Induction of Earlier Flowering in Cassava through Extended Photoperiod
Abstract
:1. Introduction
2. Materials and Methods
2.1. Location
2.2. Germplasm
2.3. Light Sources
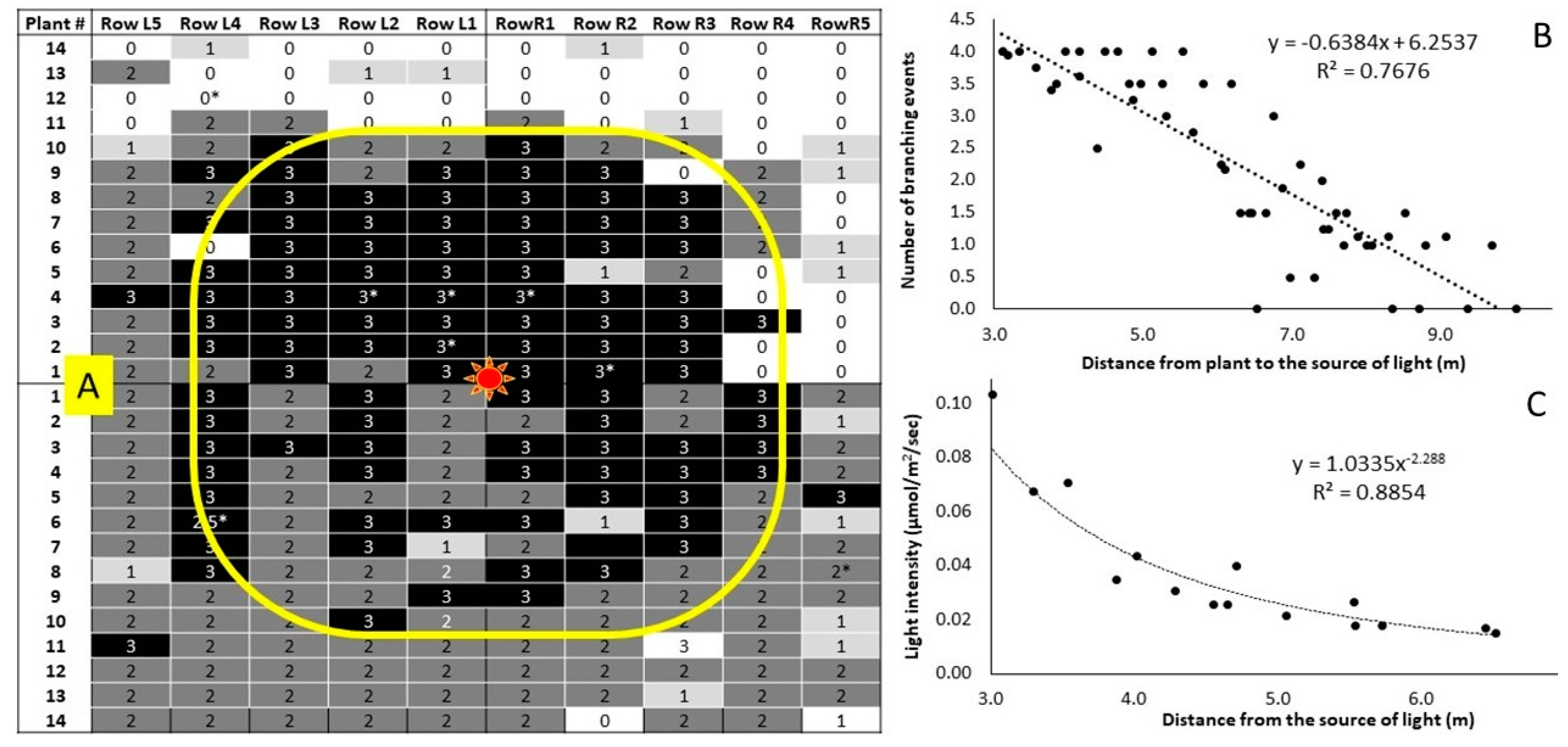
2.4. Experiment 1: Photoperiod Extension with Individual LED Lamps above each Plant
2.5. Experiment 2: Photoperiod Extension with 50W LED Lamps Illuminating a Large Area
2.6. Experiment 3: Night Breaks
2.7. Experiment 4: Validation of the Effect of Photoperiod Extension in a Crossing Nursery
2.8. Field Management
2.9. Data Recorded
2.10. Statistical Analysis
3. Results
3.1. Response to Extended Photoperiod Based on Different Sources of Red Light
3.2. Assessing the Minimum Light Intensity to Induce Earlier Flowering
3.3. The Effect of Night Breaks (Experiment 3)
3.4. The Impact of Photoperiod Extension in a Wide Range of Genotypes (Experiment 4)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stapleton, G. Global starch market outlook and competing starch raw materials for starches by product segment and region. In Proceedings of the Cassava Starch World 2012. Centre for Management Technology (CMT), Phnom Penh, Cambodia, 22–24 February 2012. [Google Scholar]
- Ceballos, H.; Hershey, C.H. Cassava. In Genetic Improvement of Tropical Species; Campos, H., Caligari, P.D.S., Eds.; Springer: Berlin, Germany, 2017; pp. 129–180. ISBN 978-3-319-59817-8. [Google Scholar]
- Ceballos, H.; Ramirez, J.; Bellotti, A.C.; Jarvis, A.; Alvarez, E. Adaptation of cassava to Changing Climates. In Crop Adaptation to Climate Change; Yadav, S., Redden, B., Hatfield, J.L., Lotze-Campen, H., Eds.; Wiley-Blackwell Publishers: Hoboken, NJ, USA, 2011; pp. 411–425. [Google Scholar]
- Alves, A.A.C. Cassava botany and physiology. In Cassava: Biology, Production and Utilization; Hillocks, R.J., Tresh, J.M., Bellotti, A.C., Eds.; CABI Publishing: Wallingford, UK, 2002; pp. 67–89. [Google Scholar]
- Kawano, K. Cassava. In Hybridization of Crop Plants; Fehr, W.R., Hadley, H.H., Eds.; ASA; CSSA: Madison, WI, USA, 1980; pp. 225–233. [Google Scholar]
- Ramos Abril, L.N.; Pineda, L.M.; Wasek, I.; Wedzony, M.; Ceballos, H. Reproductive biology in cassava: Stigma receptivity and pollen tube growth. Commun. Integr. Biol. 2019, 12, 96–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuda, W.M.G.; de Oliveira, S.; Iglesias, C. Cassava breeding. Crop Breed. Appl. Biotech. 2002, 2, 617–638. [Google Scholar] [CrossRef]
- Perera, P.I.P.; Quintero, M.; Dedicova, B.; Kularatne, J.D.J.S.; Ordoñez, C.A.; Ceballos, H. Comparative morphology, biology and histology of reproductive development in three lines of Manihot esculenta Crantz (Euphorbiaceae: Crotonoideae). Ann. Bot. Plants 2012, 5, pls046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jennings, D.L. Variation in pollen and ovule fertility in varieties of cassava, and the effect of interspecific crossing on fertility. Euphytica 1963, 12, 69–76. [Google Scholar]
