Flavonoids in Agriculture: Chemistry and Roles in, Biotic and Abiotic Stress Responses, and Microbial Associations
Abstract
1. Introduction
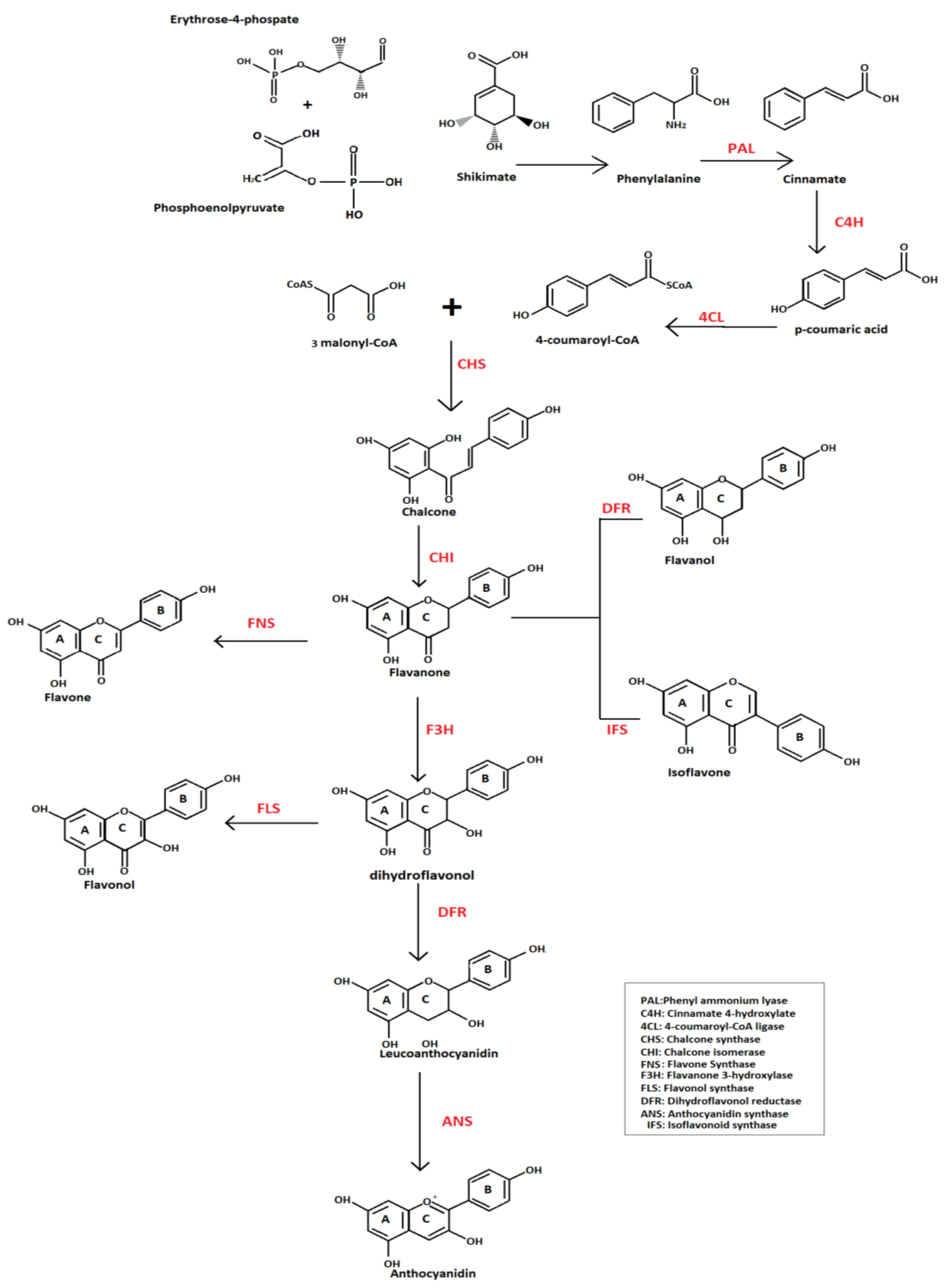
2. Biosynthesis and Classification of Flavonoids
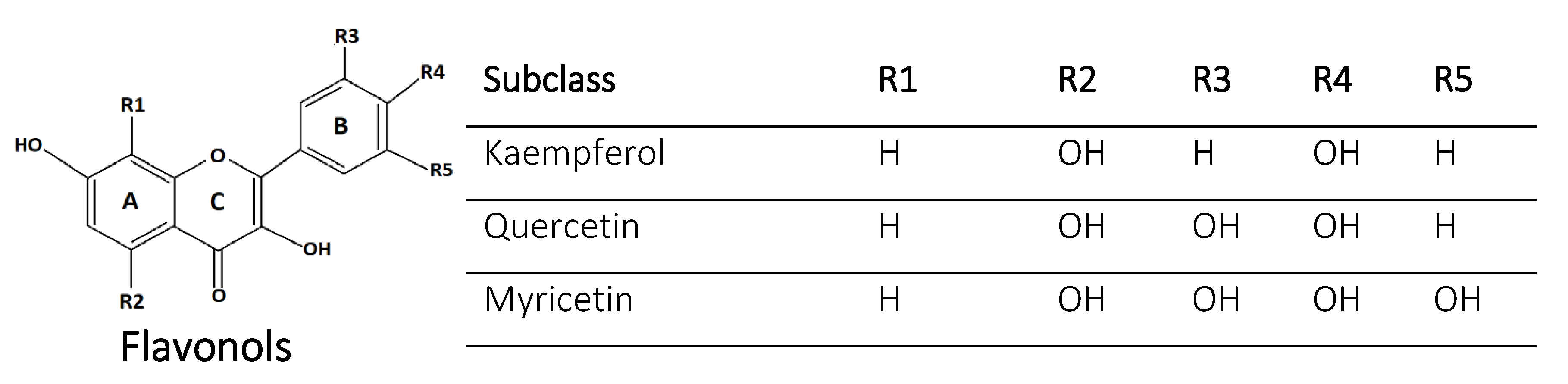
2.1. Flavonols
2.2. Flavones
2.3. Flavanones
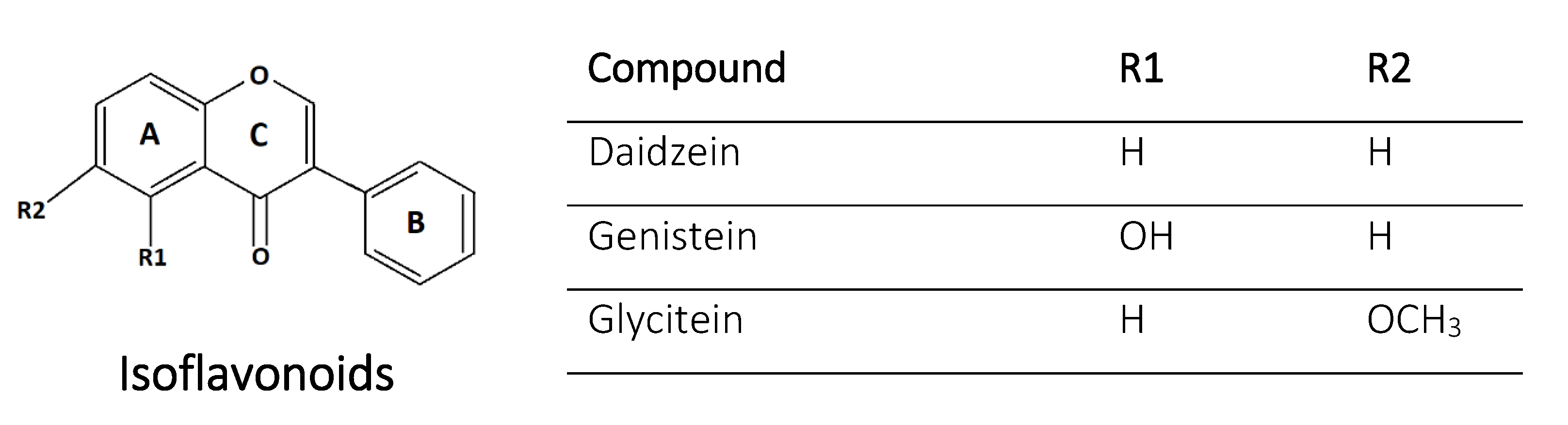
2.4. Isoflavonoids
2.5. Anthocyanidins
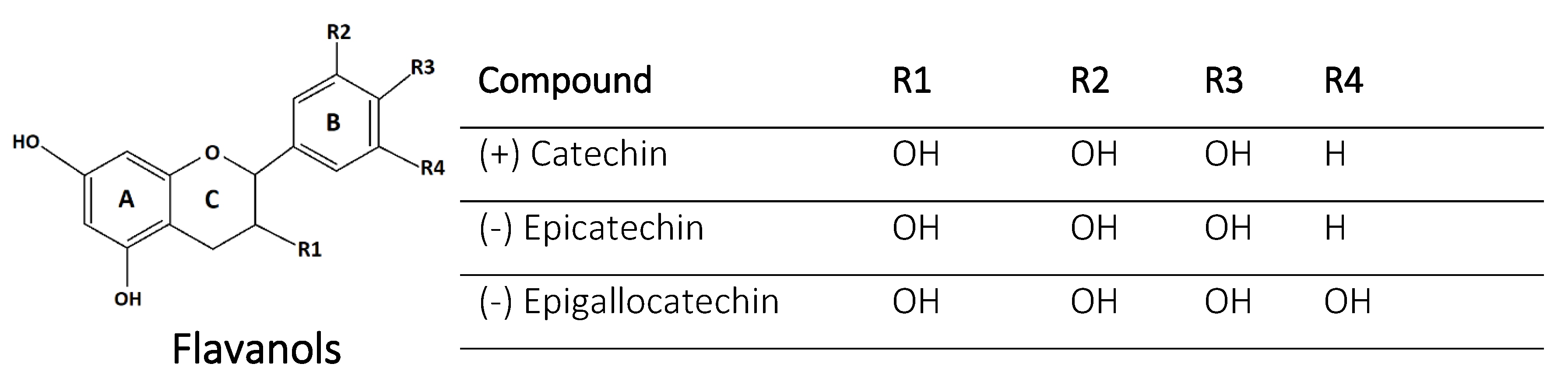
2.6. Flavanols
2.7. Chalcones
3. Role of Flavonoids in Plant Growth and Crop Yield
3.1. Flavonoids in the Rhizosphere
3.2. Flavonoids and Legume-Rhizobium Interaction
3.3. Flavonoids and Mycorrhizal Associations
4. Flavonoids and Plant Abiotic Stresses
4.1. Flavonoids as UV Scavengers
4.2. Flavonoids in Managing Salt and Drought Stress
- Substitution of biomolecules vulnerable to oxidative damage with resistant ones
- Antioxidants acting as “sacrificial agents” by reacting with reactive species to prevent them from reacting with important biomolecules [129].
5. Flavonoids against Plant Biotic Stress
5.1. Phytoalexin Flavonoids as Nematicides
5.2. Flavonoids against Pathogenic Fungi
5.3. Antibacterial Effects of Flavonoids
5.4. Flavonoids as Insect/Herbivore Repellents
6. Allelopathic/Phytotoxic Behavior of Flavonoids
7. Conclusions
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Observatory Earth. World of Change: Global Temperatures. Available online: https://earthobservatory.nasa.gov/world-of-change/global-temperatures (accessed on 24 March 2020).
- Richardson, Y.; Blin, J.; Julbe, A. A short overview on purification and conditioning of syngas produced by biomass gasification: Catalytic strategies, process intensification and new concepts. Prog. Energy Combust. Sci. 2012, 38, 765–781. [Google Scholar] [CrossRef]
- EPA. Global Emissions by Gas. Available online: https://www.epa.gov/ghgemissions/global-greenhouse-gas-emissions-data (accessed on 24 March 2020).
