Effect of Waterlogging Stress on Dry Matter Accumulation, Photosynthesis Characteristics, Yield, and Yield Components in Three Different Ecotypes of Peanut (Arachis hypogaea L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Determination of Dry Matter Accumulation
2.3. Determination of SPAD Value and Gas Exchange Parameters
2.4. Determination of Yield and Yield Components
2.5. Statistical Analysis
3. Results
3.1. Waterlogging-Stress Tolerance Index
3.2. Dry Matter Accumulation
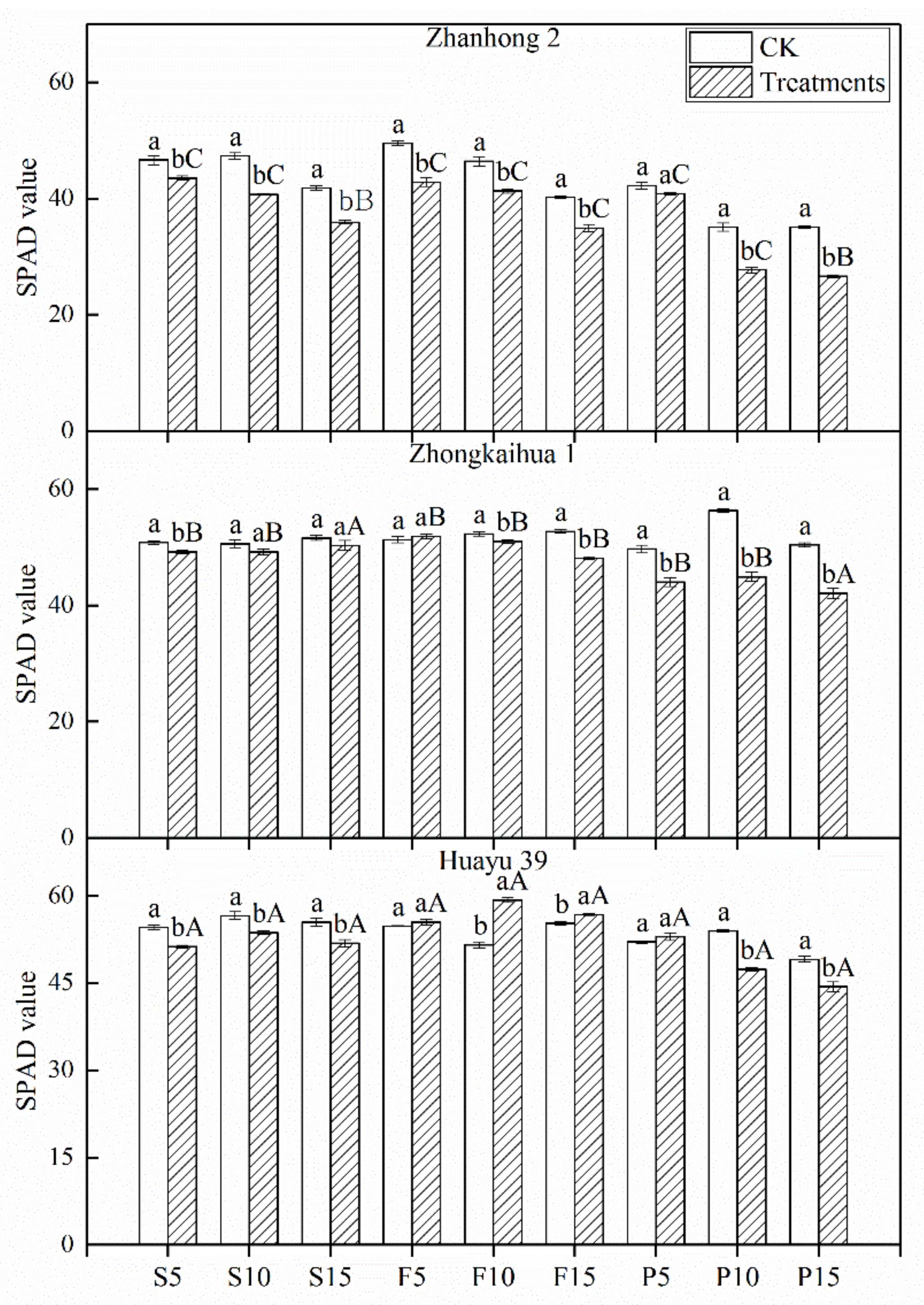
3.3. SPAD Value
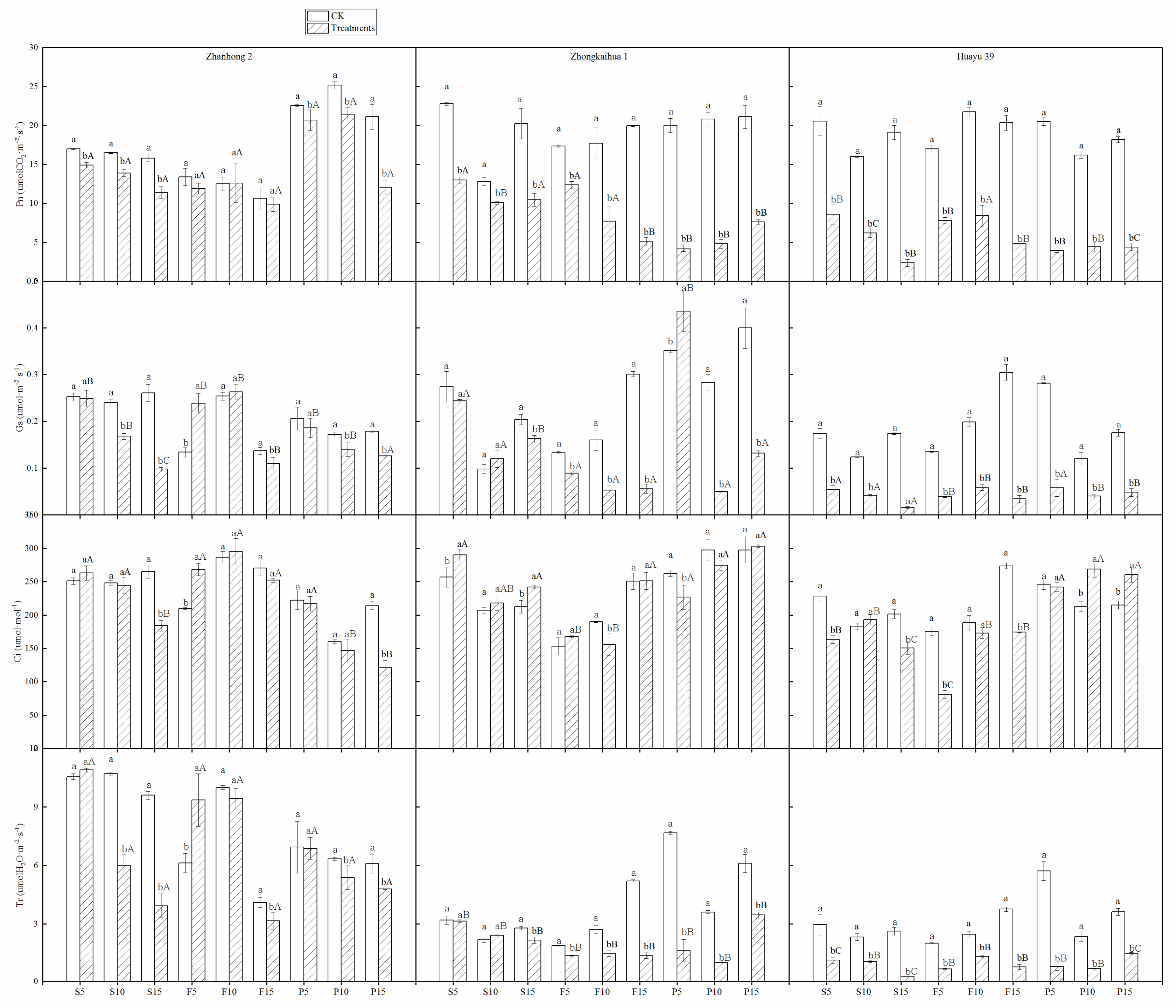
3.4. Photosynthetic Parameters
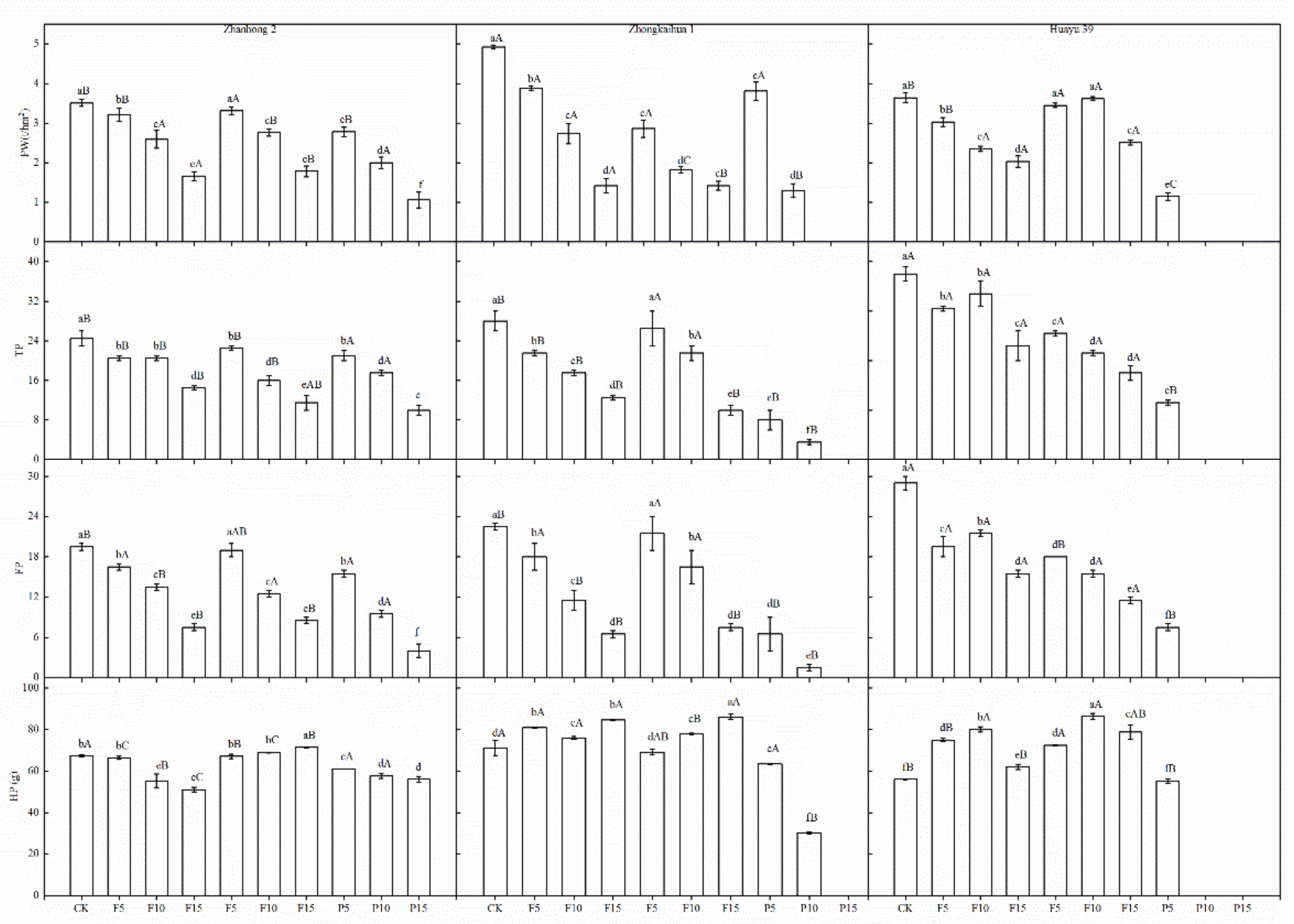
3.5. Yield and Yield Components
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ploschuk, R.A.; Miralles, D.J.; Colmer, T.D.; Ploschuk, E.L.; Striker, G.G. Waterlogging of winter crops at early and late stages: Impacts on leaf physiology, growth and yield. Front. Plant Sci. 2018, 9, 1863. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Serres, J.; Lee, S.C.; Brinton, E. Waterproofing Crops: Effective flooding survival strategies. Plant Physiol. 2012, 160, 1698–1709. [Google Scholar] [CrossRef] [PubMed]
- Khera, P.; Pandey, M.K.; Mallikarjuna, N.; Sriswathi, M.; Roorkiwal, M.; Janila, P.; Sharma, S.; Shilpa, K.; Sudini, H.; Guo, B.; et al. Genetic imprints of domestication for disease resistance, oil quality, and yield component traits in groundnut (Arachis hypogaea L.). Mol. Genet. Genom. 2019, 294, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Fukao, T.; Barrera-Figueroa, B.E.; Juntawong, P.; Peña-Castro, J.M. Submergence and waterlogging stress in plants: A review highlighting research opportunities and understudied aspects. Front. Plant Sci. 2019, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef]
- Arduini, I.; Baldanzi, M.; Pampana, S. Reduced growth and nitrogen uptake during waterlogging at tillering permanently affect yield components in late sown oats. Front. Plant Sci. 2019, 10, 1087. [Google Scholar] [CrossRef]
- Ahmed, S.; Nawata, E.; Hosokawa, M.; Domae, Y.; Sakuratani, T. Alterations in photosynthesis and some antioxidant enzymatic activities of mungbean subjected to waterlogging. Plant Sci. 2002, 163, 117–123. [Google Scholar] [CrossRef]
