Silicon-Mediated Physiological and Agronomic Responses of Maize to Drought Stress Imposed at the Vegetative and Reproductive Stages
Abstract
1. Introduction
2. Materials and Methods
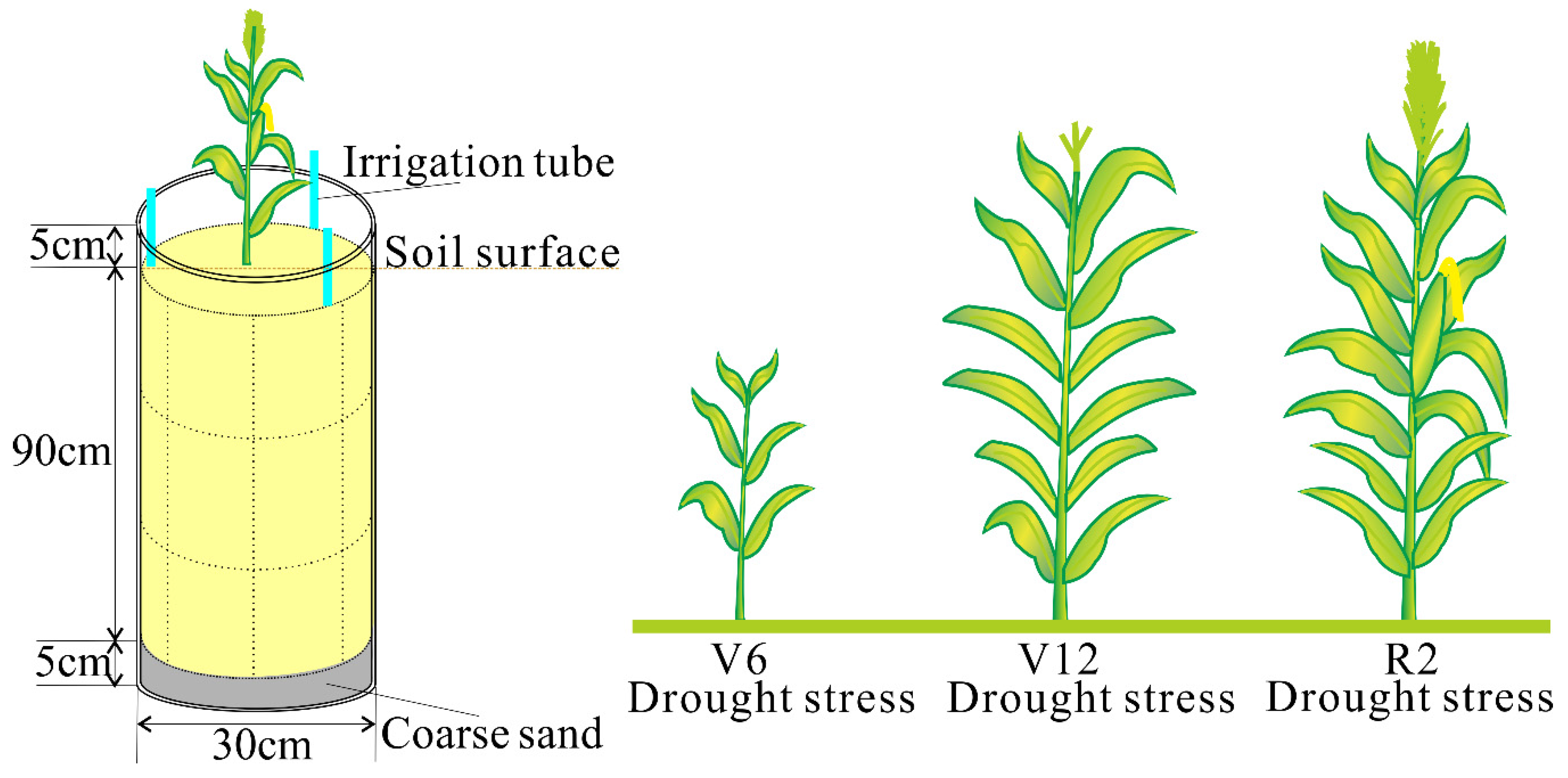
2.1. Experimental Site and Treatments
2.2. Sampling and Measurements
2.2.1. Leaf Samples
2.2.2. Dry Weight of Biomass and Grain Yield
2.2.3. Plant Height and Leaf Area
2.2.4. Photosynthetic Rate, Transpiration Rate, and Stomatal Conductance
2.3. Statistical Analysis
3. Results
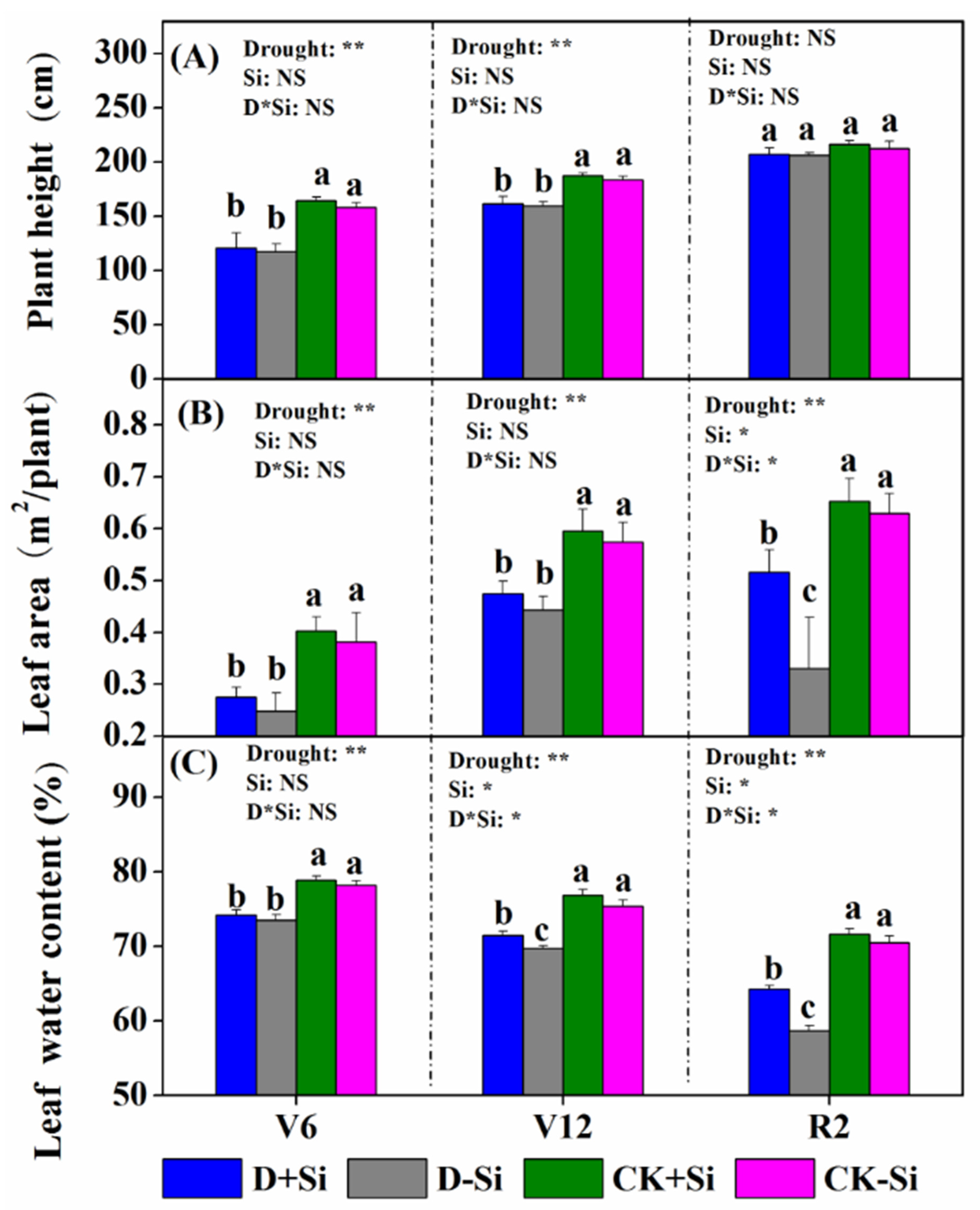
3.1. Maize Plant Growth
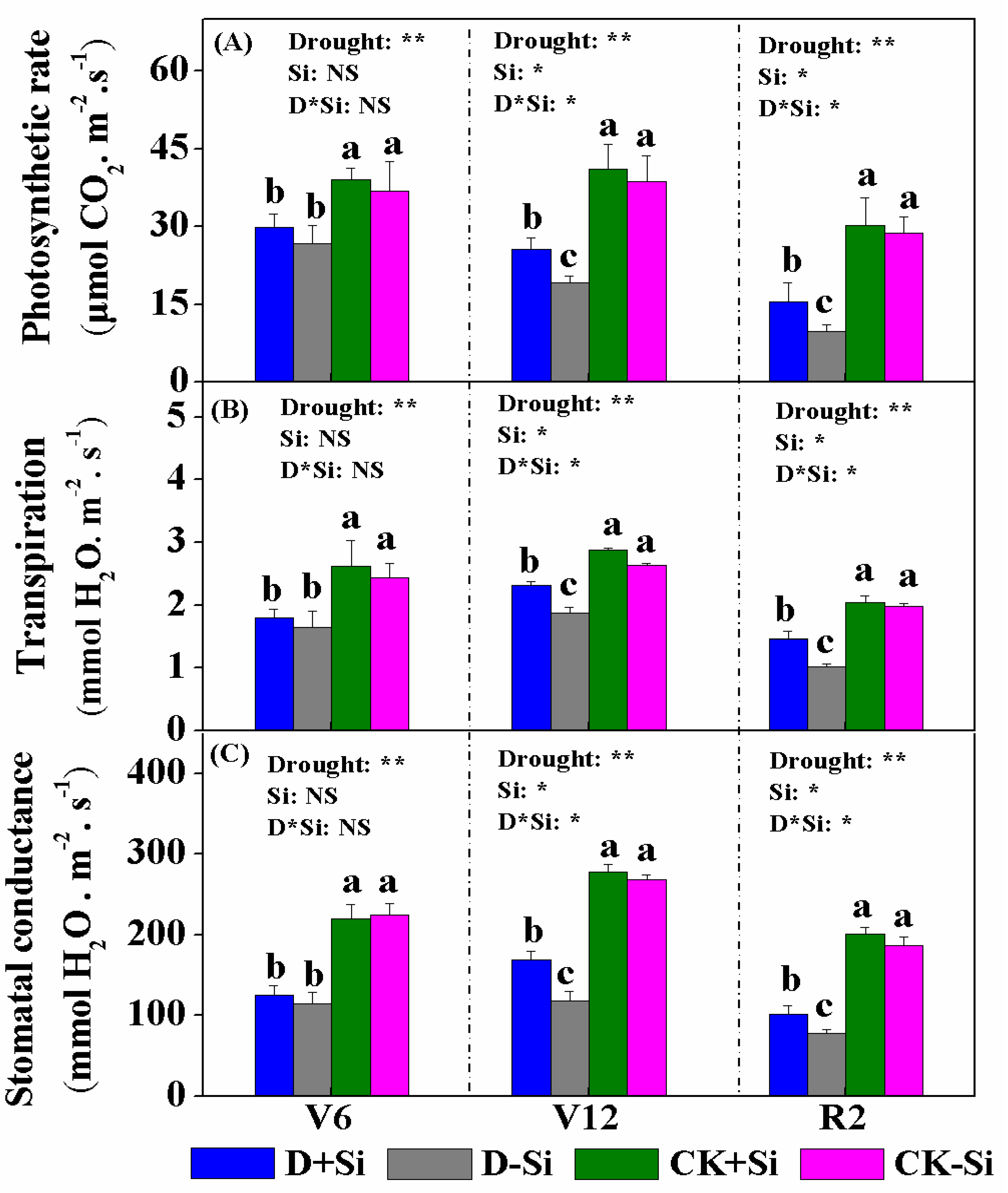
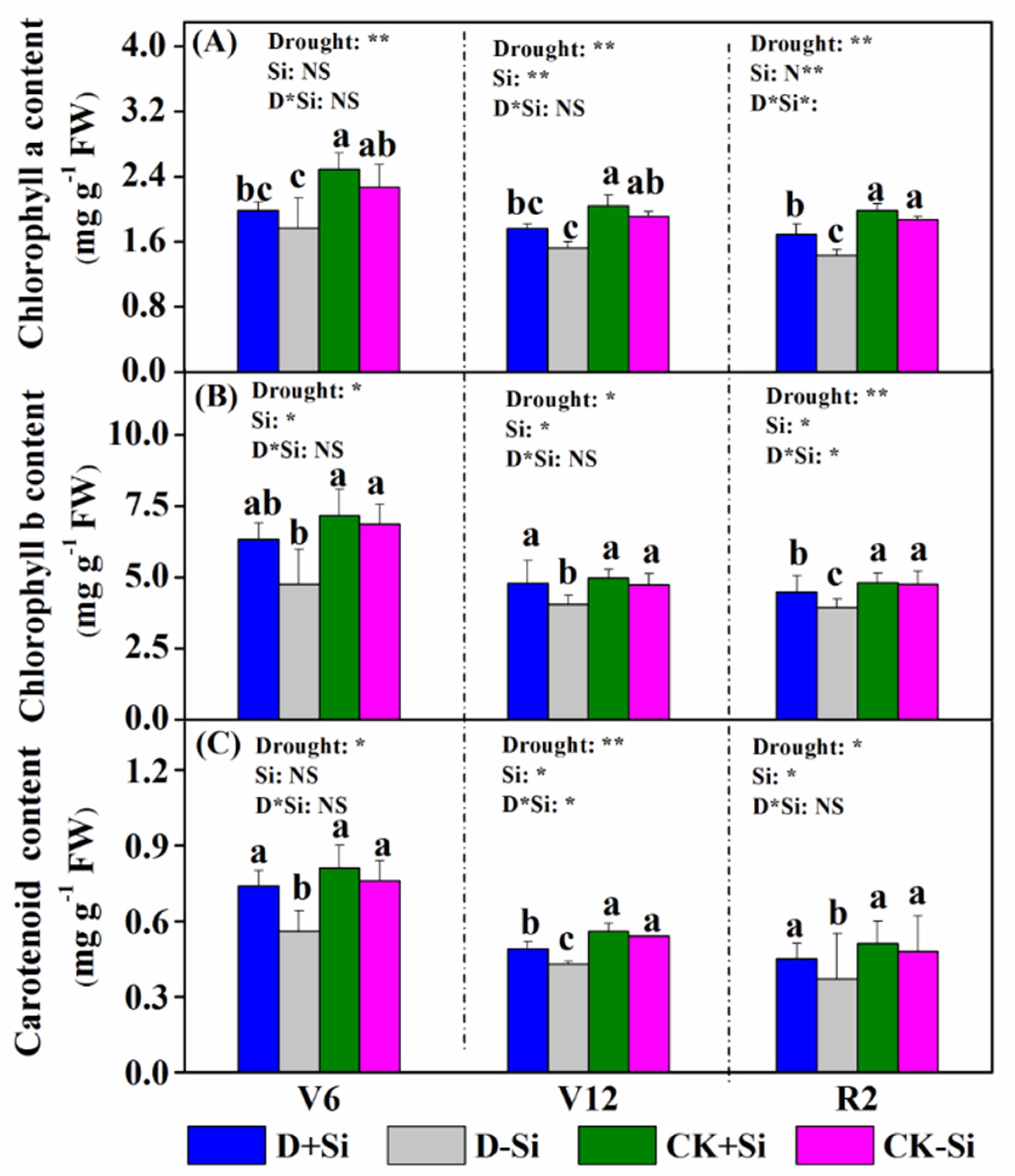
3.2. Photosynthesis and Chlorophyll Content
3.3. Osmotic Solute Contents
3.4. Superoxide Radicals and Malondialdehyde (MDA) Contents
3.5. Antioxidant Enzyme Activity
3.6. Grain Yield
3.7. Si Concentration in the Plant Tissues and Soil
3.8. Correlation Analysis
4. Discussion
4.1. Effect of Si on Maize Growth under Drought Stress
4.2. Effect of Si on Photosynthesis under Drought Stress
4.3. Effect of Si on Osmotic Adjustment under Drought Stress
4.4. Effect of Si on Antioxidant Defense under Drought Stress
4.5. Effect of Si on Maize Biomass and Yield under Drought Stress
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; SkZ, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef]
- Hussain, M.; Farooq, S.; Hasan, W.; Ul-Allah, S.; Tanveer, M.; Farooq, M.; Nawaz, A. Drought stress in sunflower: Physiological effects and its management through breeding and agronomic alternatives. Agric. Water Manag. 2018, 201, 152–166. [Google Scholar] [CrossRef]
- Vinocur, B.; Altman, A. Recent advances in engineering plant tolerance to abiotic stress, achievements and limitations. Curr. Opin. Biotechnol. 2005, 16, 123–132. [Google Scholar] [CrossRef]
- Mancosu, N.; Snyder, R.L.; Kyriakakis, G.; Spano, D. Water scarcity and future challenges for food production. Water 2015, 7, 975–992. [Google Scholar] [CrossRef]
- Ning, D.F.; Song, A.L.; Fan, F.L.; Li, Z.J.; Liang, Y.C. Effects of slag-based silicon fertilizer on rice growth and brown-spot resistance. PLoS ONE 2014, 9, e102681. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Yamaji, N. A cooperative system of silicon transport in plants. Trends Plant Sci. 2015, 20, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Conceicao, S.S.; Neto, C.F.D.; Marques, E.C.; Barbosa, A.V.C.; Galvao, J.R.; de Oliveira, T.B.; Okumura, R.S.; Martins, J.T.D.; Costa, T.C.; Gomes, E. Silicon modulates the activity of antioxidant enzymes and nitrogen compounds in sunflower plants under salt stress. Arch. Agron. Soil Sci. 2019, 65, 1237–1247. [Google Scholar] [CrossRef]
- Gong, H.J.; Zhu, X.Y.; Chen, K.M.; Wang, S.M.; Zhang, C.L. Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci. 2005, 169, 313–321. [Google Scholar] [CrossRef]
- Gao, X.; Zou, C.; Wang, L.; Zhang, F. Silicon decreases transpiration rate and conductance from stomata of maize plants. J. Plant Nutr. 2006, 29, 1637–1647. [Google Scholar] [CrossRef]
- Shen, X.F.; Zhou, Y.Y.; Duan, L.S.; Li, Z.H.; Eneji, A.E.; Li, J.M. Silicon effects on photosynthesis and antioxidant parameters of soybean seedlings under drought and ultraviolet-B radiation. Silicon effects on photosynthesis and antioxidant parameters of soybean seedlings under drought and ultraviolet-B radiation. J. Plant Physiol. 2010, 167, 1248–1252. [Google Scholar] [CrossRef]
- Chen, W.; Yao, X.Q.; Cai, K.Z.; Chen, J.N. Silicon alleviates drought stress of rice plants by improving plant water status, photosynthesis and mineral nutrient absorption. Biol. Trace Elem. Res. 2011, 142, 67–76. [Google Scholar] [CrossRef]
- Yin, L.N.; Wang, S.W.; Liu, P.; Wang, W.H.; Cao, D.; Deng, X.P. Silicon-mediated changes in polyamine and 1-aminocyclopropane-1-carboxylic acid are involved in silicon-induced drought resistance in Sorghum bicolor. Plant Physiol. Bioch. 2014, 80, 268–277. [Google Scholar] [CrossRef]
- Helaly, M.N.; Hoseiny, E.H.; El-Sheery, N.I.; Rastogi, A.; Kalaji, H.M. Regulation and physiological role of silicon in alleviating drought stress of mango. Plant Physiol. Bioch. 2017, 118, 31–44. [Google Scholar] [CrossRef]
- Hattori, T.; Inanaga, S.; Araki, H.; An, P.; Morita, S.; Luxova, M.; Lux, A. Application of silicon enhanced drought tolerance in Sorghum bicolor. Physiol. Plantarum. 2005, 123, 459–466. [Google Scholar] [CrossRef]
- Kim, Y.H.; Khan, A.L.; Waqas, M.; Lee, I.J. Silicon regulates antioxidant activities of crop plants under abiotic-induced oxidative stress, A review. Front. Plant Sci. 2017, 8, 1346. [Google Scholar] [CrossRef]