- Scialabba, N.E.-H.; Müller-Lindenlauf, M. Organic agriculture and climate change. Renew. Agric. Food Syst. 2010, 25, 158–169. [Google Scholar] [CrossRef]
- Chalker-Scott, L. Environmental significance of anthocyanins in plant stress responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- Chen, S.; Wu, F.; Li, Y.; Qian, Y.; Pan, X.; Li, F.; Wang, Y.; Wu, Z.; Fu, C.; Lin, H.; et al. NtMYB4 and NtCHS1 are critical factors in the regulation of flavonoid biosynthesis and are involved in salinity responsiveness. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Shojaie, B.; Mostajeran, A.; Ghanadian, M. Flavonoid dynamic responses to different drought conditions: Amount, type, and localization of flavonols in roots and shoots of Arabidopsis thaliana L. Turk. J. Biol. 2016, 40, 612–622. [Google Scholar] [CrossRef]
- Brunetti, C.; Di Ferdinando, M.; Fini, A.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants and developmental regulators: Relative significance in plants and humans. Int. J. Mol. Sci. 2013, 14, 3540–3555. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Singla, P.; Garg, N. Plant flavonoids: Key players in signaling, establishment, and regulation of rhizobial and mycorrhizal endosymbioses. In Mycorrhiza-Function, Diversity, State of the Art; Springer: Berlin/Heidelberg, Germany, 2017; pp. 133–176. [Google Scholar]
- Liu, C.W.; Murray, J.D. The role of flavonoids in nodulation host-range specificity: An update. Plants (Basel) 2016, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef]
- Dudek, B.; Warskulat, A.-C.; Schneider, B. The Occurrence of Flavonoids and Related Compounds in Flower Sections of Papaver nudicaule. Plants 2016, 5, 28. [Google Scholar] [CrossRef]
- Cesco, S.; Mimmo, T.; Tonon, G.; Tomasi, N.; Pinton, R.; Terzano, R.; Neumann, G.; Weisskopf, L.; Renella, G.; Landi, L.; et al. Plant-borne flavonoids released into the rhizosphere: Impact on soil bio-activities related to plant nutrition. A review. Biol. Fertil. Soils 2012, 48, 123–149. [Google Scholar] [CrossRef]
- Nabavi, S.M.; Samec, D.; Tomczyk, M.; Milella, L.; Russo, D.; Habtemariam, S.; Suntar, I.; Rastrelli, L.; Daglia, M.; Xiao, J.; et al. Flavonoid biosynthetic pathways in plants: Versatile targets for metabolic engineering. Biotechnol. Adv. 2020, 38, 107316. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, S.; Yu, O. Metabolic engineering of flavonoids in plants and microorganisms. Appl. Microbiol. Biotechnol. 2011, 91, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Aherne, S.A.; O’Brien, N.M. Dietary flavonols: Chemistry, food content, and metabolism. Nutrition 2002, 18, 75–81. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Samanta, A.; Das, G.; Das, S.K. Roles of Flavonoids in Plants. Int. J. Pharm. Sci. Technol. 2011, 6, 12–35. [Google Scholar]
- Sultana, B.; Anwar, F. Flavonols (kaempeferol, quercetin, myricetin) contents of selected fruits, vegetables and medicinal plants. Food Chem. 2008, 108, 879–884. [Google Scholar] [CrossRef]
- Crozier, A.; Lean, M.E.; McDonald, M.S.; Black, C. Quantitative analysis of the flavonoid content of commercial tomatoes, onions, lettuce, and celery. J. Agric. Food Chem. 1997, 45, 590–595. [Google Scholar] [CrossRef]
- López-Lázaro, M. Distribution and biological activities of the flavonoid luteolin. Mini Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Del Valle, I.; Webster, T.M.; Cheng, H.-Y.; Thies, J.E.; Kessler, A.; Miller, M.K.; Ball, Z.T.; MacKenzie, K.R.; Masiello, C.A.; Silberg, J.J.; et al. Soil organic matter attenuates the efficacy of flavonoid-based plant-microbe communication. Sci. Adv. 2020, 6, eaax8254. [Google Scholar] [CrossRef] [PubMed]
- Erlund, I. Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology. Nutr. Res. 2004, 24, 851–874. [Google Scholar] [CrossRef]
- Andersen, O.M.; Markham, K.R. Flavonoids: Chemistry, Biochemistry and Applications; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Arts, I.C.; van de Putte, B.; Hollman, P.C. Catechin contents of foods commonly consumed in The Netherlands. 1. Fruits, vegetables, staple foods, and processed foods. J. Agric. Food Chem. 2000, 48, 1746–1751. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Hillel, D.; Hatfield, J.L. Encyclopedia of Soils in the Environment; Elsevier: Amsterdam, The Netherlands, 2005; Volume 3. [Google Scholar]
- Dykhuizen, D. Species numbers in bacteria. Proc. Calif. Acad. Sci. 2005, 56, 62. [Google Scholar]
- Lal, R.; Francaviglia, R. Sustainable Agriculture Reviews 29: Sustainable Soil Management: Preventive and Ameliorative Strategies; Springer: Berlin/Heidelberg, Germany, 2019; Volume 29. [Google Scholar]
- Mia, M.B.; Shamsuddin, Z. Rhizobium as a crop enhancer and biofertilizer for increased cereal production. Afr. J. Biotechnol. 2010, 9, 6001–6009. [Google Scholar]
- Afzal, A.; Bano, A. Rhizobium and phosphate solubilizing bacteria improve the yield and phosphorus uptake in wheat (Triticum aestivum). Int. J. Agric. Biol. 2008, 10, 85–88. [Google Scholar]
- Hijri, M. Analysis of a large dataset of mycorrhiza inoculation field trials on potato shows highly significant increases in yield. Mycorrhiza 2016, 26, 209–214. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Biol. 2002, 5, 218–223. [Google Scholar] [CrossRef]
- Dixon, R.A.; Pasinetti, G.M. Flavonoids and isoflavonoids: From plant biology to agriculture and neuroscience. Plant Physiol. 2010, 154, 453–457. [Google Scholar] [CrossRef]
- Cetinkaya, H.; Kulak, M.; Karaman, M.; Karaman, H.S.; Kocer, F. Flavonoid accumulation behavior in response to the abiotic stress: Can a uniform mechanism be illustrated for all plants. In Flavonoids—From Biosynthesis to Human Health; Intechopen: London UK, 2017. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Vasilopoulou, E.; Hollman, P.; Chamalides, C.; Foufa, E.; Kaloudis, T.; Kromhout, D.; Miskaki, P.; Petrochilou, I.; Poulima, E.; et al. Nutritional composition and flavonoid content of edible wild greens and green pies: A potential rich source of antioxidant nutrients in the Mediterranean diet. Food Chem. 2000, 70, 319–323. [Google Scholar] [CrossRef]