- Ahmed, S.; Nawata, E.; Sakuratani, T. Changes of endogenous ABA and ACC, and their correlations to photosynthesis and water relations in mungbean (Vigna radiata (L.) Wilczak cv. KPS1) during waterlogging. Environ. Exp. Bot. 2006, 57, 278–284. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, X.; Yang, G.; Li, Z.; Lu, H.; Kong, X.; Eneji, A.E.; Dong, H. Physiological and molecular adjustment of cotton to waterlogging at peak-flowering in relation to growth and yield. Field Crops Res. 2015, 179, 164–172. [Google Scholar] [CrossRef]
- Arguello, M.N.; Mason, R.E.; Roberts, T.L.; Subramanian, N.; Acuña, A.; Addison, C.K.; Lozada, D.N.; Miller, R.G.; Gbur, E. Performance of soft red winter wheat subjected to field soil waterlogging: Grain yield and yield components. Field Crops Res. 2016, 194, 57–64. [Google Scholar] [CrossRef]
- Grassini, P.; Indaco, G.V.; Pereira, M.L.; Hall, A.J.; Trápani, N. Responses to short-term waterlogging during grain filling in sunflower. Field Crops Res. 2007, 101, 352–363. [Google Scholar] [CrossRef]
- De San Celedonio, R.P.; Abeledo, L.G.; Miralles, D.J. Identifying the critical period for waterlogging on yield and its components in wheat and barley. Plant Soil 2014, 378, 265–277. [Google Scholar] [CrossRef]
- Tian, L.; Li, J.; Bi, W.; Zuo, S.; Li, L.; Li, W.; Sun, L. Effects of waterlogging stress at different growth stages on the photosynthetic characteristics and grain yield of spring maize (Zea mays L.) under field conditions. Agric. Water Manag. 2019, 218, 250–258. [Google Scholar] [CrossRef]
- Ren, B.; Zhang, J.; Dong, S.; Liu, P.; Zhao, B. Exogenous 6-benzyladenine improves antioxidative system and carbon metabolism of summer maize waterlogged in the field. J. Agron. Crop Sci. 2018, 204, 175–184. [Google Scholar] [CrossRef]
- Wollmer, A.C.; Pitann, B.; Mühling, K.H. Waterlogging events during stem elongation or flowering affect yield of oilseed rape (Brassica napus L.) but not seed quality. J. Agron. Crop Sci. 2018, 204, 165–174. [Google Scholar] [CrossRef]
- Morishita, T.; Hara, T.; Hara, T. Important agronomic characteristics of yielding ability in common buckwheat; ecotype and ecological differentiation, preharvest sprouting resistance, shattering resistance, and lodging resistance. Breed. Sci. 2020, 70, 39–47. [Google Scholar] [CrossRef]
