How Can Weedy Rice Stand against Abiotic Stresses? A Review
Abstract
:1. Introduction
2. The Morphological Traits of Weedy Rice That Confer the Ability to Face Abiotic Stresses
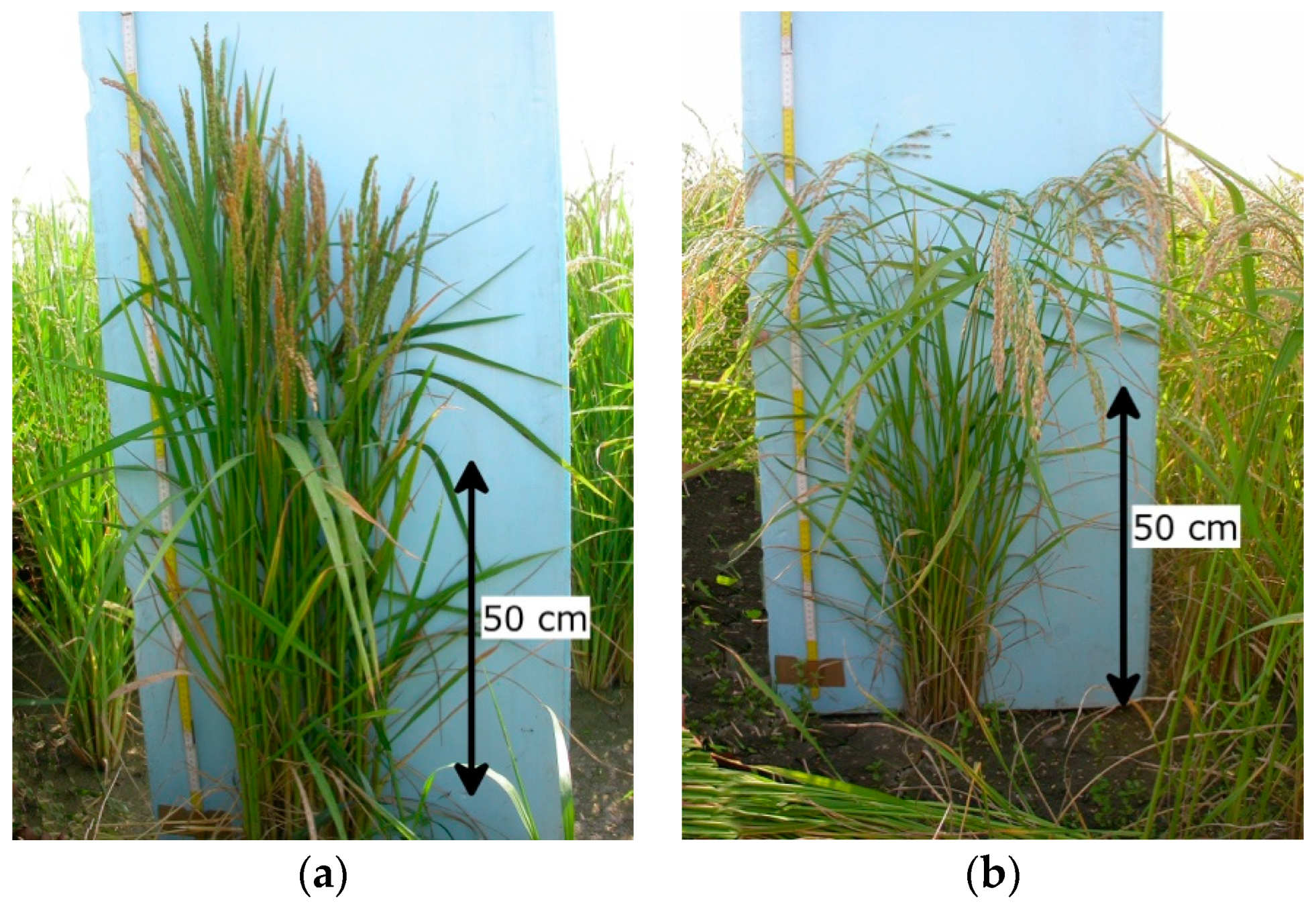
2.1. Canopy Structure and Plant Height
2.2. Tillering
2.3. Seed Morphology and Shape
2.4. Hull Coloration and Awn Presence
2.5. Red Pericarp
3. The Physiological Traits of Weedy Rice That Confer the Ability to Face Abiotic Stresses
3.1. Growth Vigor
3.2. Flowering and Ripening
3.3. Seed Shattering
3.4. Seed Dormancy and Longevity
4. Weedy Rice Behavior under Abiotic Stresses Enhanced by Climate Change
4.1. Temperature Variations
4.2. Drought and Submergence
4.3. Salinity
4.4. CO2 Increase
5. Implications for Weedy Rice Control
Author Contributions
Funding
Conflicts of Interest
References
- Mahmood-ur-Rahman; Ijaz, M.; Qamar, S.; Bukhari, S.A.; Malik, K. Abiotic stress signaling in rice crop. In Advances in Rice Research for Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Nahar, K., Biswas, J.K., Eds.; Elsevier: Duxford, UK, 2019; pp. 551–569. ISBN 978-0-12-814332-2. [Google Scholar]
- Pereira, A. Plant abiotic stress challenges from the changing environment. Front. Plant Sci. 2016, 7, 1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheeler, T.; von Braun, J. Climate change impacts on global food security. Science 2013, 341, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Knox, J.; Hess, T.; Daccache, A.; Wheeler, T. Climate change impacts on crop productivity in Africa and South Asia. Environ. Res. Lett. 2012, 7, 034032. [Google Scholar] [CrossRef]
- Zhao, X.; Fitzgerald, M. Climate change: Implications for the yield of edible rice. PLoS ONE 2013, 8, e66218. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.; Jan, T.; Riaz, M.; Fahad, S.; Arif, M.S.; Shakoor, M.B.; Amanullah; Rasul, F. Advances in rice research for abiotic stress tolerance. In Advances in Rice Research for Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Nahar, K., Biswas, J.K., Eds.; Elsevier: Duxford, UK, 2019; pp. 585–614. ISBN 978-0-12-814332-2. [Google Scholar]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef]
- Des Marais, D.L.; Hernandez, K.M.; Juenger, T.E. Genotype-by-environment interaction and plasticity: Exploring genomic responses of plants to the abiotic environment. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 5–29. [Google Scholar] [CrossRef] [Green Version]
- Fogliatto, S.; Serra, F.; Patrucco, L.; Milan, M.; Vidotto, F. Effect of different water salinity levels on the germination of imazamox-resistant and sensitive weedy rice and cultivated Rice. Agronomy 2019, 9, 658. [Google Scholar] [CrossRef] [Green Version]
- Nadir, S.; Xiong, H.-B.; Zhu, Q.; Zhang, X.-L.; Xu, H.-Y.; Li, J.; Dongchen, W.; Henry, D.; Guo, X.-Q.; Khan, S.; et al. Weedy rice in sustainable rice production. A review. Agron. Sustain. Dev. 2017, 37, 46. [Google Scholar] [CrossRef]