- Parvez, M.; Tomita-Yokotani, K.; Fujii, Y.; Konishi, T.; Iwashina, T. Effects of quercetin and its derivatives on the growth of Arabidopsis thaliana and Neurospora crassa. Biochem. Syst. Ecol. 2004, 32, 631–635. [Google Scholar] [CrossRef]
- Škerget, M.; Kotnik, P.; Hadolin, M.; Hraš, A.R.; Simonič, M.; Knez, Ž. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005, 89, 191–198. [Google Scholar] [CrossRef]
- Díaz, M.; Rossini, C. Bioactive natural products from Sapindaceae deterrent and toxic metabolites against insects. In Insecticides–Pest Engineering; Perveen, F., Ed.; InTech: Rijeka, Croatia, 2012; pp. 287–308. [Google Scholar]
- Tatsimo, S.J.N.; Tamokou, J.d.D.; Havyarimana, L.; Csupor, D.; Forgo, P.; Hohmann, J.; Kuiate, J.-R.; Tane, P. Antimicrobial and antioxidant activity of kaempferol rhamnoside derivatives from Bryophyllum pinnatum. BMC Res. Notes 2012, 5, 158. [Google Scholar] [CrossRef] [PubMed]
- Mendki, P.; Salunke, B.; Kotkar, H.; Maheshwari, V.; Mahulikar, P.; Kothari, R. Antimicrobial and Insecticidal Activities of Flavonoid Glycosides from Calotropis procera L. for Post-harvest Preservation of Pulses. Biopestic. Int. 2005, 1, 193–200. [Google Scholar]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Wu, N.-L.; Fang, J.-Y.; Chen, M.; Wu, C.-J.; Huang, C.-C.; Hung, C.-F. Chrysin protects epidermal keratinocytes from UVA- and UVB-induced damage. J. Agric. Food Chem. 2011, 59, 8391–8400. [Google Scholar] [CrossRef]
- Liggins, J.; Bluck, L.; Runswick, S.; Atkinson, C.; Coward, W.; Bingham, S. Daidzein and genistein contents of vegetables. Br. J. Nutr. 2000, 84, 717–725. [Google Scholar] [CrossRef]
- Pei, Y.; Siemann, E.; Tian, B.; Ding, J. Root flavonoids are related to enhanced AMF colonization of an invasive tree. AoB Plants 2020, 12. [Google Scholar] [CrossRef]
- Cavia-Saiz, M.; Busto, M.D.; Pilar-Izquierdo, M.C.; Ortega, N.; Perez-Mateos, M.; Muñiz, P. Antioxidant properties, radical scavenging activity and biomolecule protection capacity of flavonoid naringenin and its glycoside naringin: A comparative study. J. Sci. Food Agric. 2010, 90, 1238–1244. [Google Scholar] [CrossRef]
- Wilmsen, P.K.; Spada, D.S.; Salvador, M. Antioxidant activity of the flavonoid hesperidin in chemical and biological systems. J. Agric. Food Chem. 2005, 53, 4757–4761. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.M.; Phillips, D.A. Flavonoids released naturally from alfalfa promote development of symbiotic glomus spores in vitro. Appl. Environ. Microbiol. 1991, 57, 1485–1488. [Google Scholar] [CrossRef]
- Harborne, J.B. The flavonoids: Advances in research since 1986 (Harborne, J.B.). J. Chem. Educ. 1995, 72, A73. [Google Scholar] [CrossRef]
- Naeimi, A.F.; Alizadeh, M. Antioxidant properties of the flavonoid fisetin: An updated review of in vivo and in vitro studies. Trends Food Sci. Technol. 2017, 70, 34–44. [Google Scholar] [CrossRef]
- Ong, K.C.; Khoo, H.-E. Biological effects of myricetin. Gen. Pharmacol. Vasc. Syst. 1997, 29, 121–126. [Google Scholar] [CrossRef]
- Peters, N.; Frost, J.; Long, S. A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science 1986, 233, 977–980. [Google Scholar] [CrossRef]
- Garcia, K.; Delaux, P.M.; Cope, K.R.; Ane, J.M. Molecular signals required for the establishment and maintenance of ectomycorrhizal symbioses. New Phytol. 2015, 208, 79–87. [Google Scholar] [CrossRef]
- Rice-evans, C.A.; Miller, N.J.; Bolwell, P.G.; Bramley, P.M.; Pridham, J.B. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic. Res. 1995, 22, 375–383. [Google Scholar] [CrossRef]
- Tsanova-Savova, S.; Ribarova, F.; Gerova, M. (+)-Catechin and (−)-epicatechin in Bulgarian fruits. J. Food Compos. Anal. 2005, 18, 691–698. [Google Scholar] [CrossRef]
- Pathan, S.I.; Ceccherini, M.T.; Sunseri, F.; Lupini, A. Rhizosphere as hotspot for plant-soil-microbe interaction. In Carbon and Nitrogen Cycling in Soil; Springer: Berlin/Heidelberg, Germany, 2020; pp. 17–43. [Google Scholar]
- Mandal, S.M.; Chakraborty, D.; Dey, S. Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal. Behav. 2010, 5, 359–368. [Google Scholar] [CrossRef]
- Sugiyama, A.; Shitan, N.; Yazaki, K. Involvement of a soybean ATP-binding cassette-type transporter in the secretion of genistein, a signal flavonoid in legume-Rhizobium symbiosis. Plant Physiol. 2007, 144, 2000–2008. [Google Scholar] [CrossRef]
- Shaw, L.J.; Morris, P.; Hooker, J.E. Perception and modification of plant flavonoid signals by rhizosphere microorganisms. Environ. Microbiol. 2006, 8, 1867–1880. [Google Scholar] [CrossRef] [PubMed]
- Kovács, E.; Keresztes, Á. Effect of gamma and UV-B/C radiation on plant cells. Micron 2002, 33, 199–210. [Google Scholar] [CrossRef]
- Chaparro, J.M.; Sheflin, A.M.; Manter, D.K.; Vivanco, J.M. Manipulating the soil microbiome to increase soil health and plant fertility. Biol. Fertil. Soils 2012, 48, 489–499. [Google Scholar] [CrossRef]
- Munoz Aguilar, J.M.; Ashby, A.M.; Richards, A.J.M.; Loake, G.J.; Watson, M.D.; Shaw, C.H. Chemotaxis of Rhizobium leguminosarum biovar phaseoli towards flavonoid inducers of the symbiotic nodulation genes. Microbiology 1988, 134, 2741–2746. [Google Scholar] [CrossRef]
- Hassan, S.; Mathesius, U. The role of flavonoids in root-rhizosphere signalling: Opportunities and challenges for improving plant-microbe interactions. J. Exp. Bot. 2012, 63, 3429–3444. [Google Scholar] [CrossRef]
- Bolaños-Vásquez, M.C.; Werner, D. Effects of Rhizobium tropici, R. etli, and R. leguminosarum bv. phaseoli on nod gene-inducing flavonoids in root exudates of Phaseolus vulgaris. Mol. Plant Microbe Interact. 1997, 10, 339–346. [Google Scholar] [CrossRef]
- Stambulska, U.Y.; Bayliak, M.M. Legume-rhizobium symbiosis: Secondary metabolites, free radical processes, and effects of heavy metals. In Bioactive Molecules in Food; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–32. [Google Scholar] [CrossRef]
- Davidson, E.A. The contribution of manure and fertilizer nitrogen to atmospheric nitrous oxide since 1860. Nat. Geosci. 2009, 2, 659–662. [Google Scholar] [CrossRef]