- Jos, J.S.; Nair, R.B.; Sreekumari, M.T. Stigma receptivity and seed set in cassava. Trop. Agric. (Trinidad) 1989, 67, 257–261. [Google Scholar]
- Lentini, Z.; González, A.; Tabares, E.; Buitrago, M.E.; Wêdzony, M. Studies on gynogenesis induction in cassava (Manihot esculenta Crantz) unpollinated ovule culture. Front. Plant Sci. 2020, 11, 365. [Google Scholar] [CrossRef] [Green Version]
- Lentini, Z.; Restrepo, G.; Buitrago, M.E.; Tabares, E. Protocol for rescuing young cassava embryos. Front. Plant Sci. 2020, 11, 522. [Google Scholar] [CrossRef]
- Boonchanawiwat, A.; Sraphet, S.; Boonseng, O.; Lightfoot, D.A.; Triwitayakorn, K. QTL underlying plant and first branch height in cassava (Manihot esculenta Crantz). Field Crops Res. 2011, 121, 343–349. [Google Scholar] [CrossRef]
- Bäurle, I.; Dean, C. The timing of developmental transitions in plants. Cell 2006, 125, 655–664. [Google Scholar] [CrossRef] [Green Version]
- Ceballos, H.; Jaramillo, J.J.; Salazar, S.; Pineda, L.M.; Calle, F.; Setter, T. Induction of flowering in cassava through grafting. J. Plant Breed. Crop Sci. 2017, 9, 19–29. [Google Scholar]
- Ha, T.M. A review of plants’ flowering physiology: The control of floral induction by juvenility, temperature and photoperiod in annual and ornamental crops. Asian J. Agric. Food Sci. 2014, 2, 186–195. [Google Scholar]
- Lee, J.; Lee, I. Regulation and function of SOC1, a flowering pathway integrator. J. Exp. Bot. 2010, 61, 2247–2254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, S.B.; Amasino, R.M. Vernalization in Arabidopsis thaliana is mediated by the PHD finger protein VIN3. Nature 2004, 427, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Tournois, J. Etudes sur la sexualite du houblon. Ann. Sci. Nat. Bot. 1914, 19, 49–189. [Google Scholar]
- Knott, J.E. Effect of a localized photoperiod on spinach. Proc. Am. Soc. Hort. Sci. 1934, 31, 152–154. [Google Scholar]
- Abe, M.; Kobayashi, Y.; Yamamoto, S.; Daimon, Y.; Yamaguchi, A.; Ikeda, Y.; Ichinoki, H.; Notaguchi, M.; Goto, K.; Araki, T. FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 2005, 309, 1052–1056. [Google Scholar] [CrossRef]
- Amasino, R. Seasonal and developmental timing of flowering. Plant J. 2010, 61, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Chailakhyan, M.K. New facts for hormonal theory of plant development. Dokl. Akad. Nauk. SSSR 1936, 4, 79–83. [Google Scholar]
- Hempel, F.D.; Welch, D.R.; Feldman, L.J. Floral induction and determination: Where is flowering controlled? Trends Plant Sci. 2000, 5, 17–21. [Google Scholar] [CrossRef]
- Wigge, P.A.; Kim, M.C.; Jaeger, K.E.; Busch, W.; Schmid, M.; Lohmann, J.U.; Weigel, D. Integration of spatial and temporal information during floral induction in Arabidopsis. Science 2005, 309, 1056–1059. [Google Scholar] [CrossRef]
