Storage Conditions Deteriorate Cotton and Wheat Seeds Quality: An Assessment of Farmers’ Awareness in Pakistan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Seed Sampling, Temperature and Humidity Measurements
2.3. Knowledge and Perceptions Assessment
2.4. Germination Tests
2.5. Seed Moisture Content
2.6. Identification of Seed-Borne Pathogens
2.7. Data Analysis
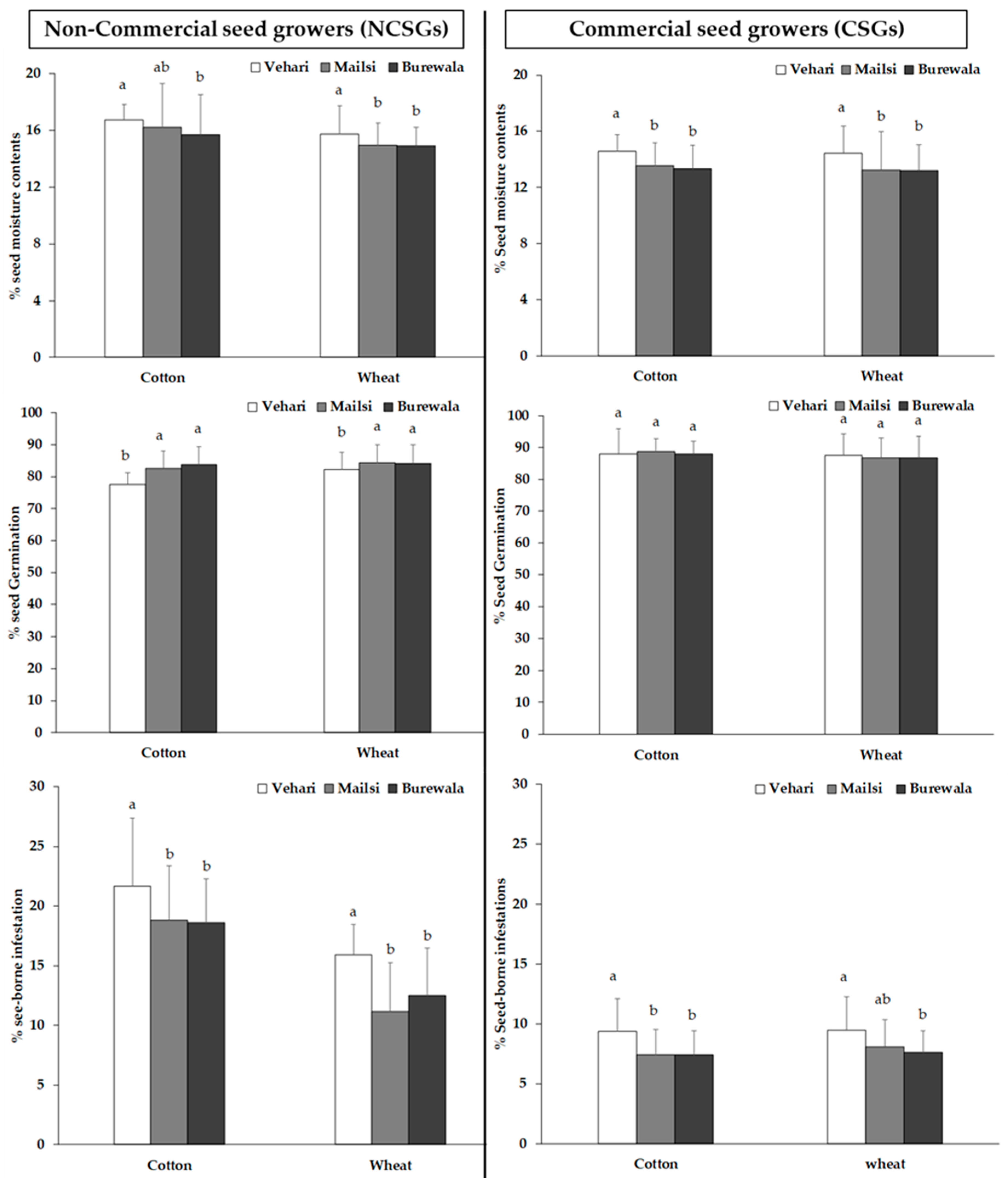
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Priestley, D.A. Seed Ageing: Implications for Seed Storage and Persistence in the Soil; Comstock: New York, NY, USA, 1986. [Google Scholar]
- Cappelli, A.; Guerrini, L.; Parenti, A.; Palladino, G.; Cini, E. Effects of wheat tempering and stone rotational speed on particle size, dough rheology and bread characteristics for a stone-milled weak flour. J. Cereal Sci. 2020, 91, 102879. [Google Scholar] [CrossRef]
- Afzal, I.; Kamran, M.; Basra, S.M.A.; Khan, S.H.U.; Mahmood, A.; Farooq, M.; Tan, D.K. Harvesting and post-harvest management approaches for preserving cottonseed quality. Ind. Crop. Prod. 2020, 155, 112842. [Google Scholar] [CrossRef]
- Heroldová, M.; Tkadlec, E. Harvesting behaviour of three central European rodents: Identifying the rodent pest in cereals. Crop. Prot. 2011, 30, 82–84. [Google Scholar] [CrossRef]
- Cappelli, A.; Cini, E.; Lorini, C.; Oliva, N.; Bonaccorsi, G. Insects as food: A review on risks assessments of Tenebrionidae and Gryllidae in relation to a first machines and plants development. Food Control. 2020, 108, 106877. [Google Scholar] [CrossRef]
- Moncini, L.; Simone, G.; Romi, M.; Cai, G.; Guerriero, G.; Whittaker, A.; Benedettelli, S.; Berni, R. Controlled nitrogen atmosphere for the preservation of functional molecules during silos storage: A case study using old Italian wheat cultivars. J. Stored Prod. Res. 2020, 88, 101638. [Google Scholar] [CrossRef]