- Kreyling, J.; Puechmaille, S.J.; Malyshev, A.V.; Valladares, F. Phenotypic plasticity closely linked to climate at origin and resulting in increased mortality under warming and frost stress in a common grass. Ecol. Evol. 2018, 9, 1344–1352. [Google Scholar] [CrossRef]
- Guan, B.; Yu, J.; Hou, A.; Han, G.; Wang, G.; Qu, F.; Xia, J.; Wang, X. The ecological adaptability of Phragmites australis to interactive effects of water level and salt stress in the Yellow River Delta. Aquat. Ecol. 2017, 51, 107–116. [Google Scholar] [CrossRef]
- Shabala, S. Physiological and cellular aspects of phytotoxicity tolerance in plants: The role of membrane transporters and implications for crop breeding for waterlogging tolerance. New Phytol. 2011, 190, 289–298. [Google Scholar] [CrossRef]
- Yin, D.; Sun, D.; Han, Z.; Ni, D.; Norris, A.; Jiang, C. PhERF2, an ethylene-responsive element binding factor, plays an essential role in waterlogging tolerance of petunia. Hortic. Res. Engl. 2019, 6, 1–11. [Google Scholar] [CrossRef]
- Akhtar, N.; Hameed, M.; Nawaz, F.; Ahmad, K.S.; Hamid, A.; Segovia-Salcedo, C.; Shahnaz, M.M. Leaf anatomical and biochemical adaptations in Typha domingensis Pers. ecotypes for salinity tolerance. Bot. Sci. 2017, 95, 807. [Google Scholar] [CrossRef]
- Hammami, H.; Saadatian, B.; Aliverdi, A. Geographical variation in breaking the seed dormancy of Persian cumin (Carum carvi L.) ecotypes and their physiological responses to salinity and drought stresses. Ind. Crop Prod. 2018, 124, 600–606. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Liu, M.; Cao, B.; Tan, H.; Wang, J.; Li, X. Responses of three different ecotypes of reed (Phragmites communis Trin.) to their natural habitats: Leaf surface micro-morphology, anatomy, chloroplast ultrastructure and physio-chemical characteristics. Plant Physiol. Biochem. 2012, 51, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Luan, H.; Guo, B.; Pan, Y.; Lv, C.; Shen, H.; Xu, R. Morpho-anatomical and physiological responses to waterlogging stress in different barley (Hordeum vulgare L.) genotypes. Plant Growth Regul. 2018, 85, 399–409. [Google Scholar] [CrossRef]
- Yin, D.; Chen, S.; Chen, F.; Guan, Z.; Fang, W. Morpho-anatomical and physiological responses of two Dendranthema species to waterlogging. Environ. Exp. Bot. 2010, 68, 122–130. [Google Scholar] [CrossRef]