- Zeevaart, J.A.D. Leaf-Produced floral signals. Curr. Opin. Plant Biol. 2008, 11, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Wigge, P. FT, a mobile developmental signal in plants. J. Plant Breed. Crop Sci. 2011, 9, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Yeoh, C.C.; Balcerowicz, M.; Laurie, R.; Macknight, R.; Putterill, J. Developing a method for customized induction of flowering. BMC Biotechnol. 2011, 11, 1–11. Available online: http://www.biomedcentral.com/1472-6750/11/36 (accessed on 31 August 2019). [CrossRef] [Green Version]
- Jung, C.; Muller, A.E. Flowering time control and applications in plant breeding. Trends Plant Sci. 2009, 14, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Doyle, M.R.; Sung, S.; Amasino, R.M. Vernalization: Winter and the timing of flowering in plants. Annu. Rev. Cell Dev. Biol. 2009, 25, 277–299. [Google Scholar] [CrossRef] [Green Version]
- McClung, C.R.; Lou, P.; Hermand, V.; Kim, J.A. The Importance of ambient temperature to growth and the induction of flowering. Front. Plant Sci. 2016, 7, 1266. [Google Scholar] [CrossRef] [PubMed]
- Searle, I.; Coupland, G. Induction of flowering by seasonal changes in photoperiod. EMBO J. 2004, 23, 1217–1222. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.-L.; Viswanath, K.K.; Tong, C.-G.; An, H.R.; Jang, S.; Chen, F.-C. Floral Induction and Flower Development of Orchids. Front. Plant Sci. 2019, 10, 1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warner, R.M.; Erwin, J.E. Prolonged high-temperature exposure differentially reduces growth and flowering of 12 Viola x Wittrockiana Gams. cvs. Sci. Hortic. 2006, 108, 295–302. [Google Scholar] [CrossRef]
- Indira, P.; Kurian, T.; Maini, S.B. Flowering behaviour in cassava (Manihot esculenta Crantz) as influenced by growth regulators. J. Root Crops 1977, 3, 37–40. [Google Scholar]
- de Bruijn, G.H. Influence of day length on the flowering of cassava. Trop. Root Tuber Crops Newsl. 1977, 10, 1–3. [Google Scholar]
- Keating, B. Environmental effects on growth and development of cassava (Manihot esculenta Crantz) with special reference to photoperiod and temperature. Cassava News 1982, 1, 10–12. [Google Scholar]
- Pellet, D.; El-Sharkawy, M.A. Cassava varietal response to phosphorus fertilization. I. Yield, biomass and gas exchange. Field Crops Res. 1993, 35, 1–11. [Google Scholar] [CrossRef]
- Tang, A.F.; Cappadocia, M.; Byrne, D. In vitro flowering in cassava (Manihot esculenta Crantz). Plant Cell Tissue Org. 1983, 2, 99–206. [Google Scholar] [CrossRef]
- Adeyemo, O.S.; Kolmos, E.; Tohme, J.; Chavarriaga, P.; Fregene, M.; Davis, S.J. Identification and characterization of the cassava Core-Clock Gene Early Flowering 4. Trop. Plant Biol. 2011, 4, 117–125. [Google Scholar] [CrossRef]
- Adeyemo, O.S.; Chavarriaga, P.; Tohme, J.; Fregene, M.; Davis, S.J.; Setter, T.L. Overexpression of arabidopsis FLOWERING LOCUS T (FT) gene improves floral development in cassava (Manihot esculenta, Crantz). PLoS ONE 2017, 12, e0181460. [Google Scholar] [CrossRef] [Green Version]
- Adeyemo, O.S.; Hyde, P.T.; Setter, T.L. Identification of FT family genes that respond to photoperiod, temperature and genotype in relation to flowering in cassava (Manihot esculenta, Crantz). Plant Reprod. 2018, 32, 181–191. [Google Scholar] [CrossRef] [Green Version]
