Long-Term Nitrogen Fertilization Impacts on Soil Bacteria, Grain Yield and Nitrogen Use Efficiency of Wheat in Semiarid Loess Plateau, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design and Treatments
2.3. Data Collection
2.3.1. Soil Sampling
2.3.2. Soil Chemical Analyses
2.3.3. Soil DNA Extraction and Amplification
2.3.4. DNA Sequencing
2.3.5. Plant Sampling and Analysis
2.4. Data Analysis
3. Results
3.1. Effects of Long-Term N Fertilization on Soil Chemical Properties
3.2. Effects of Long-Term N Fertilization on LAI, Grain Yield and NUE of Wheat
3.3. Effects of Long-Term N Fertilization on Soil Bacterial Diversity and Community Composition
3.4. Relationships of Soil Bacteria with Soil Chemical Properties, Grain Yield, and NUE
4. Discussion
4.1. Effect of Long-Term N Fertilization on Soil Chemical Properties, Wheat Yield, Crop N Uptake, and N Use Efficiency
4.2. Effect of Long-Term N Fertilization on Soil Bacteria
4.3. Relationships between Soil Bacteria and Soil Chemical Properties and Crop Factors
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sean, T.; Chris, M.; Laverne, G.; Blaire, S.; Stephanie, A.; Robert, B.; Cheryl, R. Nitrogen Fertilization Has a Stronger Effect on Soil Nitrogen-Fixing Bacterial Communities than Elevated Atmospheric CO2. Appl. Environ. Microbiol. 2014, 80, 3103–3112. [Google Scholar]
- Thomas, R.Q.; Brookshire, E.N.J.; Gerber, S. Nitrogen limitation on land: How can it occur in earth system models? Glob. Chang. Biol. 2015, 21, 1777–1793. [Google Scholar] [CrossRef] [PubMed]
- Gitelson, A.; Viña, A.; Arkebauer, T.J.; Rundquist, D.C.; Keydan, G.; Leavitt, B. Remote estimation of leaf area index and green leaf biomass in maize canopies. Geophys. Res. Lett. 2003, 30, 1248–1251. [Google Scholar] [CrossRef] [Green Version]
- Fan, T.; Xu, M.; Song, S.; Zhou, G.; Ding, L. Trends in grain yields and soil organic C in a long-term fertilization experiment in the china loess plateau. J. Plant Nutr. Soil Sci. 2008, 171, 448–457. [Google Scholar] [CrossRef]
- Fan, T.; Stewart, B.A.; Yong, W.; Junjie, L.; Guangye, Z. Long-term fertilization effects on grain yield, water-use efficiency and soil fertility in the dryland of loess plateau in china. Ecosyst. Environ. 2005, 106, 313–329. [Google Scholar] [CrossRef]
- Dai, J.; Wang, Z.; Li, F.; He, G.; Wang, S.; Li, Q.; Cao, H.; Luo, L.; Zan, Y.; Meng, X.; et al. Optimizing nitrogen input by balancing winter wheat yield and residual nitrate-N in soil in a long-term dryland field experiment in the Loess Plateau of China. Field Crop. Res. 2015, 181, 32–41. [Google Scholar] [CrossRef]
- Ikeda, S.; Sasaki, K.; Okubo, T.; Yamashita, A.; Terasawa, K.; Bao, Z.; Liu, D.; Watanabe, T.; Murase, J.; Asakawa, S.; et al. Low Nitrogen Fertilization Adapts Rice Root Microbiome to Low Nutrient Environment by Changing Biogeochemical Functions. Microbes Environ. 2014, 29, 50–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakesh, K.S.; Sukhwinder, K.B. A Review of Methods to Improve Nitrogen Use Efficiency in Agriculture. Sustainability 2018, 10, 51. [Google Scholar]
- The Food and Agriculture Organization (FAO). Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 19 January 2018).