- Yu, F.; Liang, K.; Fang, T.; Zhao, H.; Han, X.; Cai, M.; Qiu, F. A group VII ethylene response factor gene, ZmEREB180, coordinates waterlogging tolerance in maize seedlings. Plant Biotechnol. J. 2019, 17, 2286–2298. [Google Scholar] [CrossRef]
- Krishnamurthy, S.L.; Gautam, R.K.; Sharma, P.C.; Sharma, D.K. Effect of different salt stresses on agro-morphological traits and utilisation of salt stress indices for reproductive stage salt tolerance in rice. Field Crops Res. 2016, 190, 26–33. [Google Scholar] [CrossRef]
- Mann, A.; Kaur, G.; Kumar, A.; Sanwal, S.K.; Singh, J.; Sharma, P.C. Physiological response of chickpea (Cicer arietinum L.) at early seedling stage under salt stress conditions. Legume Res. 2019, 42, 625–632. [Google Scholar] [CrossRef]
- Erda, L.; Wei, X.; Hui, J.; Yinlong, X.; Yue, L.; Liping, B.; Liyong, X. Climate change impacts on crop yield and quality with CO2 fertilization in China. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 2149–2154. [Google Scholar] [CrossRef]
- Sultan, B.; Defrance, D.; Iizumi, T. Evidence of crop production losses in West Africa due to historical global warming in two crop models. Sci. Rep. 2019, 9, 12834–12915. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Jiang, Y.; Duan, B.; Xi, Z. Involvement of ABA and antioxidant system in brassinosteroid-induced water stress tolerance of grapevine (Vitis vinifera L.). Sci. Hortic Amst. 2019, 256, 108596. [Google Scholar] [CrossRef]
- Barickman, T.C.; Simpson, C.R.; Sams, C.E. Waterlogging causes early modification in the physiological performance, carotenoids, chlorophylls, proline, and soluble sugars of cucumber plants. Plants 2019, 8, 160. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Dong, S.; Liu, P.; Zhao, B.; Zhang, J. Ridge tillage improves plant growth and grain yield of waterlogged summer maize. Agric. Water Manag. 2016, 177, 392–399. [Google Scholar] [CrossRef]
- Irving, L.J.; Sheng, Y.B.; Woolley, D.; Matthew, C. Physiological effects of waterlogging on two lucerne varieties grown under glasshouse conditions. J. Agron. Crop Sci. 2007, 193, 345–356. [Google Scholar] [CrossRef]
- Najeeb, U.; Bange, M.P.; Atwell, B.J.; Tan, D.K.Y. Low incident light combined with partial waterlogging impairs photosynthesis and imposes a yield penalty in cotton. J. Agron. Crop Sci. 2016, 202, 331–341. [Google Scholar] [CrossRef]