- Delouche, J.C. Precepts of seed storage. Proc. Short Course Seedsmen Seed Technol. Lab. Miss. State Univ. 1973, 16, 30–34. [Google Scholar]
- Nagel, M.; Börner, A. The longevity of crop seeds stored under ambient conditions. Seed Sci. Res. 2010, 20. [Google Scholar] [CrossRef]
- De Vitis, M.; Hay, F.R.; Dickie, J.B.; Trivedi, C.; Choi, J.; Fiegener, R. Seed storage: Maintaining seed viability and vigor for restoration use. Restor. Ecol. 2020. [Google Scholar] [CrossRef]
- Mengistu, D.K. Farmers’ perception and knowledge on climate change and their coping strategies to the related hazards: Case study from Adiha, central Tigray, Ethiopia. Agric. Sci. 2011, 2, 138–145. [Google Scholar] [CrossRef] [Green Version]
- Wambugu, P.; Mathenge, P.; Auma, E.; Van Rheenen, H. Efficacy of traditional maize (Zea mays L.) seed storage methods in western Kenya. Afr. J. Food Agric. Nutr. Dev. 2009, 9. [Google Scholar] [CrossRef] [Green Version]
- Gari, J.A. Agro-Biodiversity Strategies to Combat Food Security and HIV/AIDS Impact in Rural Africa: Advancing Grassroots Responses for Nutrition, Health and Sustainable Livelihoods; FAO Population and Development Service: Rome, Italy, 2004. [Google Scholar]
- Delouche, J.C. Seed quality guidelines for the small farmer. In Improved Seed for the Small Farmer; CIAT (Centro Internacional de Agricultura Tropical): Cali, Colombia, 1982; pp. 26–29. [Google Scholar]
- Louwaars, N.P. Integrated Seed Supply: A flexible approach. In Production by Smallholder Farmers, Proceedings of the ILCA/ICARDA Research Planning Workshop, held at ILCA, Addis Ababa, Ethiopia, 13–15 June 1994; Hanson, J., Ed.; CIMMYT: Addis Ababa, Ethiopia, 1994; p. 58. [Google Scholar]
- Gwinner, J.; Harnisch, R.O. Mueck Manual of Handling and Conservation of Seeds after Harvest; GTZ: Eschborn, Germany, 1991. [Google Scholar]
- Food and Agriculture Organization (FAO). Crop Prospects and Food Situation. 2013. Available online: www.fao.org/docrep/019/aq119e/aq119e.pdf (accessed on 5 August 2020).