- Chien, S.H.; Teixeira, L.A.; Cantarella, H.; Rehm, G.W.; Grant, C.A.; Gearhart, M.M. Agronomic effectiveness of granular nitrogen/phosphorus fertilizers containing elemental sulfur with and without ammonium sulfate: A Review. Agron. J. 2016, 108, 1203. [Google Scholar] [CrossRef] [Green Version]
- Dobermann, A.; Ping, J.L.; Adamchuk, V.I.; Simbahan, G.C.; Ferguson, R.B. Classification of Crop Yield Variability in Irrigated Production Fields. Agron. J. 2003, 95, 1105. [Google Scholar] [CrossRef] [Green Version]
- Nolan, S.; Unkovich, M.; Shen, Y.; Li, L.; Bellotti, W. Farming systems of the Loess Plateau, Gansu Province, China. Ecosyst. Environ. 2008, 124, 13–23. [Google Scholar] [CrossRef]
- Li, H.; Zhang, W.; Zhang, F.; Du, F.; Li, L. Chemical fertilizer use and efficiency change of main grain crops in China. Plant Nutr. Fertil. Sci. 2010, 16, 1136–1143. [Google Scholar]
- Li, L.; Zhang, R.; Luo, Z.; Liang, W.; Xie, J.; Cai, L.; Bellotti, B. Evolution of soil and water conservation in rain-fed areas of China. Int. Soil Water Conserv. Res. 2014, 2, 78–90. [Google Scholar]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant acidification in major chinese croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sainjua, U.P.; Senwo, Z.N.; Nyakatawa, E.Z.; Tazisong, I.A.; Reddy, K.C. Soil carbon and nitrogen sequestration as affected by long-term tillage, cropping systems, and nitrogen fertilizer sources. Agric. Ecosyst. Environ. 2008, 127, 234–240. [Google Scholar] [CrossRef]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. Controlling Eutrophication: Nitrogen and Phosphorus. Science 2009, 323, 1014–1015. [Google Scholar] [CrossRef]
- Wei, X.R.; Hao, M.D.; Xue, X.H.; Shi, P.; Horton, R.; Wang, A.; Zhang, Y.F.; Wei, X.R. Nitrous oxide emission from highland winter wheat field after long-term fertilization. Biogeosciences 2010, 7, 3301–3310. [Google Scholar] [CrossRef] [Green Version]
- Suleiman, A.K.A.; Manoeli, L.; Boldo, J.T.; Pereira, M.G.; Roesch, L.F.W. Shifts in soil bacterial community after eight years of land-use change. Syst. Appl. Microbiol. 2013, 36, 137–144. [Google Scholar] [CrossRef]
- Garbeva, P.; van Veen, J.A.; van Elsas, J.D. Microbial diversity in soil: Selection of microbial populations by plant and soil type and implications for disease suppressiveness. Annu. Rev. Phytopathol. 2004, 42, 243–270. [Google Scholar] [CrossRef]
- Shen, W.; Lin, X.; Gao, N.; Zhang, H.; Yin, R.; Shi, W.; Duan, Z. Land use intensification affects soil microbial populations, functional diversity and related suppressiveness of cucumber Fusarium wilt in China’s Yangtze River Delta. Plant Soil 2008, 306, 117–127. [Google Scholar] [CrossRef]