- Shah, S.H.; Houborg, R.; McCabe, M.F. Response of chlorophyll, carotenoid and SPAD-502 measurement to salinity and nutrient stress in wheat (Triticum aestivum L.). Agronomy 2017, 7, 61. [Google Scholar] [CrossRef]
- Tian, L.; Bi, W.; Liu, X.; Sun, L.; Li, J. Effects of waterlogging stress on the physiological response and grain-filling characteristics of spring maize (Zea mays L.) under field conditions. Acta Physiol. Plant 2019, 41, 63. [Google Scholar] [CrossRef]
- Ren, B.; Hu, J.; Zhang, J.; Dong, S.; Liu, P.; Zhao, B. Effects of urea mixed with nitrapyrin on leaf photosynthetic and senescence characteristics of summer maize (Zea mays L.) waterlogged in the field. J. Integr. Agric. 2020, 19, 1586–1595. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Li, Z.; Guo, H. Genetic network between leaf senescence and plant immunity: Crucial regulatory nodes and new insights. Plants 2020, 9, 495. [Google Scholar] [CrossRef]
- Malik, A.I.; Colmer, T.D.; Lambers, H.; Setter, T.L.; Schortemeyer, M. Short-term waterlogging has long-term effects on the growth and physiology of wheat. New Phytol. 2002, 153, 225–236. [Google Scholar] [CrossRef]
- Herzog, M.; Striker, G.G.; Colmer, T.D.; Pedersen, O. Mechanisms of waterlogging tolerance in wheat—A review of root and shoot physiology. Plant Cell Environ. 2016, 39, 1068–1086. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Araki, H.; Takahashi, T. Poor grain filling induced by waterlogging is similar to that in abnormal early ripening in wheat in Western Japan. Field Crops Res. 2011, 123, 100–108. [Google Scholar] [CrossRef]
- Wang, X.; Huang, M.; Zhou, Q.; Cai, J.; Dai, T.; Cao, W.; Jiang, D. Physiological and proteomic mechanisms of waterlogging priming improves tolerance to waterlogging stress in wheat (Triticum aestivum L.). Environ. Exp. Bot. 2016, 132, 175–182. [Google Scholar] [CrossRef]
- Yamauchi, T.; Colmer, T.D.; Pedersen, O.; Nakazono, M. Regulation of root traits for internal aeration and tolerance to soil waterlogging-flooding stress. Plant Physiol. 2018, 176, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.; Shabbir, G.; Ahmed, M.; Shah, K.N. Phenotyping for drought resistance in bread wheat using physiological and biochemical traits. Sci. Total Environ. 2020, 729, 139082. [Google Scholar] [CrossRef]