- Delouche, J.C.; Burgos, N.R.; Gealy, D.R.; Zorrilla, G.; Labrada, R. Weedy rices—Origin, biology, ecology and control. FAO Plant Prod. Prot. Pap. 2007, 188, 1–144. [Google Scholar]
- Cui, Y.; Song, B.K.; Li, L.-F.; Li, Y.-L.; Huang, Z.; Caicedo, A.L.; Jia, Y.; Olsen, K.M. Little white lies: Pericarp color provides insights into the origins and evolution of Southeast Asian weedy rice. G3 Genes Genomes Genet. 2016, 6, 4105–4114. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Kelly, S.; Matsuo, R.; Li, L.-F.; Li, Y.; Olsen, K.M.; Jia, Y.; Caicedo, A.L. The role of standing variation in the evolution of weedines traits in South Asian weedy rice (Oryza spp.). G3 Genes Genomes Genet. 2018, 8, 3679–3690. [Google Scholar] [CrossRef] [Green Version]
- Fogliatto, S.; Vidotto, F.; Ferero, A. Germination of weedy rice in response to field conditions during winter. Weed Technol. 2011, 25, 252–261. [Google Scholar] [CrossRef]
- Grimm, A.; Fogliatto, S.; Nick, P.; Ferrero, A.; Vidotto, F. Microsatellite markers reveal multiple origins for Italian weedy rice. Ecol. Evol. 2013, 3, 4786–4798. [Google Scholar] [CrossRef] [PubMed]
- Durand-Morat, A. The implications of red rice on food security. Glob. Food Secur. 2018, 18, 62–75. [Google Scholar] [CrossRef]
- Diarra, A.; Smith, R.J.; Talbert, R.E. Interference of red rice (Oryza sativa) with rice (O. sativa). Weed Sci. 1985, 33, 644–649. [Google Scholar] [CrossRef]
- Ferrero, A. Weedy rice, biological features and control. In Weed Management for Developing Countries -FAO Plant Production and Protection Paper; R. Labrada: Rome, Italy, 2003; Volume 120, pp. 89–107. [Google Scholar]
- Huang, Z.; Young, N.D.; Reagon, M.; Hyma, K.E.; Olsen, K.M.; Jia, Y.; Caicedo, A.L. All roads lead to weediness: Patterns of genomic divergence reveal extensive recurrent weedy rice origins from South Asian Oryza. Mol. Ecol. 2017, 26, 3151–3167. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, B.S. Strategies to manage weedy rice in Asia. Crop Prot. 2013, 48, 51–56. [Google Scholar] [CrossRef]
- Kane, N.C.; Baack, E.J. The origins of weedy rice. Mol. Ecol. 2007, 16, 4423–4425. [Google Scholar] [CrossRef]
- Reagon, M.; Thurber, C.S.; Gross, B.L.; Olsen, K.M.; Jia, Y.; Caicedo, A.L. Genomic patterns of nucleotide diversity in divergent populations of U.S. weedy rice. BMC Evol. Biol. 2010, 10, 180. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, Y.; Sato, Y.-I.; Bounphanousay, C.; Kono, Y.; Tanaka, K. Genetic structure of three Oryza AA genome species (O. rufipogon, O. nivara and O. sativa) as assessed by SSR analysis on the Vientiane Plain of Laos. Conserv. Genet. 2007, 8, 149–158. [Google Scholar] [CrossRef]
- Suh, H.S.; Sato, Y.I.; Morishima, H. Genetic characterization of weedy rice (Oryza sativa L.) based on morpho-physiology, isozymes and RAPD markers. Theor. Appl. Genet. 1997, 94, 314–321. [Google Scholar] [CrossRef]