- Li, Y.; Chen, L.; Wen, H.; Zhou, T.; Zhang, T.; Gao, X. 454 Pyrosequencing Analysis of Bacterial Diversity Revealed by a Comparative Study of Soils from Mining Subsidence and Reclamation Areas. J. Microbiol. Biotechnol. 2014, 24, 313–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Ni, T.; Li, Y.; Xiong, W.; Ran, W. Responses of Bacterial Communities in Arable Soils in a Rice-Wheat Cropping System to Different Fertilizer Regimes and Sampling Times. PLoS ONE 2014, 9, e85301. [Google Scholar] [CrossRef] [PubMed]
- Gans, J.; Wolinsky, M.; Dunbar, J. Computational Improvements Reveal Great Bacterial Diversity and High Metal Toxicity in Soil. Science 2005, 309, 1387–1390. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, S.S.; Gupta, S.K.; Dadarwal, K.R. Antagonistic effect of Pseudomonas spp. on pathogenic fungi and enhancement of growth of green gram (Vigna radiata). Biol. Fertil. Soils 1999, 29, 62–68. [Google Scholar] [CrossRef]
- Vessey, J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Chu, H.; Fujii, T.; Morimoto, S.; Lin, X.; Yagi, K.; Hu, J.; Zhang, J. Community Structure of Ammonia-Oxidizing Bacteria under Long-Term Application of Mineral Fertilizer and Organic Manure in a Sandy Loam Soil. Appl. Environ. Microbiol. 2007, 73, 485–491. [Google Scholar] [CrossRef] [Green Version]
- Ning, Q.; Gu, Q.; Shen, J.; Lv, X.; Yang, J.; Zhang, X.; He, J.; Huang, J.; Wang, H.; Xu, Z.; et al. Effects of nitrogen deposition rates and frequencies on the abundance of soil nitrogen-related functional genes in temperate grassland of northern China. J. Soils Sediments 2015, 15, 694–704. [Google Scholar] [CrossRef]
- Hallin, S.; Jones, C.M.; Schloter, M.; Philippot, L. Relationship between N-cycling communities and ecosystem functioning in a 50-year-old fertilization experiment. ISME J. 2009, 3, 597–605. [Google Scholar] [CrossRef]
- Wu, H.; Zeng, G.; Liang, J.; Guo, S.; Dai, J.; Lu, L.; Wei, Z.; Xu, P.; Li, F.; Yuan, Y.; et al. Effect of early dry season induced by the Three Gorges Dam on the soil microbial biomass and bacterial community structure in the Dongting Lake wetland. Ecol. Indic. 2015, 53, 129–136. [Google Scholar] [CrossRef]
- Yao, M.; Rui, J.; Li, J.; Dai, Y.; Bai, Y.; Heděne, P.; Wang, J.; Zhang, S.; Pei, K.; Liu, C.; et al. Rate-specific responses of prokaryotic diversity and structure to nitrogen deposition in the Leymus chinensis steppe. Soil Biol. Biochem. 2014, 79, 81–90. [Google Scholar] [CrossRef]