- Gould, B.A.; Chen, Y.; Lowry, D.B. Gene regulatory divergence between locally adapted ecotypes in their native habitats. Mol. Ecol. 2018, 27, 4174–4188. [Google Scholar] [CrossRef]
- Velikova, V.; Tsonev, T.; Tattini, M.; Arena, C.; Krumova, S.; Koleva, D.; Peeva, V.; Stojchev, S.; Todinova, S.; Izzo, L.G.; et al. Physiological and structural adjustments of two ecotypes of Platanus orientalis L. from different habitats in response to drought and re-watering. Conserv. Physiol. 2018, 6. [Google Scholar] [CrossRef]
- Baduel, P.; Arnold, B.; Weisman, C.M.; Hunter, B.; Bomblies, K. Habitat-associated life history and stress-tolerance variation in Arabidopsis arenosa. Plant Physiol. 2016, 171, 437–451. [Google Scholar] [CrossRef]
- Clark, J.S.; Poore, A.G.B.; Doblin, M.A. Shaping up for stress: Physiological flexibility is key to survivorship in a habitat-forming macroalga. J. Plant Physiol. 2018, 231, 346–355. [Google Scholar] [CrossRef]
- Gill, M.B.; Zeng, F.; Shabala, L.; Böhm, J.; Zhang, G.; Zhou, M.; Shabala, S. The ability to regulate voltage-gated K+-permeable channels in the mature root epidermis is essential for waterlogging tolerance in barley. J. Exp. Bot. 2018, 69, 667–680. [Google Scholar] [CrossRef]
- Nishiuchi, S.; Yamauchi, T.; Takahashi, H.; Kotula, L.; Nakazono, M. Mechanisms for coping with submergence and waterlogging in rice. Rice 2012, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Bashar, K.K. Hormone dependent survival mechanisms of plants during post-waterlogging stress. Plant Signal. Behav. 2018, 13, e1529522. [Google Scholar] [CrossRef] [PubMed]


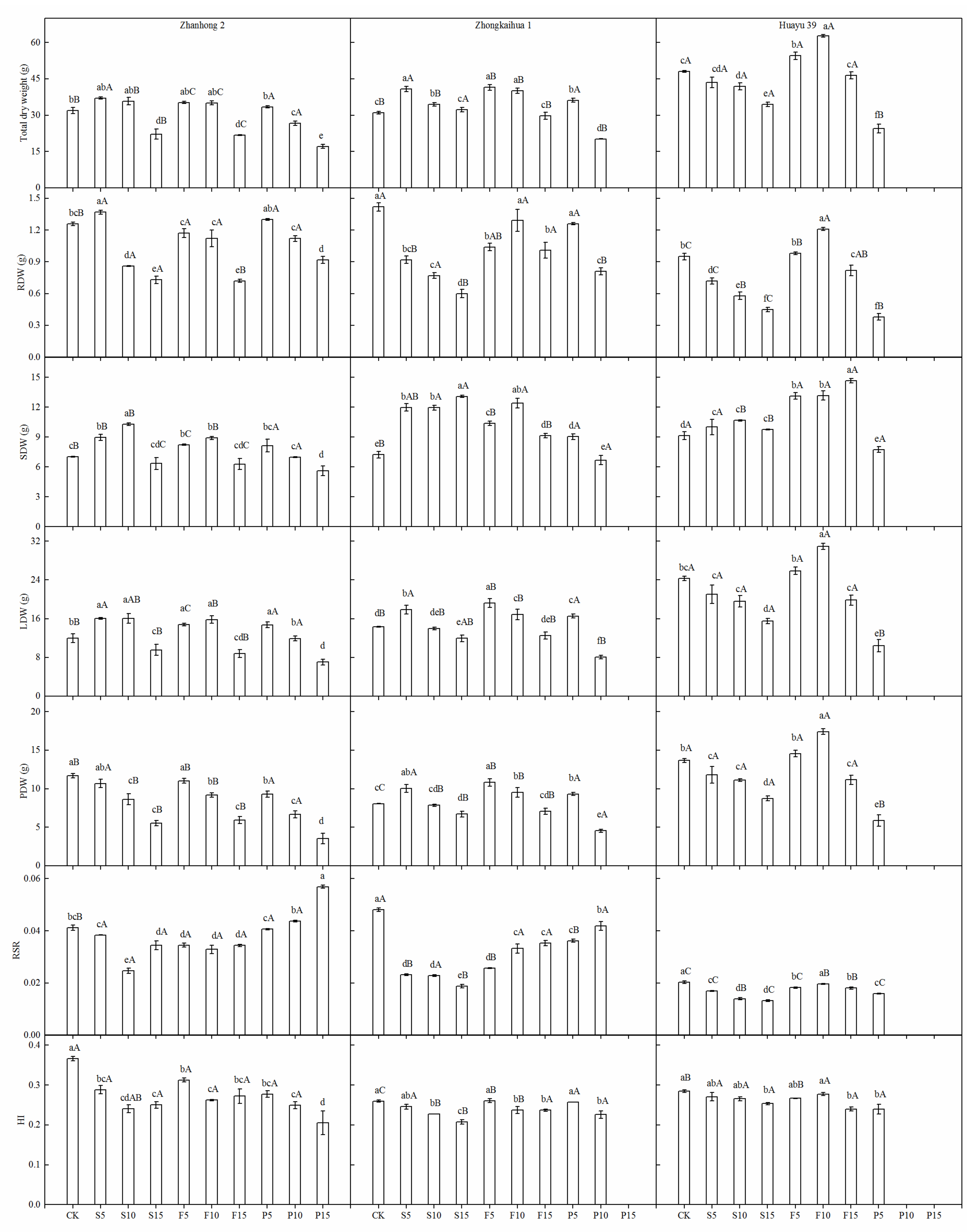
 trait vectors with a narrow angle between them indicate that a positive correlation exists between the traits (the narrower the angle, the greater the correlation); (2)
trait vectors with a narrow angle between them indicate that a positive correlation exists between the traits (the narrower the angle, the greater the correlation); (2)  trait vectors perpendicular to each other indicate no correlation between the traits; and (3)
trait vectors perpendicular to each other indicate no correlation between the traits; and (3)  trait vectors in opposing directions indicate that a negative correlation exists between the traits.
trait vectors in opposing directions indicate that a negative correlation exists between the traits.