- Agarie, S.; Uchida, H.; Agata, W.; Kubota, F.; Kaufman, P.B. Effects of silicon on transpiration and leaf conductance in rice plants (Oryza sativa L.). Plant Prod. Sci. 1998, 1, 89–95. [Google Scholar] [CrossRef]
- Sun, Q.; Liang, X.L.; Zhang, D.G.; Li, X.H.; Hao, Z.F.; Weng, J.F.; Li, M.S.; Zhang, S.H. Trends in drought tolerance in Chinese maize cultivars from the 1950s to the 2000s. Field Crops Res. 2017, 201, 175–183. [Google Scholar] [CrossRef]
- Mansouri-Far, C.; Sanavy, S.A.M.M.; Saberali, S.F. Maize yield response to deficit irrigation during low-sensitive growth stages and nitrogen rate under semi-arid climatic conditions. Agric. Water Manag. 2010, 97, 12–22. [Google Scholar] [CrossRef]
- Comas, L.H.; Trout, T.J.; DeJonge, K.C.; Zhang, H.H.; Gleason, S.M. Water productivity under strategic growth stage-based deficit irrigation in maize. Agric. Water Manag. 2019, 212, 433–440. [Google Scholar] [CrossRef]
- Trout, T.J.; DeJonge, K.C. Water productivity of maize in the US high plains. Irrig. Sci. 2017, 35, 251–266. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.Y.; Chen, J.; Chen, A.J.; Wang, L.Y.; Guo, X.Y.; Niu, Y.L.; Liu, S.R.; Mi, G.H.; Gao, Q. Reducing basal nitrogen rate to improve maize seedling growth, water and nitrogen use efficiencies under drought stress by optimizing root morphology and distribution. Agric. Water Manag. 2019, 212, 328–337. [Google Scholar] [CrossRef]
- Malcovska, S.M.; Ducaiova, Z.; Backor, M. Impact of silicon on maize seedlings exposed to short-term UV-B irradiation. Biologia 2014, 69, 1349–1355. [Google Scholar]
- Li, H.S. Principles and Techniques of Plant Physiological Biochemical Experiment; Higher Education Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence, correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 132, 93–101. [Google Scholar] [CrossRef]
- Elstner, E.F.; Heupel, A. Inhibition of nitrite formation from hydroxylammonium chloride, a simple assay for superoxide dismutase. Anal. Biochem. 1976, 70, 616–620. [Google Scholar] [CrossRef]
- Bates, I.; Waldren, R.P.; Teare, J.D. Rapid determination of free proline for water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Sgherri, C.L.M.; Loggini, B.; Puliga, S.; Navari-Izzo, F. Antioxidant system in Sporobolus stapfianus, changes in response to desiccation and rehydration. Phytochemistry 1994, 35, 561–565. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–127. [Google Scholar]