- Turlapati, S.A.; Minocha, R.; Bhiravarasa, P.S.; Tisa, L.S.; Thomas, W.K.; Minocha, S.C. Chronic N-amended soils exhibit an altered bacterial community structure in Harvard Forest, MA, USA. Ecology 2013, 83, 478–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinese Soil Taxonomy Cooperative Reseach Group. Chinese Soil Taxonomy (Revised Proposal); Institute of Soil Science/Chinese Agricultural Science and Technology Press, Academic Sinica: Beijing, China, 1995. [Google Scholar]
- Food and Agriculture or Ganisation; Food and Agriculture Organization of the United Nations: Rome, Italy, 1990.
- Wang, G.; Zhang, X.; Wang, Y.; Xu, X.; Han, Z. Key minerals influencing apple quality in Chinese orchard identified by nutritional diagnosis of leaf and soil analysis. J. Integr. Agric. 2015, 14, 864–874. [Google Scholar] [CrossRef]
- O’Kelly, B.C. Accurate Determination of Moisture Content of Organic Soils Using the Oven Drying Method. Dry. Technol. 2004, 22, 1767–1776. [Google Scholar] [CrossRef]
- Li, X.; Deng, Y.; Li, Q.; Lu, C.; Wang, J.; Zhang, H.; Zhu, J.; Zhou, J.; He, Z. Shifts of functional gene representation in wheat rhizosphere microbial communities under elevated ozone. ISME J. 2013, 7, 660–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, S. Analysis of Soil Agro-Chemistry; China Agriculture Press: Beijing, China, 1999. (In Chinese) [Google Scholar]
- National Agriculture Technoligy Extension and Service Center (NATESC). Soil Analysis Technology Specification; China Agriculture Press: Beijing, China, 2006.
- Dai, J.; Wang, Z.H.; Li, M.H.; He, G.; Li, Q.; Cao, H.B.; Wang, S.; Gao, Y.J.; Hui, X.L. Winter wheat grain yield and summer nitrate leaching: Long-term effects of nitrogen and phosphorus rates on the Loess Plateau of China. Field Crop. Res. 2016, 196, 180–190. [Google Scholar] [CrossRef]
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.J.S. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [Green Version]
- Torbjørn, R.; Tomáš, F.; Nichols, B.; Quince, C.; Frédéric, M. Vsearch: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [Green Version]
- Su, B.Y.; Song, Y.X.; Song, C.; Cui, L.; Yong, T.W.; Yang, W.T. Growth and photosynthetic responses of soybean seedlings to maize shading in relay intercropping system in Southwest China. Photosynthetica 2014, 52, 332–340. [Google Scholar] [CrossRef]
- Wang, X.Y.; Yu, Z.W. Effect of irrigation rate on absorption and translocation of nitrogen under different nitrogen fertilizer rate in wheat. Sci. Agric. Sin. 2008, 41, 3015–3024. [Google Scholar]
- Wang, C.; Lu, X.; Mori, T.; Mao, Q.; Zhou, K.; Zhou, G.; Nie, Y.; Mo, J. Responses of soil microbial community to continuous experimental nitrogen additions for 13 years in a nitrogen-rich tropical forest. Soil Biol. Biochem. 2018, 121, 103–112. [Google Scholar] [CrossRef]