- Nukenine, E.N.; Adler, C.; Reichmuth, C. Bioactivity of fenchone and Plectranthus glandulosus oil against Prostephanus truncatus and two strains of Sitophilus zeamais. J. Appl. Entomol. 2010, 134, 132–141. [Google Scholar] [CrossRef]
- Kfir, R.; Overholt, W.A.; Khan, Z.R.; Polaszek, A. Biology and Management of Economically Important Lepidopteran Cereal Stem Borers in Africa. Annu. Rev. EÈntomol. 2002, 47, 701–731. [Google Scholar] [CrossRef] [PubMed]
- Ogendo, J.O.; Deng, A.L.; Belmain, S.R.; Walker, D.J.; Musandu, A.O.; Obura, R.K. Pest status of Sitophilus zeamais Motschulsky, control methods and constraints to safe maize grain storage in western Kenya. Egert. J. Sci. Tech. 2004, 5, 175–193. [Google Scholar]
- Ojo, J.A.; Omoloye, A.A. Rearing the maize weevil, Sitophilus zeamais, on an artificial maize-cassava diet. J. Insect Sci. 2012, 12, 69. [Google Scholar] [CrossRef] [Green Version]
- Tefera, T.; Kanampiu, F.; De Groote, H.; Hellin, J.; Mugo, S.; Kimenju, S.; Beyene, Y.; Prasanna, B.M.; Shiferaw, B.; Bänziger, M. The metal silo: An effective grain storage technology for reducing post-harvest insect and pathogen losses in maize while improving smallholder farmers’ food security in developing countries. Crop. Prot. 2010, 30, 240–245. [Google Scholar] [CrossRef]
- Bekele, A.J.; Obeng-Ofori, D.; Hassanali, A. Evaluation of Ocimum kenyense (Ayobangira) as a source of repellents toxicants and protectants in storage against three major stored product insect pests. J. Appl. Entomol. 1997, 121, 169–173. [Google Scholar] [CrossRef]
- Recchia, L.; Cappelli, A.; Cini, E.; Garbati Pegna, F.; Boncinelli, P. Environmental sustainability of pasta production chains: An integrated approach for comparing local and global chains. Resources 2019, 8, 56. [Google Scholar] [CrossRef] [Green Version]
- Morse, S.; Buhler, W. Integrated Pest Management: Ideals and Realities in Developing Countries; Lynne Rienner Publishers: Boulder, CO, USA, 1997. [Google Scholar]
- Delouche, J.C.; Matthes, R.K.; Dougherty, G.M.; Boyd, A.H. Storage of seeds in sub-tropical and tropical regions. Seed Sci. Technol. 1973, 1, 671–700. [Google Scholar]
- Van Huis, A.; Meerman, F. Can we make IPM work for resource-poor farmers in sub-Saharan Africa? Int. J. Pest Manag. 1997, 43, 313–320. [Google Scholar] [CrossRef]
- Norton, G.W.; Rajotte, E.G.; Gapud, V. Participatory research in integrated pest management: Lessons from the IPM CRSP. Agric. Hum. Values 1999, 16, 431–439. [Google Scholar] [CrossRef]
- Chitere, P.O.; Omolo, B.A. Farmers’ indigenous knowledge of crop pests and their damage in western Kenya. Int. J. Pest Manag. 1993, 39, 126–132. [Google Scholar] [CrossRef]
- Devkota, M.; Devkota, K.; Acharya, S.; Shrestha, R.; McDonald, A. Establishing the value of modern seed storage methods for wheat in diverse production ecologies in Nepal. J. Stored Prod. Res. 2018, 76, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Midega, C.A.O.; Murage, A.W.; Pittchar, J.O.; Khan, Z.R. Managing storage pests of maize: Farmers’ knowledge, perceptions and practices in western Kenya. Crop. Prot. 2016, 90, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Doblado-Maldonado, A.F.; Pike, O.A.; Sweley, J.C.; Rose, D.J. Key issues and challenges in whole wheat flour milling and storage. J. Cereal Sci. 2012, 56, 119–126. [Google Scholar] [CrossRef]
- Cappelli, A.; Oliva, N.; Cini, E. Stone milling versus roller milling: A systematic review of the effects on wheat flour quality, dough rheology, and bread characteristics. Trends Food Sci. Technol. 2020, 97, 147–155. [Google Scholar] [CrossRef]
- Cappelli, A.; Guerrini, L.; Cini, E.; Parenti, A. Improving whole wheat dough tenacity and extensibility: A new kneading process. J. Cereal Sci. 2019, 90, 102852. [Google Scholar] [CrossRef]
- Harrier, J.P. Drying and Storing Sunflowers; AF-158; Kansas State University, Cooperative Extension Service: Manhattan, KS, USA, 1987. [Google Scholar]
- Thompson, T.L.; Shelton, D.P. Aeration of Stored Grain; ES, G84-692; North Dakota State University: Fargo, ND, USA, 1993. [Google Scholar]
- Noyes, R.T.; Clary, B.L.; Cuperus, G.W. Maintaining Quality of Stored Grain by Aeration; OSU Extension Facts, CES, No.1100; Oklahoma State University, Division of Agricultural Science and Natural Resources: Stillwater, OK, USA, 1998. [Google Scholar]
- Khan, M.; Damalas, C.A. Factors preventing the adoption of alternatives to chemical pest control among Pakistani cotton farmers. Int. J. Pest Manag. 2015, 61, 9–16. [Google Scholar] [CrossRef]