- Chance, B.; Maehly, A.C. Assay of catalases and peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar]
- Lu, R.K. Analytical Methods for Soil and Agro-Chemistry; Science and Technology Publishing House: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Dai, W.M.; Zhang, K.Q.; Duan, B.W. Rapid determination of silicon content in rice (Oryzasativa L). Chin. J. Rice Sci. 2005, 19, 460–462. (In Chinese) [Google Scholar]
- Cakir, R. Effect of water stress at different development stages on vegetative and reproductive growth of corn. Field Crop Res. 2004, 89, 1–16. [Google Scholar] [CrossRef]
- Pandey, J.R.K.; Maranville, P.J.W.; Chetim, M.M. Deficit irrigation and nitrogen effects on maize in a Sahelian environment II. Shoot growth, nitrogen uptake and water extraction. Agric. Water Manag. 2000, 46, 15–27. [Google Scholar] [CrossRef]
- Gong, H.J.; Chen, K.M.; Zhao, Z.G.; Chen, G.C.; Zhou, W.J. Effects of silicon on defense of wheat against oxidative stress under drought at different developmental stages. Biologia. Plantarum. 2008, 52, 592–596. [Google Scholar] [CrossRef]
- Maillard, A.; Ali, N.; Schwarzenberg, A.; Jamois, F.; Yvin, J.C.; Hosseini, S.A. Silicon transcriptionally regulates sulfur and ABA metabolism and delays leaf senescence in barley under combined sulfur deficiency and osmotic stress. Environ. Exp. Bot. 2018, 155, 394–410. [Google Scholar] [CrossRef]
- Song, A.; Ning, D.F.; Fan, F.L.; Li, Z.J.; Provance-Bowley, M.; Liang, Y.C. The potential for carbon bio-sequestration in China’s paddy rice (Oryza sativa L.) as impacted by slag-based silicate fertilizer. Sci. Rep. 2015, 5, 17354. [Google Scholar] [CrossRef]
- Meunier, J.D.; Barboni, D.; Anwar-ul-Haq, M.; Levard, C.; Chaurand, P.; Vidal, V.; Grauby, O.; Huc, R.; Laffont-Schwob, I.; Rabier, J. Effect of phytoliths for mitigating water stress in durum wheat. New Phytol. 2017, 215, 229–239. [Google Scholar] [CrossRef]
- Cattivelli, L.; Rizza, F.; Badeck, F.W.; Mazzucotelli, E.; Mastrangelo, A.M.; Francia, E.; Mare, C.; Tondelli, A.; Stanca, A.M. Drought tolerance improvement in crop plants, an integrated view from breeding to genomics. Field Crops Res. 2008, 105, 1–14. [Google Scholar] [CrossRef]