- Dai, Z.; Su, W.; Chen, H.; Barberán, A.; Zhao, H.; Yu, M.; Yu, L.; Brookes, P.C.; Schadt, C.W.; Chang, S.X.; et al. Long-term nitrogen fertilization decreases bacterial diversity and favors the growth of Actinobacteria and Proteobacteria in agro-ecosystems across the globe. Glob. Chang. Biol. 2018, 24, 3452–3461. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Li, L.; Xie, J.; Wang, X.; Coulter, J.A.; Liu, C.; Wang, L. Effect of Long-Term Nitrogen Addition on Wheat Yield, Nitrogen Use Efficiency, and Residual Soil Nitrate in a Semiarid Area of the Loess Plateau of China. Sustainability 2020, 12, 1735. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Guan, D.; Zhou, B.; Zhao, B.; Ma, M.; Qin, J.; Jiang, X.; Chen, S.; Cao, F.; Shem, D.; et al. Influence of 34-years of fertilization on bacterial communities in an intensively cultivated black soil in northeast China. Soil Biol. Biochem. 2015, 90, 42–51. [Google Scholar] [CrossRef]
- Geisseler, D.; Scow, K.M. Long-term effects of mineral fertilizers on soil microorganisms—A review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Wang, H.; Hui, X.; Wang, Z.; Qiu, W. Long-term nitrogen fertilization impacts soil fungal and bacterial community structures in a dryland soil of Loess Plateau in China. J. Soils Sediments 2017, 18, 1632–1640. [Google Scholar] [CrossRef]
- Nilsson, L.O.; Bååth, E.; Falkengren-grerup, U.; Wallander, H. Growth of Ectomycorrhizal Mycelia and Composition of Soil Microbial Communities in Oak Forest Soils along a Nitrogen Deposition Gradient. Oecologia 2007, 153, 375–384. [Google Scholar] [CrossRef]
- Lauber, C.L.; Strickland, M.S.; Bradford, M.A.; Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar] [CrossRef]
- Jenkins, S.N.; Waite, I.S.; Blackburn, A.; Husband, R.; Rushton, S.P.; Manning, D.C.; O’Donnell, A.G. Actinobacterial community dynamics in long term managed grasslands. Antonie van Leeuwenhoek 2009, 95, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Hartman, W.H.; Richardson, C.J.; Vilgalys, R.; Bruland, G.L. Environmental and anthropogenic controls over bacterial communities in wetland soils. Proc. Natl. Acad. Sci. USA 2008, 105, 17842–17847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Wertz, S.; Leigh, A.K.K.; Grayston, S.J. Effects of long-term fertilization of forest soils on potential nitrification and on the abundance and community structure of ammonia oxidizers and nitrite oxidizers. FEMS Microbiol. Ecol. 2012, 79, 142–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, Y.; Yan, W.; Shangguan, Z. Impact of long-term N additions upon coupling between soil microbial community structure and activity, and nutrient-use efficiencies. Soil Biol. Biochem. 2015, 91, 151–159. [Google Scholar] [CrossRef]