- Fogliatto, S.; Vidotto, F.; Ferrero, A. Morphological characterisation of Italian weedy rice (Oryza sativa) populations. Weed Res. 2012, 52, 60–69. [Google Scholar] [CrossRef]
- Korres, N.E.; Norsworthy, J.K.; Tehranchian, P.; Gitsopoulos, T.K.; Loka, D.A.; Oosterhuis, D.M.; Gealy, D.R.; Moss, S.R.; Burgos, N.R.; Miller, M.R.; et al. Cultivars to face climate change effects on crops and weeds: A review. Agron. Sustain. Dev. 2016, 36, 12. [Google Scholar] [CrossRef] [Green Version]
- Ziska, L.H.; Gealy, D.R.; Burgos, N.; Caicedo, A.L.; Gressel, J.; Lawton-Rauh, A.L.; Avila, L.A.; Theisen, G.; Norsworthy, J.; Ferrero, A.; et al. Weedy (red) rice: An emerging constraint to global rice production. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 129, pp. 181–228. ISBN 978-0-12-802138-5. [Google Scholar]
- Vidotto, F.; Ferrero, A.; Ducco, G. A mathematical model to predict the population dynamics of Oryza sativa var. sylvatica. Weed Res. 2001, 41, 407–420. [Google Scholar] [CrossRef]
- Fogliatto, S. Biological, Morphological, and Genetic Characterization of Italian Weedy Rice Populations. Ph.D. Thesis, Università degli Studi di Torino, Torino, Italy, 2011. [Google Scholar]
- Qiu, J.; Zhou, Y.; Mao, L.; Ye, C.; Wang, W.; Zhang, J.; Yu, Y.; Fu, F.; Wang, Y.; Qian, F.; et al. Genomic variation associated with local adaptation of weedy rice during de-domestication. Nat. Commun. 2017, 8, 15323. [Google Scholar] [CrossRef]
- He, Q.; Kim, K.; Park, Y. Population genomics identifies the origin and signatures of selection of Korean weedy rice. Plant Biotechnol. J. 2017, 15, 357–366. [Google Scholar] [CrossRef]
- De Leon, T.B.; Karn, E.; Al-Khatib, K.; Espino, L.; Blank, T.; Andaya, C.B.; Andaya, V.C.; Brim–DeForest, W. Genetic variation and possible origins of weedy rice found in California. Ecol. Evol. 2019, 9, 5835–5848. [Google Scholar] [CrossRef]
- Andres, A.; Fogliatto, S.; Ferrero, A.; Vidotto, F. Growth variability of Italian weedy rice populations grown with or without cultivated rice. Crop Sci. 2015, 55, 394–402. [Google Scholar] [CrossRef]
- Olajumoke, B.; Juraimi, A.S.; Uddin, M.D.K.; Husni, M.H.A.; Alam, M.D.A. Competitive ability of cultivated rice against weedy rice biotypes: A review. Chil. J. Agric. Res. 2016, 76, 242–251. [Google Scholar] [CrossRef]
- Atwell, B.J.; Wang, H.; Scafaro, A.P. Could abiotic stress tolerance in wild relatives of rice be used to improve Oryza sativa? Plant Sci. 2014, 215–216, 48–58. [Google Scholar] [CrossRef]
- Burgess, A.J.; Retkute, R.; Herman, T.; Murchie, E.H. Exploring relationships between canopy architecture, light distribution, and photosynthesis in contrasting rice genotypes using 3D canopy reconstruction. Front. Plant Sci. 2017, 8, 734. [Google Scholar] [CrossRef]
- Gibson, K.D.; Fischer, A.J.; Foin, T.C.; Hill, J.E. Crop traits related to weed suppression in water-seeded rice (Oryza sativa L.). Weed Sci. 2003, 51, 87–93. [Google Scholar] [CrossRef]
