 (1.51) a | −0.40 (0.71) a | 0.50 (0.83) a | −2.89 (3.09) a | 0.10 (0.26) a |
| BC1 | 8.92 (3.22) a | 3.58 (0.27) b | 8.52 (2.21) b | −0.41 (1.08) a | 12.09 (2.44) b | |
| NH4+-N High | Control | 150.76 (13.98) a | 17.54 (5.89) a | 107.40 (3.09) a | −43.36 (11.40) a | 124.94 (7.95) a |
| BC1 | 132.51 (6.69) a | 91.85 (8.47) b | 116.82 (8.98) a | −15.69 (11.70) b | 208.68 (17.36) b | |
| NO3−-N | Control | 1.98 (0.72) a | −2.25 (0.12) a | −6.99 (4.24) ab | −8.96 (3.56) b | −9.24 (4.13) a |
| BC1 | 9.98 (3.99) b | −2.79 (0.08) a | −4.73 (3.79) a | −14.71 (5.07) bc | −7.52 (3.76) a | |
| BC2 | 5.56 (1.45) ab | 6.73 (0.94) c | 2.61 (1.55) c | −2.95 (3.00) a | 9.34 (2.04) b | |
| BC3 | 7.62 (0.74) b | 0.26 (0.76) b | −11.74 (2.12) b | −19.35 (2.69) c | −11.48 (1.79) a |
| Atomic Composition (%) | |||||
|---|---|---|---|---|---|
| Element | BC1 Initial | BC1 after ORG-N Low Application (Treatment Group 1) | BC1 after ORG-N High Application (Treatment Group 2) | BC1 after NH4+-N Low Application (Treatment Group 3) | BC1 after NH4+-N High Application (Treatment Group 4) |
| C | 84.79 (1.53) | 68.13 (1.79) * | 69.65 (2.17) * | 72.17 (2.79) * | 71.12 (3.03) * |
| C-C/C-H | 67.25 (0.72) | 48.83 (0.49) * | 47.90 (2.19) * | 49.63 (3.27) * | 48.03 (2.70) * |
| C-O/C-OC | 9.38 (0.43) | 10.19 (0.66) | 11.44 (0.78) * | 10.88 (0.65) * | 11.58 (0.93) * |
| C=O | 3.63 (0.22) | 4.10 (0.27) | 4.70 (0.50) * | 4.45 (0.30) * | 5.39 (0.79) * |
| O=C-C | 2.24 (0.15) | 2.80 (0.70) | 3.41 (0.27) * | 3.46 (0.62) * | 2.91 (0.54) * |
| Shake up | 1.44 (0.36) | 1.28 (0.52) | 1.04 (0.31) | 2.53 (0.79) | 2.01 (0.14) |
| Quinone | 0.86 (0.07) | 0.94 (0.07) | 1.16 (0.25) | 1.21 (0.35) | 1.19 (0.30) |
| O | 13.49 (0.73) | 20.89 (0.29) * | 20.95 (1.60) * | 19.10 (1.82) * | 19.96 (1.62) * |
| N | 1.21 (0.04) | 1.70 (0.37) * | 3.15 (0.25) * | 1.66 (0.28) * | 1.86 (0.11) * |
| P | n.d. | 0.88 (0.19) * | 1.26 (0.24) * | 0.21 (0.07) * | 0.42 (0.03) * |
| Al | n.d. | 2.11(0.35) * | 0.99 (0.08) * | 1.74 (0.29) * | 1.51 (0.06) * |
| Ca | n.d. | 0.63 (0.12) * | 0.65 (0.07) * | 0.57 (0.03) * | 0.66 (0.04) * |
| Cl | n.d. | n.d. | n.d. | 0.14 (0.04) * | 0.32 (0.12) * |
| Fe | n.d. | 0.62 (0.20) * | 0.65 (0.11) * | 0.40 (0.07) * | 0.12 (0.01) * |
| Mg | 0.11 (0.01) | 0.41 (0.04) * | 0.27 (0.05) * | 0.37 (0.03) * | 0.51 (0.11) * |
| Si | 0.40 (0.01) | 4.31 (0.74) * | 2.09 (0.20) * | 3.35 (0.86) * | 3.18 (1.13) * |
| Sn | n.d. | 0.32 (0.03) * | 0.35 (0.05) * | 0.30 (0.06) * | 0.33 (0.07) * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanford, J.R.; Larson, R.A. Assessing Nitrogen Cycling in Corncob Biochar Amended Soil Columns for Application in Agricultural Treatment Systems. Agronomy 2020, 10, 979. https://doi.org/10.3390/agronomy10070979
Sanford JR, Larson RA. Assessing Nitrogen Cycling in Corncob Biochar Amended Soil Columns for Application in Agricultural Treatment Systems. Agronomy. 2020; 10(7):979. https://doi.org/10.3390/agronomy10070979
Chicago/Turabian StyleSanford, Joseph R., and Rebecca A. Larson. 2020. "Assessing Nitrogen Cycling in Corncob Biochar Amended Soil Columns for Application in Agricultural Treatment Systems" Agronomy 10, no. 7: 979. https://doi.org/10.3390/agronomy10070979
APA StyleSanford, J. R., & Larson, R. A. (2020). Assessing Nitrogen Cycling in Corncob Biochar Amended Soil Columns for Application in Agricultural Treatment Systems. Agronomy, 10(7), 979. https://doi.org/10.3390/agronomy10070979