- Zhang, W.J.; Xie, Z.C.; Wang, L.H.; Li, M.; Lang, D.Y.; Zhang, X.H. Silicon alleviates salt and drought stress of Glycyrrhiza uralensis seedling by altering antioxidant metabolism and osmotic adjustment. J. Plant Res. 2017, 130, 611–624. [Google Scholar] [CrossRef]
- Gong, H.J.; Chen, K.M. The regulatory role of silicon on water relations, photosynthetic gas exchange, and carboxylation activities of wheat leaves in field drought conditions. Acta Physiol. Plant. 2012, 34, 1589–1594. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress, effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Mahmood, S.; Daur, I.; Hussain, M.B.; Nazir, Q.; Al-Solaimani, S.G.; Ahmad, S.; Bakhashwain, A.A.; Elsafor, A.K. Silicon application and rhizobacterial inoculation regulate mung bean response to saline water irrigation. Clean-Soil Air Water 2017, 45, 1600436. [Google Scholar] [CrossRef]
- Liu, P.; Yin, L.N.; Deng, X.P.; Wang, S.W.; Tanaka, K.; Zhang, S.Q. Aquaporin-mediated increase in root hydraulic conductanceis involved in silicon-induced improved root water uptake under osmotic stress in Sorghum bicolor L. J. Exp. Bot. 2014, 65, 4747–4756. [Google Scholar] [CrossRef]
- Gong, H.J.; Chen, K.M.; Chen, G.C.; Wang, S.M.; Zhang, C.L. Effects of silicon on growth of wheat under drought. J. Plant Nutr. 2003, 26, 1055–1063. [Google Scholar] [CrossRef]
- Gao, X.; Zou, C.; Wang, L.; Zhang, F. Silicon improves water use efficiency in maize plants. J. Plant Nutr. 2004, 27, 1457–1470. [Google Scholar] [CrossRef]
- Puértolas, J.; Albacete, A.; Dodd, I.C. Irrigation frequency transiently alters whole plant gas exchange, water and hormone status, but irrigation volume determines cumulative growth in two herbaceous crops. Environ. Exp. Bot. 2020, 176, 104101. [Google Scholar] [CrossRef]
- Rao, D.E.; Chaitanya, K.V. Photosynthesis and antioxidative defense mechanisms in deciphering drought stress tolerance of crop plants. Biol. Plantarum. 2016, 60, 201–218. [Google Scholar] [CrossRef]
- Sonobe, K.; Hattori, T.; An, P.; Tsuji, W.; Eneji, E.; Tanaka, K.; Inanaga, S. Diurnal variations in photosynthesis, stomatal conductance and leaf water relation in sorghum grown with or without silicon under water stress. J. Plant Nutr. 2009, 32, 433–442. [Google Scholar] [CrossRef]