- Silva Souza, L.; Parreira Diniz, R.; de Jesus Neves, R.; Cunha Alves, A.A.; de Oliveira, E.J. Grafting as a strategy to increase flowering of cassava. Sci. Hortic. 2018, 240, 544–551. [Google Scholar] [CrossRef]
- Hyde, P.T.; Guan, X.; Abreu, V.; Setter, T.L. The anti-ethylene growth regulator silver thiosulfate (STS) increases flower production and longevity in cassava (Manihot esculenta Crantz). Plant Growth Regul. 2019, 90, 441–453. [Google Scholar] [CrossRef] [Green Version]
- Pineda, M.; Yu, B.; Tian, Y.; Morante, N.; Salazar, S.; Hyde, P.T.; Setter, T.L.; Ceb allos, H. Effect of pruning young branches on fruit and seed set in cassava. Front. Plant Sci. 2020, 11, 1107. [Google Scholar] [CrossRef]
- SAS. SAS/STAT 9.1User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2008. [Google Scholar]
- Ellis, R.H.; Summerfield, R.J.; Edmeades, G.O.; Roberts, E.H. Photoperiod, temperature, and the interval from sowing to tassel initiation in diverse cultivars of maize. Crop Sci. 1992, 32, 1225–1232. [Google Scholar] [CrossRef]
- Ceballos, H.; Becerra López-Lavalle, L.A.; Calle, F.; Morante, N.; Ovalle, T.M.; Hershey, C. Genetic distance and specific combining ability in cassava. Euphytica 2016, 210, 79–92. [Google Scholar] [CrossRef]
- Yan, W.; Wallace, D.H. A model of photoperiod × temperature interaction effects on plant development. Crit. Rev. Plant Sci. 1996, 15, 63–96. [Google Scholar]
- Hyde, P.; Abreu, V.; Setter, T.L. Flower initiation response to photoperiod x temperature environments in cassava. In Proceedings of the World Congress on Root and Tuber Crops, GCP21-III and ISTRC, Nanning, China, 5–10 October 2016; Volume S11–S18. [Google Scholar]
- Ceballos, H.; Pérez, J.C.; Joaqui Barandica, O.; Lenis, J.I.; Morante, N.; Calle, F.; Pino, L.; Hershey, C.H. Cassava breeding II: The value of breeding value. Front. Plant Sci. 2016, 7, 1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Source of Light | Initiation of Illumination | Light Quantity (µmol/m2/s) | ||
|---|---|---|---|---|
| 10 cm | 20 cm | 30 cm | ||
| Individual light emitting diodes (LEDs) | ||||
| 5 LEDs | 11 DAP | 0.141 (0.003) | 0.060 (0.002) | 0.041 (0.001) |
| 10 LEDs | 23 DAP | 0.177 (0.011) | 0.070 (0.002) | 0.053 (0.001) |
| LEDs Tapes | ||||
| 30 LEDs | 14 DAP | 0.685 (0.021) | 0.337 (0.003) | 0.186 (0.006) |
| 60 LEDs | 17 DAP | 1.513 (0.024) | 0.776 (0.008) | 0.388 (0.010) |
| Source of Variation | df a | Mean Squares | ||
|---|---|---|---|---|
| Days to 1st Flowering | Height 1st Branching | Branching Levels | ||
| (Number) | (cm) | (Number) | ||
| Photoperiod (P) | 2 | 93,645.0 ** | 91,588.7 ** | 30.1 ** |
| EP vs. DN | 1 | 183,085.6 ** | 148,509.3 ** | 60.2 ** |
| EP-L vs. EP-H | 1 | 4204.4 ** | 34,668.1 ** | 0.1 |
| Genotype (G) | 4 | 113,751.8 ** | 259,285.1 ** | 49.4 ** |
| P * G b | 8 | 7066.7 ** | 8675.8 ** | 3.7 ** |