- Sànchez-Olgùin, E.; Arrieta-Espinoza, G.; Espinoza Esquivel, A.M. Vegetative and reproductive development of Costa Rican weedy rice compare with commercial rice (Oryza sativa). Planta Daninha 2007, 25, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Coppola, E.; Giorgi, F. An assessment of temperature and precipitation change projections over Italy from recent global and regional climate model simulations. Int. J. Climatol. 2010, 30, 11–32. [Google Scholar] [CrossRef]
- Kwon, S.L.; Smith, R.J.; Talbert, R.E. Comparative growth and development of red rice (Oryza sativa) and rice (O. sativa). Weed Sci. 1992, 40, 57–62. [Google Scholar] [CrossRef]
- Ferrero, A.; Vidotto, F. Weeds and weed management in Italian rice field. In Agro-Economicals Traits of Rice Cultivation in Europe and India; Ferrero, A., Vidotto, F., Eds.; Edizioni Mercurio: Vercelli, Italy, 2007; pp. 55–72. ISBN 978-88-86960-83-0. [Google Scholar]
- Fogliatto, S.; Ferrero, A.; Vidotto, F. Current and future scenarios of glyphosate use in Europe: Are there alternatives. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 163, pp. 219–278. ISBN 978-0-12-820769-7. [Google Scholar]
- Reagon, M.; Thurber, C.S.; Olsen, K.M.; Jia, Y.; Caceido, A.L. The long and the short of it: SD1 polymorphism and the evolution of growth trait divergence in U.S. weedy rice. Mol. Ecol. 2011, 20, 3743–3756. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, B.S. Effect of shade on growth and yield of weedy rice (Oryza sativa L.) biotypes and a rice (Oryza sativa L.) cultivar from Asia. J. Crop Improv. 2013, 27, 272–280. [Google Scholar] [CrossRef]
- Noldin, J.A.; Chandler, J.M.; McCauley, G.N. Red rice (Oryza sativa) biology. I. Characterization of red rice ecotypes. Weed Technol. 1999, 13, 12–18. [Google Scholar] [CrossRef]
- Noldin, J.A.; Chandler, J.M.; Ketchersid, M.L.; McCauley, G.N. Red Rice (Oryza sativa) Biology. II. Ecotype sensitivity to herbicides. Weed Technol. 1999, 13, 19–24. [Google Scholar] [CrossRef]
- Burgos, N.R.; Norman, R.J.; Gealy, D.R.; Black, H. Competitive N uptake between rice and weedy rice. Field Crops Res. 2006, 99, 96–105. [Google Scholar] [CrossRef]
- Sales, M.A.; Burgos, N.R.; Shivrain, V.K.; Murphy, B.; Gbur, E.E. Morphological and physiological responses of weedy red rice (Oryza sativa L.) and Cultivated Rice (O. sativa) to N Supply. Am. J. Plant Sci. 2011, 2, 569–577. [Google Scholar] [CrossRef]
- Dai, L.; Song, X.; He, B.; Valverde, B.E.; Qiang, S. Enhanced photosynthesis endows seedling growth vigour contributing to the competitive dominance of weedy rice over cultivated rice. Pest Manag. Sci. 2017, 73, 1410–1420. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.K.; White, J.R.; Bridgham, S.D.; Pastor, J. Climate change effects on carbon and nitrogen mineralization in peatlands through changes in soil quality. Glob. Chang. Biol. 2004, 10, 1053–1064. [Google Scholar] [CrossRef]