- Saeed, M.F.; Baćanović, J.; Finckh, M.R.; Bruns, C.; Schmidt, H. Seed health of organic peas and faba beans and its effects on the health of the harvested grains. J. Plant Dis. Prot. 2017, 124, 331–337. [Google Scholar] [CrossRef]
- Commander, L.; Merritt, D.J.; Rokich, D.P.; Dixon, K.W. The role of after-ripening in promoting germination of arid zone seeds: A study on six Australian species. Bot. J. Linn. Soc. 2009, 161, 411–421. [Google Scholar] [CrossRef] [Green Version]
- ISTA. International rules for seed testing. Seed Sci. Technol. 1996, 24, 335. [Google Scholar]
- Barnett, H.L.; Hunter, B.B. Illustrated Genera of Imperfect Fungi; Burgess Publishing Company: Minneapolis, MN, USA, 1972; p. 241. [Google Scholar]
- Nirenberg, H. Untersuchungen uber die morphologische und biologischi Differenzierung in der Fusarium-Sektion Liseeola. In Mitteilungen aus der Biologischen Bundesanstalt für Land- und Forstwirtschaft; Kommissionsverlag Paul Parey Buchverlag: Berlin, Germany, 1976; Heft 169; pp. 1–117. [Google Scholar]
- Nelson, P.E.; Toussoun, T.A.; Marasas, W.F.O. Fusarium spp.: An Illustrated Manual for Identification; The Pennsylvania University: Philadelphia, PA, USA, 1983. [Google Scholar]
- Pampel, F.C. Logistic Regression: A Primer. In Quantitative Applications in the Social Sciences; Sage: Southend Oaks, CA, USA, 2000. [Google Scholar]
- Agresti, A. An Introduction to Categorical Data Analysis; Wiley & Sons: New York, NY, USA, 1996. [Google Scholar]
- Long, J.S. Regression Models for Categorical and Limited Dependent Variables; Sage: Thousand Oaks, CA, USA, 1997. [Google Scholar]
- McDonald, M.B. Seed deterioration: Physiology, repair and assessment. Seed Sci. Technol. 1999, 27, 177–237. [Google Scholar]
- Ellis, R.H.; Osei-Bonsu, K.; Roberts, E.H. The Influence of Genotype, Temperature and Moisture on Seed Longevity in Chickpea, Cowpea and Soya bean. Ann. Bot. 1982, 50, 69–82. [Google Scholar] [CrossRef]
- Amaral, A.S.; Bicca, L.H.F. Storage of onion seed in sealed cans. Lavoura Arrozeira 1982, 35, 16–20. [Google Scholar]
- Thomazelli, L.F.; Da Silva, R.F.; Sediyama, C.S. How to preserve onion seed quality. Rev. Agropec. Catarin. 1990, 3, 7–8. [Google Scholar]
- Shelar, V.R.; Patil, R.B.; Gawade, N.D. Onion seed viability influenced by different storage containers. Onion Newsl. Trop. 1992, 4, 39–42. [Google Scholar]
- Vijayakumar, A.; Palanisamy, V.; Jayaraj, T.; Arumugam, R. Effect of seed treatments and containers on the storability of onion seed. South Indian Hortic. 1991, 39, 296–299. [Google Scholar]
- Doijode, S.D. Effect of silica gel and storage containers on seed viability and vigour in onion (Allium cepa L.). Seed Res. 1995, 23, 67–70. [Google Scholar]
- Bam, R.K.; Craufurd, P.Q.; Dorward, P.T.; Asiedu, E.A.; Kumaga, F.K.; Ofori, K. Introducing improved cultivars: Understanding farmers’ seed drying and storage practices in central Ghana. Exp. Agric. 2007, 43, 301–317. [Google Scholar] [CrossRef]
- Horky, J. The effect of temperature on the long term storage of dry seeds of some selected vegetables. Zahradnictvi 1991, 18, 29–33. [Google Scholar]
- Ellis, R.H.; Hong, T.D.; Astley, D.; Pinnegar, A.E.; Kraak, H.L. Survival of dry and ultra-dry seeds of carrot, groundnut, lettuce, oilseed rape, and onion during five years; hermetic storage at two temperatures. Seed Sci. Technol. 1996, 24, 347–358. [Google Scholar]
- Walters, C.; Kameswara Rao, N.; Hu, X. Optimizing seed water content to improve longevity in ex situ genebanks. Seed Sci. Res. 1998, 8, 15–22. [Google Scholar]
- Vertucci, C.W.; Roos, E.E. Theoretical basis of protocols for seed storage. II. The influence of temperature on optimal moisture levels. Seed Sci. Res. 1993, 3, 201–213. [Google Scholar] [CrossRef]
- Dearman, J.; Brocklehurst, P.A.; Drew, R.L.K. Effects of osmotic priming and ageing on onion seed germination. Ann. Appl. Biol. 1986, 108, 639–648. [Google Scholar] [CrossRef]
- Van Campenhout, B.; Vandevelde, S.; Walukano, W.; Van Asten, P. Agricultural Extension Messages Using Video on Portable Devices Increased Knowledge about Seed Selection, Storage and Handling among Smallholder Potato Farmers in Southwestern Uganda. PLoS ONE 2017, 12, e0169557. [Google Scholar] [CrossRef] [Green Version]
- Munyambonera, E.; Nampewo, D.; Adong, A.; Mayanja, M. Access and Use of Credit in Uganda: Unlocking the Dilemma of Financing Small Holder Farmers. 2012. Available online: http://ageconsearch.umn.edu/bitstream/150229/2/policybrief25.pdf (accessed on 15 November 2019).