- Latef, A.A.A.; Tran, L.S.P. Impacts of priming with silicon on the growth and tolerance of maize plants to alkaline stress. Front. Plant Sci. 2016, 7, 243. [Google Scholar]
- Mansour, M.M.F.; Ali, E.F. Evaluation of proline functions in saline conditions. Phytochemistry 2017, 140, 52–68. [Google Scholar] [CrossRef]
- Pei, Z.F.; Ming, D.F.; Liu, D.; Wan, G.L.; Geng, X.X.; Gong, H.J.; Zhou, W.J. Silicon improves the tolerance to water-deficit stress induced by polyethylene glycol in wheat (Triticum aestivum L.) seedlings. J. Plant Growth Regul. 2010, 29, 106–115. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Huynh, W.Q.; Kronzucker, H.J. The role of silicon in higher plants under salinity and drought stress. Front. Plant Sci. 2016, 7, 1072. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, Y.; Yao, H.J.; Wu, J.W.; Sun, H. Silicon improves seed germination and alleviates oxidative stress of bud seedlings in tomato under water deficit stress. Plant Physiol. Bioch. 2014, 78, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Zhao, W.; Zhu, X. Silicon improves photosynthesis and strengthens enzyme activities in the C3 succulent xerophyte zygophyllum xanthoxylum under drought stress. J. Plant Physiol. 2016, 199, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Puértolas, J.; Larsen, E.K.; Davies, W.J.; Dodd, I.C. Applying ‘drought’ to potted plants by maintaining suboptimal soil moisture improves plant water relations. J. Exp. Bot. 2017, 68, 2413–2424. [Google Scholar] [CrossRef]
- Lavinsky, A.O.; Detmann, K.C.; Reis, J.V.; Avila, R.T.; Sanglard, M.L.; Pereira, L.F.; Sanglard, L.M.V.P.; Rodrigues, F.A.; Araujo, W.L.; DaMatta, F.M. Silicon improves rice grain yield and photosynthesis specifically when supplied during the reproductive growth stage. J. Plant Physiol. 2016, 206, 125–132. [Google Scholar] [CrossRef]
- Tollenaar, M.; Ahmadzadeh, A.; Lee, E.A. Physiological basis of heterosis for grain yield in maize. Crop Sci. 2004, 44, 2086–2094. [Google Scholar] [CrossRef]
- Detmann, K.C.; Araujo, W.L.; Martins, S.C.V.; Sanglard, L.M.V.P.; Reis, J.V.; Detmann, E.; Rodrigues, F.A.; Nunes-Nesi, A.; Fernie, A.R.; DaMatta, F.M. Silicon nutrition increases grain yield, which, in turn, exerts a feed-forward stimulation of photosynthetic rates via enhanced mesophyll conductance and alters primary metabolism in rice. New Phytol. 2012, 196, 752–762. [Google Scholar] [CrossRef]