 trait vectors with a narrow angle between them indicate that a positive correlation exists between the traits (the narrower the angle, the greater the correlation); (2)
trait vectors with a narrow angle between them indicate that a positive correlation exists between the traits (the narrower the angle, the greater the correlation); (2)  trait vectors perpendicular to each other indicate no correlation between the traits; and (3)
trait vectors perpendicular to each other indicate no correlation between the traits; and (3)  trait vectors in opposing directions indicate that a negative correlation exists between the traits.
trait vectors in opposing directions indicate that a negative correlation exists between the traits.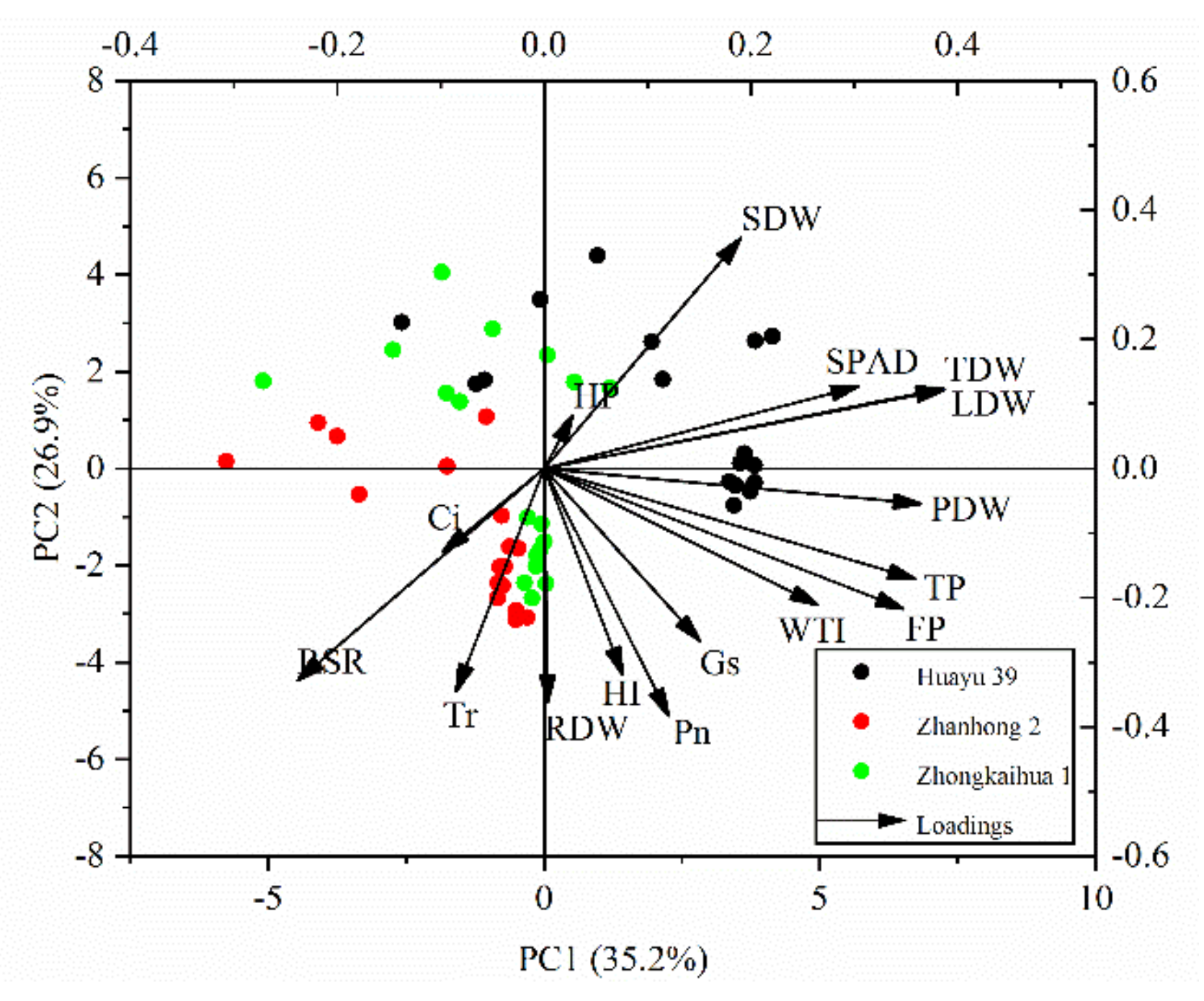
| Ecotypes | Variety | Variety Source | Localities | Geographic Coordinates | Annual Rainfall (mm) | Experimental Site Location | |
|---|---|---|---|---|---|---|---|
| Latitude N | Longitude E | ||||||
| Zhanhong 2 | Vulgaris | Zhanyou 30 × Wengyuanzhusi | Zhanjiang, Guangdong Province, PR China | 21°12′ N | 110°04′ E | 2031.7 | Guangzhou, Guangdong Province, PR China (23°15′ N, 113°36′ E) |
| Zhongkaihua 1 | Vulgaris | Zhanyou 41 × Yueyou 193 | Guangzhou, Guangdong Province, PR China | 23°06′ N | 113°15′ E | 1759.0 | |
| Huayu 39 | Vulgaris | Baisha 1016 × Flora | Jinan, Shandong Province, PR China | 36°40′ N | 117°00′ E | 880.0 | |
| Ecotypes | Abbreviation | Treatment | |
|---|---|---|---|
| Zhanhong 2 Zhongkaihua 1 Huayu 39 | CK | No waterlogging | |
| Treatments | S5 | Waterlogging for 5 days at the seedling stage | |
| S10 | Waterlogging for 10 days at the seedling stage | ||
| S15 | Waterlogging for 15 days at the seedling stage | ||
| F5 | Waterlogging for 5 days at the flowering and pegging stage | ||
| F10 | Waterlogging for 10 days at the flowering and pegging stage | ||
| F15 | Waterlogging for 15 days at the flowering and pegging stage | ||
| P5 | Waterlogging for 5 days at the pod-filling stage | ||
| P10 | Waterlogging for 10 days at the pod-filling stage | ||
| P15 | Waterlogging for 15 days at the pod-filling stage | ||
| Index | SPAD | Pn | Gs | Ci | Tr | TDW | RDW | SDW | LDW | PDW | RSR | HI | TP | FP | HP | WTI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SPAD | 1 | |||||||||||||||
| Pn | −0.065 | 1 | ||||||||||||||
| Gs | 0.02 | 0.502 | 1 | |||||||||||||
| Ci | −0.036 | 0.059 | −0.122 | 1 | ||||||||||||
| Tr | −0.425 | 0.465 | 0.268 | 0.408 | 1 | |||||||||||