- Hartwig, U.A.; Joseph, C.M.; Phillips, D.A. Flavonoids released naturally from alfalfa seeds enhance growth rate of Rhizobium meliloti. Plant Physiol. 1991, 95, 797–803. [Google Scholar] [CrossRef]
- Maxwell, C.A.; Hartwig, U.A.; Joseph, C.M.; Phillips, D.A. A chalcone and two related flavonoids released from alfalfa roots induce nod genes of Rhizobium meliloti. Plant Physiol. 1989, 91, 842–847. [Google Scholar] [CrossRef]
- Dolatabadian, A.; Sanavy, S.A.M.M.; Ghanati, F.; Gresshoff, P.M. Morphological and physiological response of soybean treated with the microsymbiont Bradyrhizobium japonicum pre-incubated with genistein. S. Afr. J. Bot. 2012, 79, 9–18. [Google Scholar] [CrossRef]
- Subramanian, S.; Stacey, G.; Yu, O. Endogenous isoflavones are essential for the establishment of symbiosis between soybean and Bradyrhizobium japonicum. Plant J. 2006, 48, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Jones, F.R.; Tisdale, W. Effect of soil temperature upon the development of nodules on the roots of certain legumes. J. Agric. Res. 1921, 22, 17–37. [Google Scholar]
- Lynch, D.; Smith, D. Soybean (Glycine max) modulation and N2-fixation as affected by exposure to a low root-zone temperature. Physiol. Plant. 1993, 88, 212–220. [Google Scholar] [CrossRef]
- Pan, B.; Zhang, F.; Smith, D.L. Genistein addition to the rooting medium of soybean at the onset of nitrogen fixation increases nodulation. J. Plant Nutr. 1998, 21, 1631–1639. [Google Scholar] [CrossRef]
- Gibson, A. Factors in the physical and biological environment affecting nodulation and nitrogen fixation by legumes. Plant Soil 1971, 35, 139–152. [Google Scholar] [CrossRef]
- Layzell, D.; Rochman, P.; Canvin, D. Low root temperatures and nitrogenase activity in soybean. Can. J. Bot. 1984, 62, 965–971. [Google Scholar] [CrossRef]
- Zhang, F.; Smith, D.L. Preincubation of bradyrhizobium japonicum with genistein accelerates nodule development of soybean at suboptimal root zone temperatures. Plant Physiol. 1995, 108, 961–968. [Google Scholar] [CrossRef]
- Kasper, S.; Christoffersen, B.; Soti, P.; Racelis, A. Abiotic and biotic limitations to nodulation by leguminous cover crops in South Texas. Agriculture 2019, 9, 209. [Google Scholar] [CrossRef]
- Hemida, M.; Issa, A.A.; Ohyam, T. Impact of harsh environmental conditions on nodule formation and dinitrogen fixation of legumes. In Advances in Biology and Ecology of Nitrogen Fixation; Intechopen: London, UK, 2014. [Google Scholar] [CrossRef]
- Zhang, F.; Smith, D.L. Application of genistein to inocula and soil to overcome low spring soil temperature inhibition of soybean nodulation and nitrogen fixation. Plant Soil 1997, 192, 141–151. [Google Scholar] [CrossRef]
- Belkheir, A.M.; Zhou, X.; Smith, D.L. Variability in yield and yield component responses to genistein pre-incubated Bradyrhizobium japonicum by soybean [Glycine max (L.) Merr] cultivars. Plant soil 2001, 229, 41–46. [Google Scholar] [CrossRef]
- Belkheir, A.; Zhou, X.; Smith, D. Response of soybean [Glycine max (L.) Merr.] cultivars to genistein-preincubated bradyrhizobium japonicum: Nodulation and dry matter accumulation under Canadian short-season conditions. J. Agron. Crop Sci. 2000, 185, 167–175. [Google Scholar] [CrossRef]
- Morrison, M.; Cober, E.; Saleem, M.; McLaughlin, N.; Fregeau-Reid, J.; Ma, B.; Yan, W.; Woodrow, L. Changes in isoflavone concentration with 58 years of genetic improvement of short-season soybean cultivars in Canada. Crop Sci. 2008, 48, 2201–2208. [Google Scholar] [CrossRef]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Mohammadi, K.; Khalesro, S.; Sohrabi, Y.; Heidari, G. A review: Beneficial effects of the mycorrhizal fungi for plant growth. J. Appl. Environ. Biol. Sci. 2011, 1, 310–319. [Google Scholar]
- Parniske, M. Arbuscular mycorrhiza: The mother of plant root endosymbioses. Nat. Rev. Microbiol. 2008, 6, 763–775. [Google Scholar] [CrossRef]
- Vierheilig, H.; Bago, B.; Albrecht, C.; Poulin, M.-J.; Piché, Y. Flavonoids and arbuscular-mycorrhizal fungi. In Flavonoids in the Living System; Manthey, J.A., Buslig, B.S., Eds.; Springer: Boston, MA, USA, 1998; pp. 9–33. [Google Scholar] [CrossRef]
- Becard, G.; Taylor, L.P.; Douds, D.D.; Pfeffer, P.E.; Doner, L.W. Flavonoids are not necessary plant signal compounds in arbuscular mycorrhizal symbioses. MPMI Mol. Plant Microbe Interact. 1995, 8, 252–258. [Google Scholar] [CrossRef]
- Siqueira, J.; Safir, G.; Nair, M. Stimulation of vesicular-arbuscular mycorrhiza formation and growth of white clover by flavonoid compounds. New Phytol. 1991, 118, 87–93. [Google Scholar] [CrossRef]
- Davies, F.T.; Calderón, C.M.; Huaman, Z. Influence of arbuscular mycorrhizae indigenous to peru and a flavonoid on growth, yield, and leaf elemental concentration of ‘yungay’potatoes. HortScience 2005, 40, 381–385. [Google Scholar] [CrossRef]
- Davies, F.T., Jr.; Calderón, C.M.; Huaman, Z.; Gómez, R. Influence of a flavonoid (formononetin) on mycorrhizal activity and potato crop productivity in the highlands of Peru. Sci. Hortic. 2005, 106, 318–329. [Google Scholar] [CrossRef]
- De Almeida Ribeiro, P.R.; dos SANTOS, J.V.; de Carvalho, T.S.; da Silva, J.S.; de Resende, P.M.; de Souza Moreira, F.M. Formononetin associated with phosphorus influences soybean symbiosis with mycorrhizal fungi and Bradyrhizobium. Biosci. J. 2016, 32. [Google Scholar]
- Scervino, J.M.; Ponce, M.A.; Erra-Bassells, R.; Bompadre, J.; Vierheilig, H.; Ocampo, J.A.; Godeas, A. The effect of flavones and flavonols on colonization of tomato plants by arbuscular mycorrhizal fungi of the genera Gigaspora and Glomus. Can. J. microbiol. 2007, 53, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, R.; Mori, T.; Saito, K. Alternation of flavonoid accumulation under drought stress in Arabidopsis thaliana. Plant Signal. Behav. 2014, 9, e29518. [Google Scholar] [CrossRef]
- Dakora, F.D.; Phillips, D.A. Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil 2002, 245, 35–47. [Google Scholar] [CrossRef]
- Gupta, R.; Chakrabarty, S. Gibberellic acid in plant: Still a mystery unresolved. Plant Signal. Behav. 2013, 8, e25504. [Google Scholar] [CrossRef]