- Benard, R.; Frankwell, D.; Ngalapa, H. Assessment of Information Needs of Rice Farmers in Tanzania; A Case Study of Kilombero District, Morogoro. 2014. Available online: https://digitalcommons.unl.edu/libphilprac/1071/?utm_source=digitalcommons.unl.edu%2Flibphilprac%2F1071&utm_medium=PDF&utm_campaign=PDFCoverPages (accessed on 22 January 2014).
- RLDC. Improving Rice Profitability through Increased Profitability and Better Marketing Focusing on Tanzania’s Central Corridor. 2009. Available online: http://www.rldp.org/downloads/rice_strategy.pdf (accessed on 15 November 2019).
- Lwoga, E. Application of Knowledge Management Approaches and Information and Communication Technologies to Manage Indigenous Knowledge in the Agricultural Sector in Selected Districts of Tanzania. Ph.D. Thesis, University of Kwazulu-Natal, Durban, South Africa, 2009. [Google Scholar]
- Shaffril, H.A.M.; Samah, B.A.; Hassan, M.A.; Silva, J.L. Socio-economic factors that impinge computer usage in administration works among village leaders in Malaysia. Sci. Res. Essays 2010, 5, 3623–3633. [Google Scholar]
- Samah, M.S.; Yassin, S.M.; Shaffril, H.A.M.; Othman, M.S.; Samah, B.A.; Samah, A.A.; Ramli, S.A. Receiving the agriculture information through mass media and interpersonal sources among the rural community. Am. J. Agric. Biol. Sci. 2011, 6, 451–454. [Google Scholar]
- Mtega, W.; Benard, R. The state of rural information and communication services in Tanzania: A meta-analysis. Int. J. Inform. Commun. Technol. Res. 2013, 3, 64–73. [Google Scholar]
- Ingle, R.W.; Raut, J.G. Effect of fungicidal sprays on incidence of seed-borne fungi and germination of pearl millet. Seed Res. 1994, 22, 85–87. [Google Scholar]
- ICRISAT. Improved Cultivation Practices for Groundnut. 2007. Available online: http//www.oar.icrisat.org/id/eprint/400 (accessed on 29 August 2019).
- Safránková, I.; Marková, J.; Kmoch, M. Seed mycoflora of malting varieties and lines of spring barley. J. Crop Sci. 2010, 56, 138–144. [Google Scholar]
- Manjaro, M. Millions are hungry while food supplies rot in India. Eagle News, 8 July 2012; 3. [Google Scholar]
- Schmidt, L. Guide to Handling of Tropical and Subtropical Forest Seed; Danida Forest Seed Centre: Humlebaek, Denmark, 2000; p. 511. [Google Scholar]
- Ferguson, J.M.; Keys, R.D.; Laughlin, M.C.; Warren, J.M. Seed quality: A guide for seed producers, farmers and home gardeners. J. Agric. Biol. 2004, 3, 519–520. [Google Scholar]
- Bishaw, Z.; Niane, A.A.; Gastel, V.; Anthony, J.G. Technical Guidelines for Quality Seed Production; International Center for Agricultural Research in the Dry Areas (ICARDA): Allepo, Syria, 2006; p. 23. [Google Scholar]
- Gioria, M.; Osborne, B. Similarities in the impact of three large invasive plant species on soil seed bank communities. Biol. Invasions 2010, 12, 1671–1683. [Google Scholar] [CrossRef]
- Majid, M.A. Seed Health Status of Okra of Two Sadar Thana of Mymensingh and Thakurgaon. Master’s Thesis, Bangladesh Agricultural University (BAU), Mymensingh, Bangladesh, 1996. [Google Scholar]
- Quayum, M.A. Influence of Date of Harvest on the Incidence of Seed Borne Fungal Pathogens in Okra. Master’s Thesis, Bangladesh Agricultural University (BAU), Mymensingh, Bangladesh, 1999. [Google Scholar]
- Alam, A.K.M.A. Studies on the Quality of Vegetable Seeds Available in the Market. Master’s Thesis, Bangladesh Agricultural University (BAU), Mymensingh, Bangladesh, 2001. [Google Scholar]