| G1 (EP vs. DN) | 1 | 101,094.1 ** | 50,471.0 ** | 52.0 ** |
| G2 (EP vs. DN) | 1 | 1904.0 * | 298.2 | 0.7 * |
| G3 (EP vs. DN) | 1 | 16,708.8 ** | 58,476.7 ** | 4.4 ** |
| G4 (EP vs. DN) | 1 | 72,422.5 ** | 89,036.4 ** | 16.1 ** |
| G5 (EP vs. DN) | 1 | 38,736.1 ** | 13,573.3 ** | 10.2 ** |
| Error | 285 | 295.3 | 606.8 | 0.2 |
| Total | 299 | |||
| Genotype | 2016/17 | 2017/18 | 2018/19 | |||
|---|---|---|---|---|---|---|
| DN | EP | DN | EP | DN | EP | |
| Regression Coefficients on Number of Flowering Events on DAP (Standard Error of Coefficient) | ||||||
| GM 971-2 | 0.190 (0.014) | 0.521 (0.026) | 0.114 (0.006) | 0.176 (0.007) | 0.102 (0.004) | 0.151 (0.004) |
| CM 4919-1 | 0.037 (0.007) | 0.497 (0.029) | No flowering | 0.156 (0.008) | 0.009 (0.002) | 0.166 (0.005) |
| SM 3348-29 | 0.006 (0.000) | 0.215 (0.034) | 0.017 (0.003) | 0.085 (0.006) | No flowering | 0.098 (0.005) |
| GM 3893-65 | No flowering | 0.040 (0.009) | 0.017 (0.004) | 0.045 (0.005) | No flowering | 0.007 (0.002) |
| SM 3409-43 | No flowering | 0.162 (0.021) | No flowering | 0.097 (0.006) | N.A. | N.A. |
| Average Number of Flowering Events (or Branching Levels) per Plant 200 Days after Planting b,c | ||||||
| GM 971-2 | 2.00b | 2.58a ** | 2.30b | 3.70a ** | 2.10b | 3.10a ** |
| CM 4919-1 | 0.40d | 2.38ab ** | 0.00c | 3.50a ** | 0.20c | 2.90a ** |
| SM 3348-29 | 0.05d | 1.18c ** | 0.40c | 2.20b ** | 0.00c | 1.60b ** |
| GM 3893-65 | 0.00d | 0.23d* | 0.40c | 1.71b ** | 0.00c | 0.00c NS |
| SM 3409-43 | 0.00d | 0.88c ** | 0.00c | 2.50b ** | N.A. | N.A. |
| Across | 0.43 | 1.37 ** | 0.58 | 2.60 ** | 0.58 | 1.90 ** |
| Average Number of Days from Planting to 2nd Branching (% of Plants that Flowered) | ||||||
| GM 971-2 | 152.2 (95%) | 118.2 (100%) | 137.6 (80%) | 107.0 (100%) | 169.1 (90%) | 126.7 (100%) |
| CM 4919-1 | No flowering | 124.9 (100%) | No flowering | 119.3 (100%) | No flowering | 136.0 (100%) |
| SM 3348-29 | No flowering | 167.5 (21%) | No flowering | 169.7 (90%) | No flowering | 198.4 (100%) |
| GM 3893-65 | No flowering | No flowering | No flowering | 194.3 (60%) | No flowering | No flowering |
| SM 3409-43 | No flowering | 169.3 (10%) | No flowering | 168.1 (100%) | N.A. | N.A. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pineda, M.; Morante, N.; Salazar, S.; Cuásquer, J.; Hyde, P.T.; Setter, T.L.; Ceballos, H. Induction of Earlier Flowering in Cassava through Extended Photoperiod. Agronomy 2020, 10, 1273. https://doi.org/10.3390/agronomy10091273
Pineda M, Morante N, Salazar S, Cuásquer J, Hyde PT, Setter TL, Ceballos H. Induction of Earlier Flowering in Cassava through Extended Photoperiod. Agronomy. 2020; 10(9):1273. https://doi.org/10.3390/agronomy10091273
Chicago/Turabian StylePineda, Marcela, Nelson Morante, Sandra Salazar, Juan Cuásquer, Peter T. Hyde, Tim L. Setter, and Hernán Ceballos. 2020. "Induction of Earlier Flowering in Cassava through Extended Photoperiod" Agronomy 10, no. 9: 1273. https://doi.org/10.3390/agronomy10091273