- Fierer, N.; Lauber, C.L.; Ramirez, K.S.; Zaneveld, J.; Bradford, M.A.; Knight, R. Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J. 2012, 6, 1007–1017. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Song, Z.; Zhuang, D.; Wang, J.; Xie, S.; Liu, G. Urea fertilization decreases soil bacterial diversity, but improves microbial biomass, respiration, and N-cycling potential in a semiarid grassland. Biol. Fertil. Soils 2019, 55, 229–242. [Google Scholar] [CrossRef]
- Shen, C.; Xiong, J.; Zhang, H.; Feng, Y.; Lin, X.; Li, X.; Liang, W.; Chu, H. Soil pH drives the spatial distribution of bacterial communities along elevation on Changbai Mountain. Soil Biol. Biochem. 2012, 57, 204–211. [Google Scholar] [CrossRef]
- Xiong, J.; Liu, Y.; Lin, X.; Zhang, H.; Zeng, J.; Hou, J.; Yang, Y.; Yao, T.; Knight, R.; Chu, H. Geographic distance and ph drive bacterial distribution in alkaline lake sediments across tibetan plateau. Environ. Microbiol. 2012, 14, 2457–2466. [Google Scholar] [CrossRef] [Green Version]
- Dong, W.-Y.; Zhang, X.Y.; Dai, X.Q.; Fu, X.L.; Yang, F.T.; Liu, X.Y.; Sun, X.M.; Wen, X.F.; Schaeffer, S. Changes in soil microbial community composition in response to fertilization of paddy soils in subtropical China. Appl. Soil Ecol. 2014, 84, 140–147. [Google Scholar] [CrossRef]
- Ramirez, K.S.; Craine, J.M.; Fierer, N.B. Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Glob. Chang. Biol. 2012, 18, 1918–1927. [Google Scholar] [CrossRef]
- Zhalnina, K.; Dias, R.; Quadros, P.D.; Davis-Richardson, A.; Camargo, F.A.O.; Clark, I.M.; McGrath, S.P.; Hirsch, P.R.; Triplett, E.W. Soil pH determines microbial diversity and composition in the park grass experiment. Microb. Ecol. 2015, 69, 395–406. [Google Scholar] [CrossRef]
- Malhi, S.S.; Soon, Y.K.; Grant, C.A.; Lemke, R.; Lupwayi, N. Influence of controlled-Release urea on seed yield and N concentration, and N use efficiency of small grain crops grown on Dark Gray Luvisols. Can. J. Soil Sci. 2010, 90, 363–372. [Google Scholar] [CrossRef]
- Nemergut, D.R.; Townsend, A.R.; Sattin, S.R.; Freeman, K.R.; Fierer, N. The effects of chronic nitrogen fertilization on alpine tundra soil microbial communities: Implications for carbon and nitrogen cycling. Environ. Microbiol. 2008, 10, 3093–3105. [Google Scholar] [CrossRef] [PubMed]
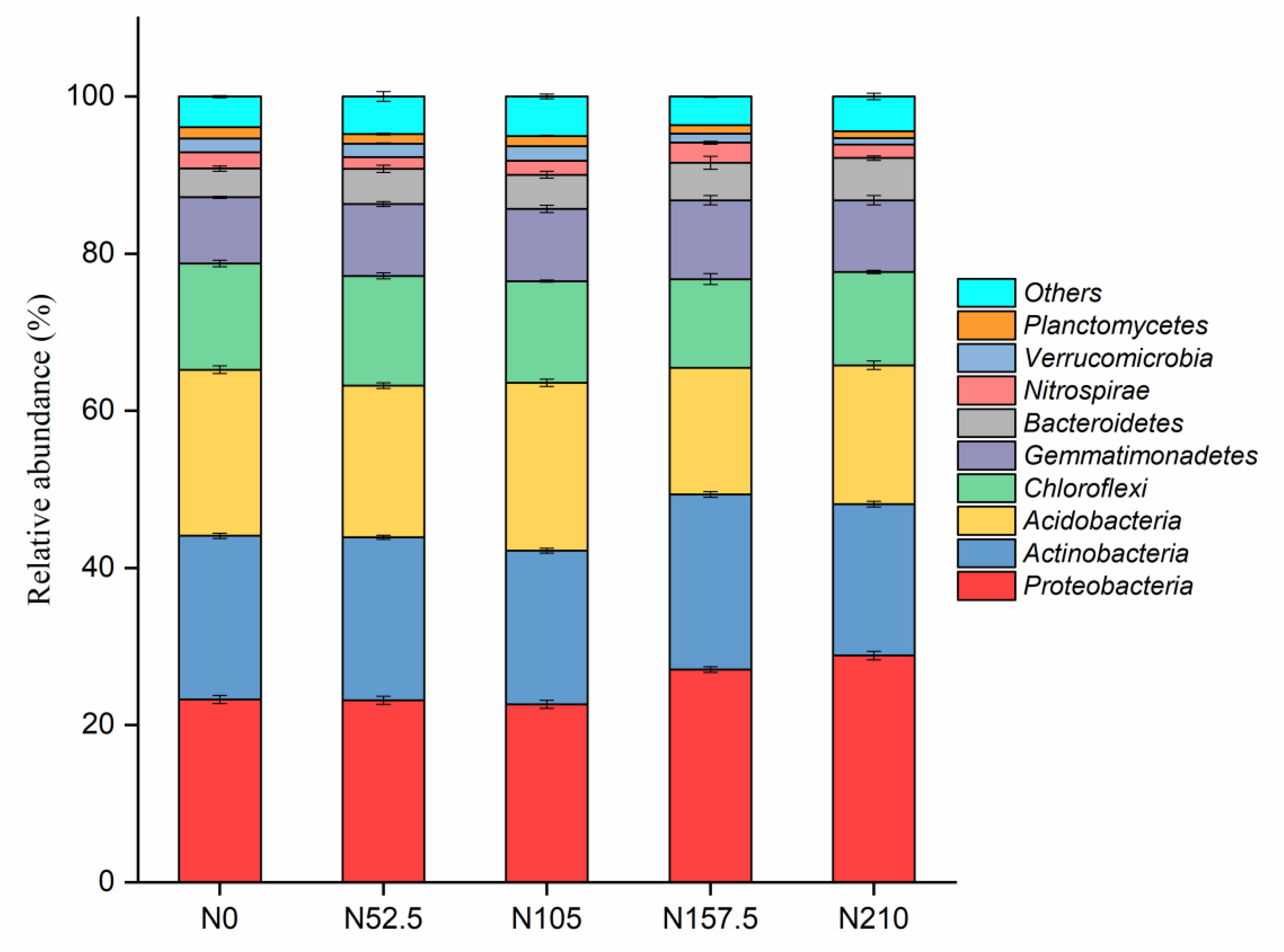
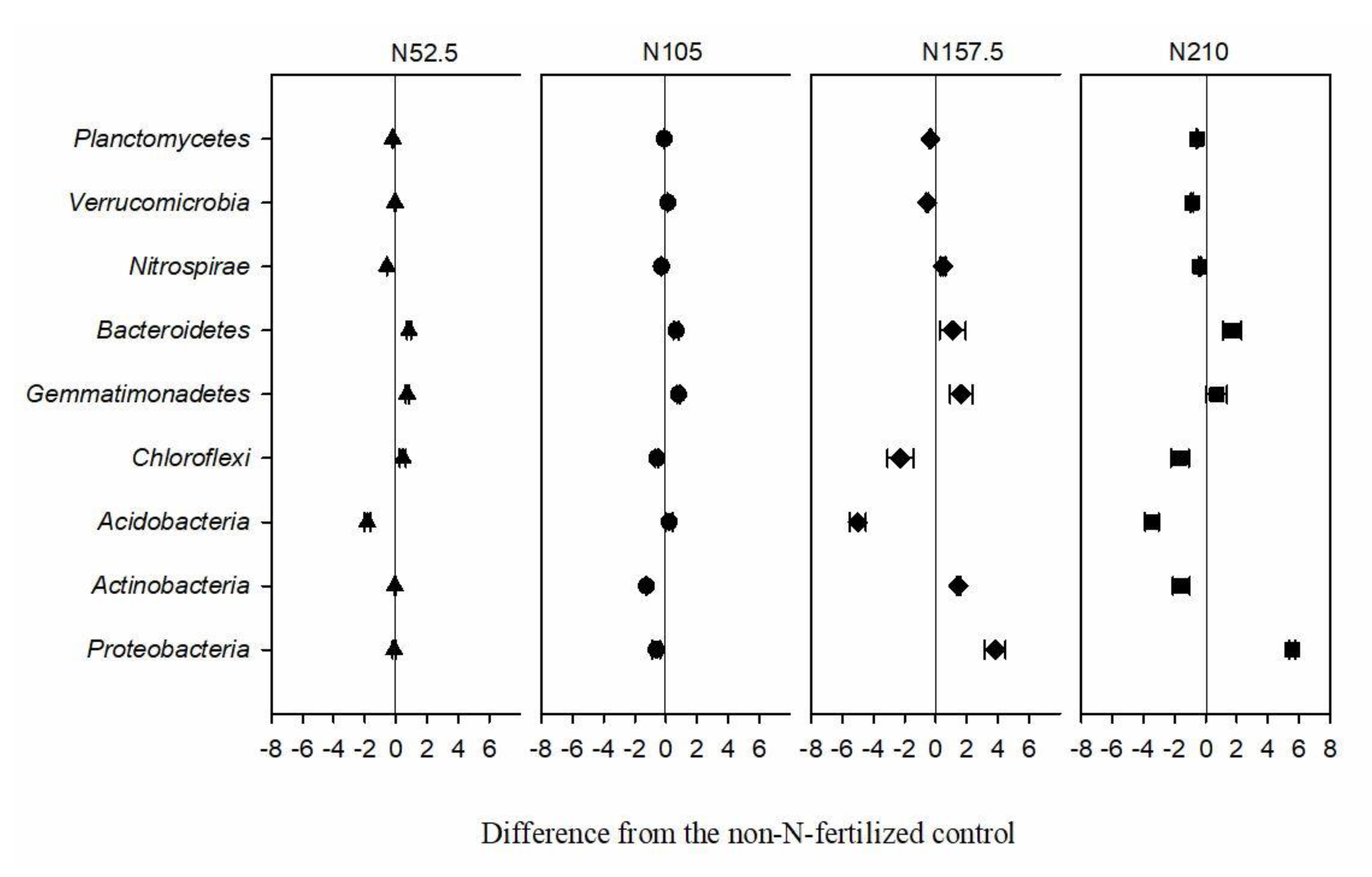




| Treatment | pH | TN (g kg−1) | NH4-N (mg kg−1) | NO3-N (mg kg−1) | TP (g kg−1) | AP (mg kg−1) | SM (%) |
|---|---|---|---|---|---|---|---|
| N0 | 8.09 ± 0.04ab | 0.92 ± 0.04a | 11.61 ± 0.20b | 15.74 ± 0.12d | 0.60 ± 0.03a | 21.52 ± 1.44b | 11.63 ± 0.91a |