- Tomasi, N.; Weisskopf, L.; Renella, G.; Landi, L.; Pinton, R.; Varanini, Z.; Nannipieri, P.; Torrent, J.; Martinoia, E.; Cesco, S. Flavonoids of white lupin roots participate in phosphorus mobilization from soil. Soil Biol. Biochem. 2008, 40, 1971–1974. [Google Scholar] [CrossRef]
- Cesco, S.; Neumann, G.; Tomasi, N.; Pinton, R.; Weisskopf, L. Release of plant-borne flavonoids into the rhizosphere and their role in plant nutrition. Plant Soil 2010, 329, 1–25. [Google Scholar] [CrossRef]
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: A systems biology perspective. BMC Plant Biol. 2011, 11, 163. [Google Scholar] [CrossRef]
- Haak, D.C.; Fukao, T.; Grene, R.; Hua, Z.; Ivanov, R.; Perrella, G.; Li, S. Multilevel regulation of abiotic stress responses in plants. Front. Plant Sci. 2017, 8, 1564. [Google Scholar] [CrossRef]
- Nihorimbere, V.; Ongena, M.; Smargiassi, M.; Thonart, P. Beneficial effect of the rhizosphere microbial community for plant growth and health. Biotechnol. Agron. Soc. Environ. 2011, 15, 327–337. [Google Scholar]
- Walker, T.S.; Bais, H.P.; Grotewold, E.; Vivanco, J.M. Root exudation and rhizosphere biology. Plant Physiol. 2003, 132, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Gao, H.; Fang, X.; Ye, L.; Zhou, Y.; Yang, H. Effects of allyl isothiocyanate treatment on postharvest quality and the activities of antioxidant enzymes of mulberry fruit. Postharvest Biol. Technol. 2015, 108, 61–67. [Google Scholar] [CrossRef]
- Solovchenko, A.; Schmitz-Eiberger, M. Significance of skin flavonoids for UV-B-protection in apple fruits. J. Exp. Bot. 2003, 54, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Mariz-Ponte, N.; Mendes, R.; Sario, S.; de Oliveira, J.F.; Melo, P.; Santos, C. Tomato plants use non-enzymatic antioxidant pathways to cope with moderate UV-A/B irradiation: A contribution to the use of UV-A/B in horticulture. J. Plant Physiol. 2018, 221, 32–42. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Solovchenko, A.E.; Gitelson, A.A. Reflectance spectral features and non-destructive estimation of chlorophyll, carotenoid and anthocyanin content in apple fruit. Postharvest Biol. Technol. 2003, 27, 197–211. [Google Scholar] [CrossRef]
- Schmitz-Hoerner, R.; Weissenböck, G. Contribution of phenolic compounds to the UV-B screening capacity of developing barley primary leaves in relation to DNA damage and repair under elevated UV-B levels. Phytochemistry 2003, 64, 243–255. [Google Scholar] [CrossRef]
- Tossi, V.; Lombardo, C.; Cassia, R.; Lamattina, L. RETRACTED: Nitric oxide and flavonoids are systemically induced by UV-B in maize leaves. Plant Sci. 2012, 193–194, 103–109. [Google Scholar] [CrossRef]
- Pathan, M.S.; Lee, J.-D.; Shannon, J.G.; Nguyen, H.T. Recent advances in breeding for drought and salt stress tolerance in soybean. In Advances in Molecular Breeding Toward Drought and Salt Tolerant Crops; Jenks, M.A., Hasegawa, P.M., Jain, S.M., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 739–773. [Google Scholar] [CrossRef]
- Ilangumaran, G.; Smith, D.L. Plant growth promoting rhizobacteria in amelioration of salinity stress: A systems biology perspective. Front. Plant Sci. 2017, 8, 1768. [Google Scholar] [CrossRef]
- Greenway, H.; Munns, R. Mechanisms of salt tolerance in nonhalophytes. Ann. Rev. Plant Physiol. 1980, 31, 149–190. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Perri, S.; Entekhabi, D.; Molini, A. Plant osmoregulation as an emergent water-saving adaptation. Water Resour. Res. 2018, 54, 2781–2798. [Google Scholar] [CrossRef]
- Bernstein, N. Plants and salt: Plant response and adaptations to salinity. In Model Ecosystems in Extreme Environments; Elsevier: Amsterdam, The Netherlands, 2019; pp. 101–112. [Google Scholar]
- Jones, G.W.; Gorham, J. Intra-and Inter-Cellular Compartmentation of Ions. In Salinity: Environment-Plants-Molecules; Springer: Dordrecht, The Netherlands, 2002; pp. 159–180. [Google Scholar]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Ann. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Koyro, H.-W. Effect of salinity on growth, photosynthesis, water relations and solute composition of the potential cash crop halophyte Plantago coronopus (L.). Environ. Exp. Bot. 2006, 56, 136–146. [Google Scholar] [CrossRef]
- Rhodes, D.; Nadolska-Orczyk, A.; Rich, P. Salinity, osmolytes and compatible solutes. In Salinity: Environment-Plants-Molecules; Springer: Dordrecht, The Netherlands, 2002; pp. 181–204. [Google Scholar]
- Al Hassan, M.; MartÍNez Fuertes, M.; Ramos SÁNchez, F.J.; Vicente, O.; Boscaiu, M. Effects of salt and water stress on plant growth and on accumulation of osmolytes and antioxidant compounds in cherry tomato. Not. Bot. Horti Agrobot. Cluj Napoca 2015, 43, 1–11. [Google Scholar] [CrossRef]
- AbdElgawad, H.; Zinta, G.; Hegab, M.M.; Pandey, R.; Asard, H.; Abuelsoud, W. High salinity induces different oxidative stress and antioxidant responses in maize seedlings organs. Front. Plant Sci. 2016, 7, 276. [Google Scholar] [CrossRef]
- Wang, H.-M.; Xiao, X.-R.; Yang, M.-Y.; Gao, Z.-L.; Zang, J.; Fu, X.-M.; Chen, Y.-H. Effects of salt stress on antioxidant defense system in the root of Kandelia candel. Bot. Stud. 2014, 55, 57. [Google Scholar] [CrossRef]
- Abogadallah, G.M. Antioxidative defense under salt stress. Plant Signal. Behav. 2010, 5, 369–374. [Google Scholar] [CrossRef]
- Engwa, G.A. Free radicals and the role of plant phytochemicals as antioxidants against oxidative stress-related diseases. In Phytochemicals: Source of Antioxidants and Role in Disease Prevention. BoD–Books on Demand; Intechopen: London, UK, 2018; pp. 49–74. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Smith, A.M.; Ratcliffe, R.G.; Sweetlove, L.J. Activation and function of mitochondrial uncoupling protein in plants. J. Biol. Chem. 2004, 279, 51944–51952. [Google Scholar] [CrossRef]
- Pietta, P.-G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Lü, J.M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell. Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef] [PubMed]
- Di Ferdinando, M.; Brunetti, C.; Fini, A.; Tattini, M. Flavonoids as antioxidants in plants under abiotic stresses. In Abiotic Stress Responses in Plants; Springer: New York, NY, USA, 2012; pp. 159–179. [Google Scholar] [CrossRef]