- Shivrain, V.K.; Burgos, N.R.; Scott, R.C.; Gbur, E.E., Jr.; Estorninos, L.E., Jr.; McClelland, M.R. Diversity of weedy red rice (Oryza sativa L.) in Arkansas, U.S.A. in relation to weed management. Crop Prot. 2010, 29, 721–730. [Google Scholar] [CrossRef]
- Leishman, M.R. Does the seed size/number trade-off model determine plant community structure? An assessment of the model mechanisms and their generality. Oikos 2001, 93, 294–302. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Johnson, D.E. Weedy Rice (Oryza sativa) I. Grain characteristics and growth response to competition of weedy rice variants from five Asian countries. Weed Sci. 2010, 58, 374–380. [Google Scholar] [CrossRef]
- Westoby, M.; Jurado, E.; Leishman, M. Comparative evolutionary ecology of seed size. Trends Ecol. Evol. 1992, 7, 368–372. [Google Scholar] [CrossRef]
- Gealy, D.R.; Agrama, H.A.; Eizenga, G.C. Exploring genetic and spatial structure of U.S. weedy red rice (Oryza sativa) in relation to rice relatives worldwide. Weed Sci. 2009, 57, 627–643. [Google Scholar] [CrossRef]
- Gealy, D.H.; Agrama, H.; Jia, M.H. Genetic analysis of atypical U.S. red rice phenotypes: Indications of prior gene flow in rice fields? Weed Sci. 2012, 60, 451–461. [Google Scholar] [CrossRef]
- Vigueira, C.C.; Li, W.; Olsen, K.M. The role of Bh4 in parallel evolution of hull colour in domesticated and weedy rice. J. Evol. Biol. 2013, 26, 1738–1749. [Google Scholar] [CrossRef]
- Shrestha, S.; Sharma, G.; Burgos, N.R.; Tseng, T.-M. Competitive ability of weedy rice: Toward breeding weed-suppressive rice cultivars. J. Crop Improv. 2020, 34, 455–469. [Google Scholar] [CrossRef]
- Tseng, T.M.; Burgos, N.R.; Shivrain, V.K.; Alcober, E.A.; Mauromoustakos, A. Inter- and intrapopulation variation in dormancy of Oryza sativa (weedy red rice) and allelic variation in dormancy-linked loci. Weed Res. 2013, 53, 440–451. [Google Scholar] [CrossRef]
- Grimm, A. Dolce Vita in the Rice Paddy—Characterization of Weedy Rice Groups in Northern Italy and Investigation of Their Evolutionary Origins, Karlsruher Institut für Technologie (KIT). Ph.D. Thesis, Universität Karlsruhe, Karlsruhe, Germany, 2014. [Google Scholar]
- Yang, C.; Zeng, D.; Qin, R.; Alamin, M.d.; Jin, X.; Shi, C. Rice gene, BBH/Lsi1, regulates the color of rice hull by reducing the absorption and deposition of silicon and accumulating excess flavonoid. Plant Growth Regul. 2018, 85, 133–142. [Google Scholar] [CrossRef]
- Koes, R.; Verweij, W.; Quattrocchio, F. Flavonoids: A colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci. 2005, 10, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Prathepha, P. Seed morphological traits and genotypic diversity of weedy rice (Oryza sativa f. spontanea) populations found in the Thai Hom Mali rice fields of north-eastern Thailand. Weed Biol. Manag. 2009, 9, 1–9. [Google Scholar] [CrossRef]