- Jamadar, M.M.; Ashok, S.; Shamrao, J.; Sajjan, A.; Jahangidar, S. Studies on seed mycoflora and their effect on germination of colour graded okra [Abelmoschus esculentus (L.) Moench]. J. Crop Res. 2001, 22, 479–484. [Google Scholar]
- Kalsa, K.K. Farmers’ attitudes and practices towards variety and certified seed use, seed replacement and seed storage in wheat growing areas of Ethiopia. Afr. J. Sci. Technol. Innov. Dev. 2019, 11, 107–120. [Google Scholar] [CrossRef]
- Gwinner, J.; Harnisch, R.; Müch, O. Fumonisins: History, worldwide occurrence and impact. In Fumonisins in Food; Plenum Press: New York, NY, USA, 1996; p. 17. [Google Scholar]
- Nautiyal, P.C. Groundnut post harvest operation. J. Agric. Sci. 2007, 2, 1–102. [Google Scholar]
- Malaker, P.K.; Mian, I.H.; Bhuiyan, K.A.; Akanda, A.M.; Reza, M.M.A. Effect of storage containers and time on seed quality of wheat. J. Plant Prot. 2008, 3, 469–477. [Google Scholar] [CrossRef] [Green Version]
- Oyekale, K.; Daniel, I.O.; Ajala, M.O.; Sanni, L.O. Potential Longevity of Maize Seeds under Storage in Humid Tropical Seed Stores. Nat. Sci. 2012, 10, 114–124. [Google Scholar]
- Timsina, K.; Dahal, P.; Bradford, K.J.; Kunusoth, K.; Van Asbrouck, J.; Pandey, I.; Bajracharya, J.; Shivakoti, G. Impacts of a new post-harvest drying technology on the horticultural seed value chain in Nepal. In Proceedings of the 29th International Horticultural Congress, Queensland, Australia, 17–22 August 2014. [Google Scholar]
- Timsina, K.P.; Shivakoti, G.P. Vegetables production and marketing: Practice and perception of vegetable seed producers and fresh growers in Nepal. Agric. Food Secur. 2018, 7, 11. [Google Scholar] [CrossRef]
- Oudwater, N.; Martin, A. Methods and issues in exploring local knowledge of soils. Geoderma 2003, 111, 387–401. [Google Scholar] [CrossRef]
- Niemeijer, D.; Mazzucato, V. Moving beyond indigenous soil taxonomies: Local theories of soils for sustainable development. Geoderma 2003, 111, 403–424. [Google Scholar] [CrossRef]

| Variable | Frequency | Percentage |
|---|---|---|
| Area | ||
| Vehari (VE) | 48 | 30.8 |
| Mailsi (MA) | 56 | 35.9 |
| Burewala (BU) | 52 | 33.3 |
| Growers | ||
| Non-commercial seed growers | 108 | 69.2 |
| Commercial seed growers | 48 | 30.8 |
| Age | ||
| 20–30 years | 42 | 26.9 |
| 31–40 years | 57 | 36.5 |
| 41–50 years | 26 | 16.7 |
| 51–60 years | 31 | 19.9 |
| Education | ||
| Primary | 58 | 37.2 |
| Middle to intermediate | 36 | 23.1 |
| University graduate | 45 | 28.8 |
| Master and above | 17 | 10.9 |
| Farming experience | ||
| 1 to 10 years | 57 | 36.5 |
| 11 to 20 years | 80 | 51.3 |
| 21 to 30 years | 19 | 12.2 |
| Total farmland | ||
| 1 to 10 acres | 55 | 35.3 |
| 11 to 20 acres | 90 | 57.7 |
| 21 to 30 acres | 6 | 3.8 |
| Above 30 acres | 5 | 3.2 |
| Variable | CSGs (n = 48) | NCSGs (n = 108) | ||||
|---|---|---|---|---|---|---|
| BU | MA | VE | BU | MA | VE | |
| Place of seed storage | ||||||
| Living room | 22.9 | 8.3 | 0.0 | 14.8 | 12.0 | 8.3 |
| Open area | 10.4 | 8.3 | 8.3 | 11.1 | 12.0 | 7.4 |
| Kitchen | 0.0 | 2.1 | 0.0 | 3.7 | 4.6 | 7.4 |
| Store | 0.0 | 16.7 | 22.9 | 3.7 | 7.4 | 7.4 |
| Storage container | ||||||
| Polythene bags | 0.0 | 0.0 | 0.0 | 10.2 | 9.3 | 10.2 |
| Jute bags | 6.3 | 10.4 | 6.3 | 8.3 | 7.4 | 8.3 |
| Cotton bags | 12.5 | 20.8 | 20.8 | 3.7 | 7.4 | 4.6 |
| Closed bins | 14.6 | 4.2 | 4.2 | 4.6 | 6.5 | 3.7 |
| Covered heaps | 0.0 | 0.0 | 0.0 | 6.5 | 5.6 | 3.7 |
| Appropriate temperature for seed storage | ||||||
| 0 to 10 | 18.8 | 22.9 | 10.4 | 6.5 | 5.6 | 4.6 |