- Kaliamoortiiy, S.; Rao, A. Effect of salinity on anthocyanin accumulation in the root of maize. Science 1994, 248, 1637–1638. [Google Scholar]
- Sugiyama, A.; Yazaki, K. Flavonoids in plant rhizospheres: Secretion, fate and their effects on biological communication. Plant Biotechnol. 2014, 31, 431–443. [Google Scholar] [CrossRef]
- Miransari, M.; Smith, D. Overcoming the stressful effects of salinity and acidity on soybean nodulation and yields using signal molecule genistein under field conditions. J. Plant Nutr. 2007, 30, 1967–1992. [Google Scholar] [CrossRef]
- Miransari, M.; Smith, D. Alleviating salt stress on soybean (Glycine max (L.) Merr.)–Bradyrhizobium japonicum symbiosis, using signal molecule genistein. Eur. J. Soil Biol. 2009, 45, 146–152. [Google Scholar] [CrossRef]
- Ma, D.; Sun, D.; Wang, C.; Li, Y.; Guo, T. Expression of flavonoid biosynthesis genes and accumulation of flavonoid in wheat leaves in response to drought stress. Plant Physiol. Biochem. 2014, 80, 60–66. [Google Scholar] [CrossRef]
- Varela, M.C.; Arslan, I.; Reginato, M.A.; Cenzano, A.M.; Luna, M.V. Phenolic compounds as indicators of drought resistance in shrubs from Patagonian shrublands (Argentina). Plant Physiol. Biochem. 2016, 104, 81–91. [Google Scholar] [CrossRef]
- Koes, R.E.; Quattrocchio, F.; Mol, J.N. The flavonoid biosynthetic pathway in plants: Function and evolution. BioEssays 1994, 16, 123–132. [Google Scholar] [CrossRef]
- Maddox, C.E.; Laur, L.M.; Tian, L. Antibacterial activity of phenolic compounds against the phytopathogen Xylella fastidiosa. Curr. Microbiol. 2010, 60, 53. [Google Scholar] [CrossRef]
- Rattan, R.S. Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Prot. 2010, 29, 913–920. [Google Scholar] [CrossRef]
- Kaplan, D.; Keen, N.; Thomason, I. Studies on the mode of action of glyceollin in soybean incompatibility to the root knot nematode, Meloidogyne incognita. Physiol. Plant Pathol. 1980, 16, 319–325. [Google Scholar] [CrossRef]
- Chin, S.; Behm, C.A.; Mathesius, U. Functions of flavonoids in plant(-)nematode interactions. Plants (Basel) 2018, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-S.; Barker, K.R. Glyceollin I in soybean-cyst nematode interactions: Spatial and temporal distribution in roots of resistant and susceptible soybeans. Plant Physiol. 1991, 96, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Soriano, I.; Asenstorfer, R.; Schmidt, O.; Riley, I. Inducible flavone in oats (Avena sativa) is a novel defense against plant-parasitic nematodes. Phytopathology 2004, 94, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Fawe, A.; Abou-Zaid, M.; Menzies, J.; Bélanger, R. Silicon-mediated accumulation of flavonoid phytoalexins in cucumber. Phytopathology 1998, 88, 396–401. [Google Scholar] [CrossRef]
- del Río, J.A.; Gómez, P.; Baidez, A.G.; Arcas, M.C.; Botía, J.M.; Ortuño, A. Changes in the levels of polymethoxyflavones and flavanones as part of the defense mechanism of citrus sinensis (Cv. Valencia Late) fruits against phytophthora citrophthora. J. Agric. Food Chem. 2004, 52, 1913–1917. [Google Scholar] [CrossRef]
- Ortuno, A.; Arcas, M.; Botia, J.; Fuster, M.; Del Río, J. Increasing resistance against Phytophthora citrophthora in tangelo Nova fruits by modulating polymethoxyflavones levels. J. Agric. Food Chem. 2002, 50, 2836–2839. [Google Scholar] [CrossRef]
- Boué, S.M.; Carter, C.H.; Ehrlich, K.C.; Cleveland, T.E. Induction of the soybean phytoalexins coumestrol and glyceollin by Aspergillus. J. Agric. Food Chem. 2000, 48, 2167–2172. [Google Scholar] [CrossRef]
- Cushnie, T.P.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef]
- Gnanamanickam, S.S.; Patil, S.S. Accumulation of antibacterial isoflavonoids in hypersensitively responding bean leaf tissues inoculated with Pseudomonas phaseolicola. Physiol. Plant Pathol. 1977, 10, 159–168. [Google Scholar] [CrossRef]
- Lyon, F.M.; Wood, R. Production of phaseollin, coumestrol and related compounds in bean leaves inoculated with Pseudomonas spp. Physiol. Plant Pathol. 1975, 6, 117–124. [Google Scholar] [CrossRef]
- Keen, N.; Kennedy, B. Hydroxyphaseollin and related isoflavanoids in the hypersensitive resistance reaction of soybeans to Pseudomonas glycinea. Physiol. Plant Pathol. 1974, 4, 173–185. [Google Scholar] [CrossRef]
- Wyman, J.G. Antibacterial activity of selected isoflavonoids. Phytopathology 1978, 68, 583. [Google Scholar] [CrossRef]
- Tanaka, H.; Sato, M.; Oh-Uchi, T.; Yamaguchi, R.; Etoh, H.; Shimizu, H.; Sako, M.; Takeuchi, H. Antibacterial properties of a new isoflavonoid from Erythrina poeppigiana against methicillin-resistant Staphylococcus aureus. Phytomedicine 2004, 11, 331–337. [Google Scholar] [CrossRef] [PubMed]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.-M.; Zhu, S.; Kachroo, P.; Kachroo, A. Signal regulators of systemic acquired resistance. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef]
- Tridiptasari, A.; Brawijaya, U.; Leksono, A.S.; Siswanto, D. Antifeedant Effect of Moringa oleifera (L.) Leaf and Seed Extract on Growth and Feeding Activity of Spodoptera litura (Fab.) (Lepidoptera: Noctuidae). J. Exp. Life Sci. 2019, 9, 25–31. [Google Scholar] [CrossRef]
- Aljbory, Z.; Chen, M.S. Indirect plant defense against insect herbivores: A review. Insect Sci. 2018, 25, 2–23. [Google Scholar] [CrossRef]
- Rosell, G.; Quero, C.; Coll, J.; Guerrero, A. Biorational insecticides in pest management. J. Pestic. Sci. 2008, 33, 103–121. [Google Scholar] [CrossRef]
- Quiroz, A.; Mendez, L.; Mutis, A.; Hormazabal, E.; Ortega, F.; Birkett, M.A.; Parra, L. Antifeedant activity of red clover root isoflavonoids on Hylastinus obscurus. J. Soil Sci. Plant Nutr. 2017, 17, 231–239. [Google Scholar] [CrossRef]
- Goławska, S.; Lukasik, I. Antifeedant activity of luteolin and genistein against the pea aphid, Acyrthosiphon pisum. J. Pest. Sci. (2004) 2012, 85, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.T.; Dowd, P.F. Differentially enhanced insect resistance, at a cost, in Arabidopsis thaliana constitutively expressing a transcription factor of defensive metabolites. J. Agric. Food Chem. 2004, 52, 5135–5138. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, M.S. Importance of flavonoids in insect–plant interactions: Feeding and oviposition. Phytochemistry 2001, 56, 245–252. [Google Scholar] [CrossRef]