| Indicators | Protocol | References |
|---|---|---|
| Leaf water content (LWC) | Leaf was oven-dried to constant weight at 80 °C to determine the dry weight. LWC was calculated by the equation: LWC(%) = 100 × (Leaf fresh weight − Leaf dry weight)/Leaf fresh weight | [8] |
| Chlorophyll content | Extracted in 80% acetone solution, filtrate was measured at 663 nm, 646 nm and 470 nm | [23] |
| Malondialdehyde (MDA) | Thiobarbituric acid (TBA) method, supernatant was measured at 532 nm, 450 nm and 600 nm | [24] |
| Superoxide radical (O2·−) | Hydroxylamine oxidation method | [25] |
| Proline | Acidic ninhydrin method | [26] |
| Soluble sugar | Anthrone–sulfuric acid method | [23] |
| Soluble protein | Coomassie brilliant blue method. | [23] |
| Superoxide dismutase activity (SOD) | Nitro-blue tetrazolium reduction method | [27] |
| Catalase activity (CAT) | Measured as the reduction in absorbance at 240 nm due to the reduction in H2O2 | [28] |
| Peroxidase activity (POD) | Based on the determination of guaiacol oxidation at 470 nm by H2O2 | [29] |
| Plant-available Si content in the soil | Extracted by 0.25 M citric acid and analyzed by silicon molybdenum blue spectrophotometry | [30] |
| Si content in plants | Extracted by 50% NaOH, autoclaved at 125 °C for 1 h, assayed using silicon molybdenum blue spectrophotometry | [31] |
| Factor | Maize Yield (g plot-1) | Straw Biomass (g plot−1) | Harvest Index |
|---|---|---|---|
| +Si-D-V6 | 190 ± 6.97 b | 139 ± 9.87 c | 0.58 ± 0.03 a |
| +Si-D-V12 | 179 ± 6.66 b | 166 ± 5.09 b | 0.52 ± 0.02 b |
| +Si-D-R2 | 143 ± 9.95 c | 219 ± 14.7 a | 0.40 ± 0.05 d |
| +Si-CK | 214 ± 9.19 a | 237 ± 13.2 a | 0.47 ± 0.02 bc |
| -Si-D-V6 | 169 ± 10.3 bc | 135 ± 6.20 c | 0.55 ± 0.03 ab |
| -Si-D-V12 | 106 ± 9.94 d | 165 ± 10.7 b | 0.39 ± 0.07 d |
| -Si-D-R2 | 79.1 ± 8.99 e | 207 ± 8.61 a | 0.28 ± 0.01 e |
| -Si-CK | 198 ± 11.3 ab | 217 ± 3.97 a | 0.46 ± 0.02 c |
| Two-Way ANOVA analysis | |||
| Silicon (Si) | ** | NS | ** |
| Drought (D) | ** | ** | ** |
| Si × Drought | ** | NS | ** |
| Factor | Leaf (%) | Stem (%) | Plant-Available Silicon in the Soil (mg kg−1) |
|---|---|---|---|
| +Si-D-V6 | 7.84 ± 0.66 a | 1.49 ± 0.16 a | 438 ± 26.8 a |
| +Si-D-V12 | 7.70 ± 0.14 a | 1.32 ± 0.24 ab | 432 ± 12.9 a |
| +Si-D-R2 | 7.88 ± 0.88 a | 1.12 ± 0.05 bc | 412 ± 22.7 ab |