| N52.5 | 7.94 ± 0.03b | 0.97 ± 0.13a | 12.05 ± 0.51b | 19.77 ± 0.55c | 0.69 ± 0.09a | 23.74 ± 0.54b | 11.57 ± 0.46a |
| N105 | 7.87 ± 0.03bc | 1.10 ± 0.02a | 12.68 ± 1.47b | 26.08 ± 0.63b | 0.77 ± 0.06a | 26.65 ± 0.21a | 11.45 ± 0.27a |
| N157.5 | 7.81 ± 0.01c | 1.05 ± 0.04a | 18.09 ± 0.23a | 46.58 ± 0.68a | 0.77 ± 0.08a | 23.55 ± 0.68b | 10.75 ± 0.5a |
| N210 | 7.83 ± 0.05c | 0.99 ± 0a | 18.68 ± 0.79a | 47.35 ± 0.42a | 0.76 ± 0.07a | 23.94 ± 0.84b | 10.55 ± 0.79a |
| ANOVA p-value | 0.001 | 0.369 | <0.001 | <0.001 | 0.429 | 0.022 | 0.503 |
| Growth Stage | Dependent Variable | N Rate (kg ha−1) | ANOVA (p-Value) | |||||
|---|---|---|---|---|---|---|---|---|
| N0 | N52.5 | N105 | N157.5 | N210 | ||||
| Tillering | N uptake | aboveground | 46.64 ± 3.39c b | 62.61 ± 2.61b | 71.72 ± 1.06ab | 73.35 ± 2.13a | 78.45 ± 4.28a | <0.001 |
| Anthesis | N uptake | leaf | 5.14 ± 0.60b | 11.43 ± 2.19ab | 17.38 ± 2.16a | 20 ± 4.69a | 19.57 ± 3.18a | 0.020 |
| stem + sheath | 14.44 ± 0.22b | 21.50 ± 1.15a | 25.18 ± 0.67a | 27.18 ± 3.95a | 25.47 ± 1.68a | 0.008 | ||
| spike | 29.61 ± 2.18c | 41.67 ± 0.46b | 50.97 ± 2.00a | 58.06 ± 1.43a | 56.06 ± 5.11a | <0.001 | ||
| aboveground | 52.25 ± 3.44b | 81.93 ± 4.5ab | 103.31 ± 4.01a | 112.88 ± 18.73a | 110.11 ± 9.08a | 0.007 | ||
| LAI | 1.82 ± 0.05d | 3.56 ± 0.10b | 3.05 ± 0.12c | 3.93 ± 0.09a | 3.86 ± 0.12ab | <0.001 | ||
| Maturity | N uptake | leaf | 2.80 ± 0.22b | 8.53 ± 1.28a | 8.71 ± 1.43a | 9.34 ± 1.26a | 7.40 ± 0.85a | 0.011 |
| stem+ sheath | 5.48 ± 0.38d | 8.09 ± 0.52c | 12.36 ± 0.48a | 10.80 ± 0.37b | 11.18 ± 0.36ab | <0.001 | ||
| glume+spike-stalk | 5.27 ± 0.48b | 11.68 ± 0.78a | 11.97 ± 0.85a | 13.97 ± 0.49a | 14.21 ± 1.60a | <0.001 | ||
| grain | 33.92 ± 1.56c | 41.23 ± 1.49b | 53.17 ± 0.30a | 54.15 ± 2.47a | 57.21 ± 2.75a | <0.001 | ||
| straw | 16.37 ± 0.26c | 35.56 ± 2.86b | 39.57 ± 2.12ab | 43.30 ± 1.72a | 39.61 ± 1.03ab | <0.001 | ||
| aboveground | 45.72 ± 0.87c | 74.31 ± 2.92b | 88.17 ± 2.96a | 90.86 ± 0.29a | 88.23 ± 1.75a | <0.001 | ||
| GY (kg ha−1) | 1473.00 ± 30.20c | 1734.40 ± 104.91b | 2117.50 ± 74.05a | 2209.07 ± 73.88a | 2204.97 ± 30.09a | <0.001 | ||
| NUE (%) | — | 27.19 ± 2.70a | 27.73 ± 3.53a | 23.79 ± 1.47ab | 17.02 ± 1.00b | 0.045 | ||
| Treatment | PD Whole Tree | Chao1 | Goods Coverage | Observed Species | Shannon | Simpson | OTUs |
|---|---|---|---|---|---|---|---|
| N0 | 100.2 ± 0.1b | 3696.0 ± 55.1ab | 1.0 ± 0.0a | 2921.8 ± 35.1ab | 9.9 ± 0.0a | 1.0 ± 0.0a | 3054.3 ± 18.1bc |
| N52.5 | 98.8 ± 0.2c | 3729.6 ± 28.7ab | 1.0 ± 0.0a | 2940.1 ± 26.9ab | 9.9 ± 0.0a | 1.0 ± 0.0ab | 3112.3 ± 60.5ab |
| N105 | 101.9 ± 0.4a | 3807.6 ± 51.2a | 1.0 ± 0.0a | 3016.0 ± 17.5a | 9.9 ± 0.0a | 1.0 ± 0.0ab | 3207.7 ± 29.7a |