| 11 to 20 | 12.5 | 8.3 | 14.6 | 8.3 | 7.4 | 6.5 |
| 21 to 30 | 2.1 | 4.2 | 6.3 | 18.5 | 23.1 | 19.4 |
| Appropriate humidity for seed storage | ||||||
| 50 to 60 | 32.5 | 30.0 | 22.5 | 18.5 | 21.3 | 13.9 |
| 61 to 70 | 7.5 | 10.0 | 10.0 | 8.3 | 7.4 | 6.5 |
| 71 to 80 | 0.0 | 2.5 | 5.0 | 3.7 | 7.4 | 3.7 |
| 81 to 90 | 0.0 | 0.0 | 0.0 | 2.8 | 0.0 | 6.5 |
| Appropriate seed moisture for harvest | ||||||
| 5 to 10 | 22.9 | 22.9 | 18.8 | 14.8 | 13.0 | 4.6 |
| 11 to 15 | 10.4 | 12.5 | 12.5 | 4.6 | 17.6 | 10.2 |
| 16 to 20 | 0.0 | 0.0 | 0.0 | 8.3 | 4.6 | 13.0 |
| 21 to 25 | 0.0 | 0.0 | 0.0 | 5.6 | 0.9 | 2.8 |
| Grading of storage conditions | ||||||
| Bad | 33.3 | 29.2 | 14.6 | 31.5 | 35.2 | 25.9 |
| Moderate | 0.0 | 6.3 | 10.4 | 0.0 | 0.9 | 2.8 |
| Good | 0.0 | 0.0 | 6.3 | 1.9 | 0.0 | 1.9 |
| Variable | Yes | No | Yes | No |
|---|---|---|---|---|
| FQ | FQ | Percent | Percent | |
| Knowledge | ||||
| Do you have training on seed storage? | 39 | 117 | 25.0 | 75.0 |
| Can we use sealed containers/closed polythene bags? | 95 | 61 | 60.9 | 39.1 |
| Do you know about seed-borne diseases? | 59 | 97 | 37.8 | 62.2 |
| Do seed-borne diseases reduce crop yield? | 53 | 103 | 34.0 | 66.0 |
| Are crop diseases spread through seeds? | 63 | 93 | 40.4 | 59.6 |
| Do you have a diseases problem in your crops? | 45 | 111 | 28.8 | 71.2 |
| Practice | ||||
| Do you clean seeds before storing them? | 91 | 65 | 58.3 | 41.7 |
| Do you perform sorting of seeds damaged/diseased? | 24 | 132 | 15.4 | 84.6 |
| Do you maintain varieties separately? | 86 | 70 | 55.1 | 44.9 |
| Do you maintain temperature in the store? | 27 | 129 | 17.3 | 82.7 |
| Do maintain moisture/humidity in the store | 27 | 129 | 17.3 | 82.7 |
| Do you clean/spray the store before storage? | 99 | 57 | 63.5 | 36.5 |
| Do you calculate the seed rate after the germination test? | 20 | 136 | 12.8 | 87.2 |
| Do you contact the extension agents? | 47 | 109 | 30.1 | 69.9 |
| Social participation to update knowledge | 64 | 92 | 410 | 59.0 |
| Do you control the temperature of the storeroom? | 15 | 141 | 9.6 | 90.4 |
| Do you control the humidity of the storeroom? | 60 | 96 | 38.5 | 61.5 |
| Do you dress seeds with fungicides before storage? | 109 | 47 | 69.9 | 30.1 |
| Do you check the seed moisture at harvest of seeds? | 128 | 28 | 82.1 | 17.9 |
| Do you check the weather before seed harvest? | 135 | 21 | 86.5 | 13.5 |
| Do you check the level of seed maturity at harvest? | 137 | 19 | 87.8 | 12.2 |
| Do you perform germination tests before sowing? | 52 | 104 | 33.3 | 66.7 |
| Do you keep records of the stored seeds? | 22 | 134 | 14.1 | 85.9 |
| Do you adopt new varieties immediately if available in market? | 52 | 104 | 33.3 | 66.7 |
| Practice | Age (Years) | Education (Level) | Farming Experience (Years) | |
|---|---|---|---|---|
| Graduation | Master | |||
| Controlling the temperature of the storeroom | 0.025 (0.045) ** | −1.8 (1.476) | 0.594 (0.991) | 0.075 (0.059) |
| Controlling the humidity of the storeroom | 0.002 (0.023) | −1.094 (0.757) | −0.388 (0.667) | 0.088 (0.034) ** |
| Seed dressing fungicides before storage | −0.014 (0.02) | 1.547 (0.778) * | 0.466 (0.679) | −0.013 (0.029) |
| Checking seed maturity before harvesting | 0.01 (0.28) | 0.85 (1.096) | 2.39 (1.234) * | −0.022 (0.04) |
| Practice | Total Farmland (Acres) | Training for Seed Storage | Contact Extension Agent |
|---|---|---|---|
| Controlling the temperature of the storeroom | 0.146 (0.051) ** | −1.629 (0.803) * | −0.826 (0.837) |