- Lattanzio, V.; Lattanzio, V.M.; Cardinali, A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. Phytochem. Adv. Res. 2006, 661, 23–67. [Google Scholar]
- Nenaah, G.E. Toxic and antifeedant activities of prenylated flavonoids isolated from Tephrosia apollinea L. against three major coleopteran pests of stored grains with reference to their structure–activity relationship. Nat. Prod. Res. 2014, 28, 2245–2252. [Google Scholar] [CrossRef]
- Weston, L.A.; Duke, S.O. Weed and crop allelopathy. Crit. Rev. Plant Sci. 2003, 22, 367–389. [Google Scholar] [CrossRef]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2005, 144, 31–43. [Google Scholar] [CrossRef]
- Young, S.L.; Pierce, F.J. Automation: The Future of Weed Control; Springer Science and Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Molisch, H. Der Einfluss Einer Pflanze Auf die Andere, Allelopathie; Fischer: Jena, Germany, 1937. [Google Scholar]
- Rice, E. Allelopathy, 2nd ed.; Acad. Press Inc.: Orlando, FL, USA, 1984; p. 422. [Google Scholar]
- Rob, M.M.; Hossen, K.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic activity and identification of phytotoxic substances from schumannianthus dichotomus. Plants 2020, 9, 102. [Google Scholar] [CrossRef]
- Jabran, K.; Mahajan, G.; Sardana, V.; Chauhan, B.S. Allelopathy for weed control in agricultural systems. Crop Prot. 2015, 72, 57–65. [Google Scholar] [CrossRef]
- Alsaadawi, I.S.; Sarbout, A.K.; Al-Shamma, L.M. Differential allelopathic potential of sunflower (Helianthus annuus L.) genotypes on weeds and wheat (Triticum aestivum L.) crop. Arch. Agron. Soil Sci. 2012, 58, 1139–1148. [Google Scholar] [CrossRef]
- Palma-Tenango, M.; Soto-Hernández, M.; Aguirre-Hernández, E. Flavonoids in agriculture. In Flavonoids—From Biosynthesis to Human Health; Intech: Rijeka, Croatia, 2017. [Google Scholar] [CrossRef]
- Weston, L.A.; Mathesius, U. Flavonoids: Their structure, biosynthesis and role in the rhizosphere, including allelopathy. J. Chem. Ecol. 2013, 39, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gao, J.-M.; Liu, W.-T.; Tang, J.-C.; Zhang, X.-C.; Jin, Z.-G.; Xu, Y.-P.; Shao, M.-A. Allelopathic substances from walnut (Juglans regia L.) leaves. Allelopath. J. 2008, 21, 425–431. [Google Scholar]
- Yan, Z.; Guo, H.; Yang, J.; Liu, Q.; Jin, H.; Xu, R.; Cui, H.; Qin, B. Phytotoxic flavonoids from roots of Stellera chamaejasme L. (Thymelaeaceae). Phytochemistry 2014, 106, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Kaab, S.B.; Rebey, I.B.; Hanafi, M.; Hammi, K.M.; Smaoui, A.; Fauconnier, M.L.; De Clerck, C.; Jijakli, M.H.; Ksouri, R. Screening of Tunisian plant extracts for herbicidal activity and formulation of a bioherbicide based on Cynara cardunculus. S. Afr. J. Bot. 2020, 128, 67–76. [Google Scholar] [CrossRef]
- Al-obaidi, A.F. Phytotoxicity of plantago major extracts on germination and seedling growth of purslane (portulaca oleracea). In Seed Dormancy and Germination; IntechOpen: London, UK, 2020. [Google Scholar]




| Compound | Flavonoid Class | Structure Substitution | Food Sources | Role in Plants | References | |
|---|---|---|---|---|---|---|
| 1 | Quercetin | Flavonols | 3,5,7,3′,4′-OH | Onions, apples, berries | Antioxidant, Allelopathic | [38,39] |
| 2 | Kaempferol | Flavonols | 3,5,7,4′-OH | Tea, broccoli, cabbage, beans, tomato, strawberries and grapes | Antioxidant, antibacterial, insect repellent, abiotic stress mitigation | [40,41,42,43] |
| 3 | Chrysin | Flavones | 5,7-OH | Honey, propolis | Antioxidant, UV-A/B Resistance, AMF symbiosis | [40,44,45] |
| 4 | Apigenin | Flavones | 5,7,4′-OH | Parsley, Pepper | Antioxidant, AMF spore germination (symbiosis), phytoalexin | [46,47] |
| 5 | Naringenin | Flavanone | 5,7,4′-OH | Grape, apple, orange | AntioxidantAMF Hyphal growth (Symbiosis) | [25,48] |
| 6 | Hesperidin | Flavanone | 5,3′-OH, 4′OMe, 7-rutinose | Citrus, orange juice | Antioxidant | [25,49] |
| 7 | Genistein | Isoflavonoid | 5,7,4′-OH | Currants, raisin, legumes | Nodule induction, signaling | [24,46] |
| 8 | Daidzein | Isoflavonoid | 7,4′-OH | Currants, raisin, legumes | Nodule induction, signaling, chelation | [46,50] |
| 9 | Apigeninidin | Anthocyanin | 7,4′-OH | Flowers, fruits | Color pigmentation, pollinator attractant, UV-B absorber | [19,25,51] |
| 10 | Fisetin | Flavonols | 3,7,4′,5′-OH | Apple, strawberry, onion, cucumber | Antioxidant | [25,52] |
| 11 | Myricetin | Flavonols | 3,5,7,3′,4′,5′-OH | Berries, tea, wine | Antioxidant | [53] |
| 12 | Luteolin | Flavones | 5,7,3′,4′-OH | Broccoli, chilli, onion leaves bilimbi fruit and leaves, carrot, local celery | Nod gene inducer | [54] |
| 13 | Rutin | Flavones | 5,7,3′,4′-OH, 3-rutinose | Parsley, Pepper, carrot | Mycorrhizae symbiosis, abiotic stress mitigation | [22,55] |
| 14 | (+)-catechin | Flavanol | 3,5,7,3′,5′-OH | Grapes, pears, apples | Antioxidant, ROS scavengers | [26,56,57] |
| 15 | (−)-epicatechin. | Flavanol | 3,5,7,3′,5′-OH | Strawberry, apple | Antioxidant | [56,57] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, A.; Smith, D.L. Flavonoids in Agriculture: Chemistry and Roles in, Biotic and Abiotic Stress Responses, and Microbial Associations. Agronomy 2020, 10, 1209. https://doi.org/10.3390/agronomy10081209
Shah A, Smith DL. Flavonoids in Agriculture: Chemistry and Roles in, Biotic and Abiotic Stress Responses, and Microbial Associations. Agronomy. 2020; 10(8):1209. https://doi.org/10.3390/agronomy10081209
Chicago/Turabian StyleShah, Ateeq, and Donald L. Smith. 2020. "Flavonoids in Agriculture: Chemistry and Roles in, Biotic and Abiotic Stress Responses, and Microbial Associations" Agronomy 10, no. 8: 1209. https://doi.org/10.3390/agronomy10081209
APA StyleShah, A., & Smith, D. L. (2020). Flavonoids in Agriculture: Chemistry and Roles in, Biotic and Abiotic Stress Responses, and Microbial Associations. Agronomy, 10(8), 1209. https://doi.org/10.3390/agronomy10081209