| TDW | 0.644 | 0.033 | 0.191 | −0.249 | −0.265 | 1 | ||||||||||
| RDW | −0.061 | 0.543 | 0.317 | 0.188 | 0.475 | −0.113 | 1 | |||||||||
| SDW | 0.504 | −0.428 | −0.235 | −0.25 | −0.454 | 0.681 | −0.394 | 1 | ||||||||
| LDW | 0.657 | 0.075 | 0.175 | −0.261 | −0.337 | 0.973 | −0.135 | 0.596 | 1 | |||||||
| PDW | 0.456 | 0.216 | 0.415 | −0.142 | 0.069 | 0.872 | 0.081 | 0.368 | 0.797 | 1 | ||||||
| RSR | −0.472 | 0.37 | 0.116 | 0.207 | 0.461 | −0.694 | 0.763 | −0.724 | −0.675 | −0.492 | 1 | |||||
| HI | −0.159 | 0.366 | 0.499 | 0.158 | 0.6 | 0.053 | 0.384 | −0.377 | −0.069 | 0.531 | 0.191 | 1 | ||||
| TP | 0.522 | 0.56 | 0.421 | −0.105 | 0.007 | 0.622 | 0.175 | 0.031 | 0.666 | 0.685 | −0.258 | 0.31 | 1 | |||
| FP | 0.523 | 0.603 | 0.421 | −0.007 | 0.08 | 0.581 | 0.306 | −0.032 | 0.616 | 0.681 | −0.157 | 0.378 | 0.978 | 1 | ||
| HP | 0.22 | −0.169 | −0.094 | −0.005 | −0.06 | 0.131 | 0.209 | 0.431 | 0.017 | 0.055 | 0.021 | −0.03 | −0.085 | −0.063 | 1 | |
| WTI | 0.511 | 0.606 | 0.335 | −0.094 | 0.023 | 0.36 | 0.38 | −0.108 | 0.433 | 0.37 | 0.044 | 0.132 | 0.719 | 0.755 | −0.035 | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, R.; Chen, L.; Wang, X.; Cao, J.; Li, X.; Xu, X.; Xia, Q.; Chen, T.; Zhang, L. Effect of Waterlogging Stress on Dry Matter Accumulation, Photosynthesis Characteristics, Yield, and Yield Components in Three Different Ecotypes of Peanut (Arachis hypogaea L.). Agronomy 2020, 10, 1244. https://doi.org/10.3390/agronomy10091244
Zeng R, Chen L, Wang X, Cao J, Li X, Xu X, Xia Q, Chen T, Zhang L. Effect of Waterlogging Stress on Dry Matter Accumulation, Photosynthesis Characteristics, Yield, and Yield Components in Three Different Ecotypes of Peanut (Arachis hypogaea L.). Agronomy. 2020; 10(9):1244. https://doi.org/10.3390/agronomy10091244
Chicago/Turabian StyleZeng, Ruier, Lei Chen, Xinyue Wang, Jing Cao, Xi Li, Xueyu Xu, Qing Xia, Tingting Chen, and Lei Zhang. 2020. "Effect of Waterlogging Stress on Dry Matter Accumulation, Photosynthesis Characteristics, Yield, and Yield Components in Three Different Ecotypes of Peanut (Arachis hypogaea L.)" Agronomy 10, no. 9: 1244. https://doi.org/10.3390/agronomy10091244
APA StyleZeng, R., Chen, L., Wang, X., Cao, J., Li, X., Xu, X., Xia, Q., Chen, T., & Zhang, L. (2020). Effect of Waterlogging Stress on Dry Matter Accumulation, Photosynthesis Characteristics, Yield, and Yield Components in Three Different Ecotypes of Peanut (Arachis hypogaea L.). Agronomy, 10(9), 1244. https://doi.org/10.3390/agronomy10091244