| Using sealed containers/closed polythene bags | 0.003 (0.026) | 1.550 (0.453) *** | 0.723 (0.469) |
| Keeping records of stored seeds | 0.059 (0.030) * | 0.069 (0.607) | 0.319 (0.648) |
| Sorting damaged and diseased seeds | 0.078 (0.030) ** | −0.361 (0.566) | 0.478 (0.587) |
| Cleaning or spraying the store to disinfect it | 0.163 (0.047) *** | −1.918 (0.711) ** | 1.69 (0.623) ** |
| Variable | Total Farmland | Training for Seed Storage | Contact Extension Agent | Social Participation | |
|---|---|---|---|---|---|
| Storage place | Living room | −0.127 (0.046) ** | 0.360 (0.560) | 0.164 (0.610) | −0.062 (0.480) |
| Open area | −0.123 (0.048) ** | 0.627 (0.600) | 0.808 (0.670) | −0.110 (0.494) | |
| Kitchen | −0.229 (0.071) *** | 0.027 (0.798) | −0.198 (0.861) | 0.783 (0.675) | |
| Store | Reference category | ||||
| Containers used for storage | Jute bags | 0.341 (0.081) *** | −0.513 (0.895) | 0.909 (0.891) | −0.343 (0.614) |
| Cotton bags | 0.377 (0.081) *** | −0.624 (0.881) | 1.163 (0.886) | −1.091 (0.613) | |
| Closed bins | 0.348 (0.083) *** | −0.356 (0.946) | 1.213 (0.944) | −0.427 (0.661) | |
| Covered heap | 0.316 (0.089) *** | −1.670 (1.028) | 0.740 (1.061) | −0.341 (0.765) | |
| Polythene bags | Reference category | ||||
| Appropriate temperature for store | 16–30 °C | −0.051 (0.033) | 0.537 (0.555) | −1.058 (0.611) | 1.500 (0.561) ** |
| 31–45 °C | −0.037 (0.042) | 3.344 (0.839) *** | −0.825 (0.722) | 2.419 (0.624) *** | |
| 0–15 °C | Reference category | ||||
| Appropriate humidity for store | 61–70% | −0.012 (0.029) | 0.178 (0.543) | −1.753 (0.630) ** | 0.605 (0.440) |
| 71–80% | 0.003 (0.042) | 1.875 (1.162) | −0.408 (0.876) | 1.814 (0.688) ** | |
| 81–90% | −0.036 (0.08) | 1.088 (1.417) | −1.958 (1.527) | 0.694 (0.822) | |
| 50–60% | Reference category | ||||
| Knowledge of seed moisture at harvest | 11–15% | 0.007 (0.027) | 0.212 (0.487) | 0.285 (0.552) | −0.500 (0.399) |
| 16–20% | −0.057 (0.047) | 2.504 (0.878) ** | 0.161 (0.648) | 0.932 (0.575) | |
| 21–25% | −0.135 (0.091) | 0.408 (0.995) | 0.109 (0.947) | −0.430 (0.755) | |
| 5 to 10 | Reference category | ||||
| Grading of store conditions | Bad | −0.159 (0.058) ** | 2.521 (1.35) | −1.526 (1.317) | −0.837 (1.378) |
| Medium | −0.115 (0.063) | 1.275 (1.49) | −1.48 (1.484) | −0.675 (1.485) | |
| Good | Reference category |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeed, M.F.; Jamal, A.; Ahmad, I.; Ali, S.; Shah, G.M.; Husnain, S.K.; Farooq, A.; Wang, J. Storage Conditions Deteriorate Cotton and Wheat Seeds Quality: An Assessment of Farmers’ Awareness in Pakistan. Agronomy 2020, 10, 1246. https://doi.org/10.3390/agronomy10091246
Saeed MF, Jamal A, Ahmad I, Ali S, Shah GM, Husnain SK, Farooq A, Wang J. Storage Conditions Deteriorate Cotton and Wheat Seeds Quality: An Assessment of Farmers’ Awareness in Pakistan. Agronomy. 2020; 10(9):1246. https://doi.org/10.3390/agronomy10091246
Chicago/Turabian StyleSaeed, Muhammad Farhan, Aftab Jamal, Iftikhar Ahmad, Sajjad Ali, Ghulam Mustafa Shah, Syed Kamil Husnain, Amjad Farooq, and Jingkuan Wang. 2020. "Storage Conditions Deteriorate Cotton and Wheat Seeds Quality: An Assessment of Farmers’ Awareness in Pakistan" Agronomy 10, no. 9: 1246. https://doi.org/10.3390/agronomy10091246
APA StyleSaeed, M. F., Jamal, A., Ahmad, I., Ali, S., Shah, G. M., Husnain, S. K., Farooq, A., & Wang, J. (2020). Storage Conditions Deteriorate Cotton and Wheat Seeds Quality: An Assessment of Farmers’ Awareness in Pakistan. Agronomy, 10(9), 1246. https://doi.org/10.3390/agronomy10091246