| N157.5 | 99.4 ± 0.2bc | 3629.6 ± 17.5b | 1.0 ± 0.0a | 2914.7 ± 32.2ab | 9.9 ± 0.0a | 1.0 ± 0.0ab | 3095.3 ± 35.4abc |
| N210 | 97.4 ± 0.5d | 3628.1 ± 23.1b | 1.0 ± 0.0a | 2874.9 ± 57.6b | 9.8 ± 0.1a | 1.0 ± 0.0b | 2963.0 ± 46.8c |
| ANOVA p-value | <0.001 | 0.038 | 0.475 | 0.165 | 0.281 | 0.147 | 0.021 |
| Dependent Variable | PD Whole Tree | Chao1 | Goods Coverage | Observed Species | Shannon | Simpson | OTUs |
|---|---|---|---|---|---|---|---|
| pH | 0.246 | 0.144 | −0.318 | 0.170 | 0.317 | 0.539 * | 0.000 |
| TN (g kg−1) | 0.283 | 0.369 | 0.051 | 0.349 | 0.394 | −0.168 | 0.402 |
| NH4 (mg kg−1) | −0.585 * | −0.582 * | 0.468 | −0.487 | −0.571 * | −0.377 | −0.469 |
| NO3 (mg kg−1) | −0.468 | −0.527 * | 0.411 | −0.335 | −0.404 | −0.320 | −0.361 |
| TP (g kg−1) | 0.090 | −0.019 | −0.440 | 0.337 | 0.054 | −0.181 | 0.059 |
| AP (mg kg−1) | 0.244 | 0.371 | −0.170 | 0.336 | −0.076 | −0.343 | 0.385 |
| SM (%) | 0.273 | 0.073 | −0.018 | 0.162 | 0.061 | 0.315 | 0.273 |
| LAI | −0.522 * | −0.303 | 0.337 | −0.205 | −0.319 | −0.524 * | −0.119 |
| GY (kg ha−1) | 0.204 | 0.258 | −0.121 | 0.323 | 0.225 | −0.181 | 0.555 * |
| NUE (%) | 0.518 | 0.368 | −0.144 | 0.427 | 0.462 | 0.381 | 0.657 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, A.; Li, L.; Coulter, J.A.; Xie, J.; Gopalakrishnan, S.; Zhang, R.; Luo, Z.; Cai, L.; Liu, C.; Wang, L.; et al. Long-Term Nitrogen Fertilization Impacts on Soil Bacteria, Grain Yield and Nitrogen Use Efficiency of Wheat in Semiarid Loess Plateau, China. Agronomy 2020, 10, 1175. https://doi.org/10.3390/agronomy10081175
Xu A, Li L, Coulter JA, Xie J, Gopalakrishnan S, Zhang R, Luo Z, Cai L, Liu C, Wang L, et al. Long-Term Nitrogen Fertilization Impacts on Soil Bacteria, Grain Yield and Nitrogen Use Efficiency of Wheat in Semiarid Loess Plateau, China. Agronomy. 2020; 10(8):1175. https://doi.org/10.3390/agronomy10081175
Chicago/Turabian StyleXu, Aixia, Lingling Li, Jeffrey A. Coulter, Junhong Xie, Subramaniam Gopalakrishnan, Renzhi Zhang, Zhuzhu Luo, Liqun Cai, Chang Liu, Linlin Wang, and et al. 2020. "Long-Term Nitrogen Fertilization Impacts on Soil Bacteria, Grain Yield and Nitrogen Use Efficiency of Wheat in Semiarid Loess Plateau, China" Agronomy 10, no. 8: 1175. https://doi.org/10.3390/agronomy10081175
APA StyleXu, A., Li, L., Coulter, J. A., Xie, J., Gopalakrishnan, S., Zhang, R., Luo, Z., Cai, L., Liu, C., Wang, L., & Khan, S. (2020). Long-Term Nitrogen Fertilization Impacts on Soil Bacteria, Grain Yield and Nitrogen Use Efficiency of Wheat in Semiarid Loess Plateau, China. Agronomy, 10(8), 1175. https://doi.org/10.3390/agronomy10081175