| +Si-CK | 8.20 ± 0.45 a | 1.26 ± 0.07 abc | 394 ± 1.60 b |
| -Si-D-V6 | 6.69 ± 0.45 b | 1.45 ± 0.09 ab | 419 ± 10.6 a |
| -Si-D-V12 | 6.56 ± 0.43 b | 1.01 ± 0.26 cd | 375 ± 17.5 bc |
| -Si-D-R2 | 5.74 ± 0.86 b | 0.95 ± 0.13 d | 352 ± 21.3 c |
| -Si-CK | 6.58 ± 0.55 b | 1.20 ± 0.10 bc | 364 ± 13.3 c |
| Two-Way ANOVA analysis | |||
| Silicon (Si) | ** | * | ** |
| Drought (D) | NS | * | ** |
| Si × Drought | NS | NS | NS |
| Parameters | V6 Stage | V12 Stage | R2 Stage |
|---|---|---|---|
| Plant height | 0.766 | 0.78 | 0.816 |
| Leaf area | 0.836 | 0.881 | 0.992** |
| Chlorophyll content | 0.924* | 0.998** | 0.994** |
| Photosynthetic rate | 0.837 | 0.901* | 0.966* |
| Transpiration rate | 0.829 | 0.961* | 0.987* |
| Soluble protein | 0.423 | 0.739 | 0.200 |
| Soluble sugar | 0.162 | 0.137 | 0.521 |
| Proline | −0.674 | −0.755 | −0.977* |
| SOD | 0.918* | 0.932* | 0.925* |
| CAT | 0.831 | 0.976* | 0.987* |
| POD | 0.971* | 0.994* | 0.919* |
| O2·− | −0.890 | −0.907 | −0.832 |
| MDA | −0.957* | −0.959* | −0.989* |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ning, D.; Qin, A.; Liu, Z.; Duan, A.; Xiao, J.; Zhang, J.; Liu, Z.; Zhao, B.; Liu, Z. Silicon-Mediated Physiological and Agronomic Responses of Maize to Drought Stress Imposed at the Vegetative and Reproductive Stages. Agronomy 2020, 10, 1136. https://doi.org/10.3390/agronomy10081136
Ning D, Qin A, Liu Z, Duan A, Xiao J, Zhang J, Liu Z, Zhao B, Liu Z. Silicon-Mediated Physiological and Agronomic Responses of Maize to Drought Stress Imposed at the Vegetative and Reproductive Stages. Agronomy. 2020; 10(8):1136. https://doi.org/10.3390/agronomy10081136
Chicago/Turabian StyleNing, Dongfeng, Anzhen Qin, Zhandong Liu, Aiwang Duan, Junfu Xiao, Jiyang Zhang, Zugui Liu, Ben Zhao, and Zhanjun Liu. 2020. "Silicon-Mediated Physiological and Agronomic Responses of Maize to Drought Stress Imposed at the Vegetative and Reproductive Stages" Agronomy 10, no. 8: 1136. https://doi.org/10.3390/agronomy10081136
APA StyleNing, D., Qin, A., Liu, Z., Duan, A., Xiao, J., Zhang, J., Liu, Z., Zhao, B., & Liu, Z. (2020). Silicon-Mediated Physiological and Agronomic Responses of Maize to Drought Stress Imposed at the Vegetative and Reproductive Stages. Agronomy, 10(8), 1136. https://doi.org/10.3390/agronomy10081136