- Sweeney, M.T.; Thomson, M.J.; Pfeil, B.E.; McCouch, S. Caught red-handed: Rc encodes a basic helix-loop-helix protein conditioning red pericarp in rice. Plant Cell 2006, 18, 283–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, X.-Y.; Foley, M.E.; Horvath, D.P.; Anderson, J.V.; Feng, J.; Zhang, L.; Mowry, C.R.; Ye, H.; Suttle, J.C.; Kadowaki, K.; et al. association between seed dormancy and pericarp color is controlled by a pleiotropic gene that regulates abscisic acid and flavonoid synthesis in weedy red rice. Genetics 2011, 189, 1515–1524. [Google Scholar] [CrossRef] [Green Version]
- Grimm, A.; Sahi, V.P.; Amann, S.; Vidotto, F.; Fogliatto, S.; Devos, K.M.; Ferrero, A.; Nick, P. Italian weedy rice—A case of de-domestication? Ecol. Evol. 2020, 10, 8449–8464. [Google Scholar] [CrossRef]
- Lee, D.; Lupotto, E.; Powell, W. G-string slippage turns white rice red. Genome 2008, 52, 490–493. [Google Scholar] [CrossRef]
- McCouch, S.; Sweeney, M.; Li, J.; Jiang, H.; Thomson, M.; Septiningsih, E.; Moncada, P.; Xiao, J.; Coburn, J.; Fraker, E.; et al. Identification and transfer of trait-enhancing alleles from wild species. In Rice Genetics V: Proceedings of the Fifth International Rice Genetics Symposium, 19–23 November 2005, Manila, Philippines; World Scientific: Singapore; World Scientific: Los Banos, Philippines, 2007; pp. 209–234. [Google Scholar]
- Gross, B.L.; Reagon, M.; Hsu, S.-C.; Caicedo, A.L.; Jia, Y.; Olsen, K.M. Seeing red: The origin of grain pigmentation in US weedy rice. Mol. Ecol. 2010, 19, 3380–3393. [Google Scholar] [CrossRef] [Green Version]
- Gu, X.-Y.; Kianian, S.F.; Foley, M.E. Seed dormancy imposed by covering tissues interrelates to shattering and seed morphological characteristics in weedy rice. Crop Sci. 2005, 45, 948–955. [Google Scholar] [CrossRef] [Green Version]
- Wedger, M.J.; Olsen, K.M. Evolving insights on weedy rice. Ecol. Genet. Genom. 2018, 7–8, 23–26. [Google Scholar] [CrossRef]
- Islam, M.d.S.; Coronejo, S.; Subudhi, P.K. Whole-genome sequencing reveals uniqueness of black-hulled and straw-hulled weedy rice genomes. Theor. Appl. Genet. 2020, 133, 2461–2475. [Google Scholar] [CrossRef] [PubMed]
- Rathore, M.; Singh, R.; Kumar, B.; Chauhan, B.S. Characterization of functional trait diversity among Indian cultivated and weedy rice populations. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Thurber, C.S.; Reagon, M.; Olsen, K.M.; Jia, Y.; Caicedo, A.L. The evolution of flowering strategies in US weedy rice. Am. J. Bot. 2014, 101, 1737–1747. [Google Scholar] [CrossRef] [Green Version]
- Saragih, A.A.; Puteh, A.B.; Ismail, M.R.; Mondal, M.M.A. Pollen quality traits of cultivated (Oryza sativa L. ssp. indica) and weedy (Oryza sativa var. nivara) rice to water stress at reproductive stage. Aust. J. Crop Sci. 2013, 7, 1106–1112. [Google Scholar]
- Craig, S.M.; Reagon, M.; Resnick, L.E.; Caicedo, A.L. Allele distributions at hybrid incompatibility loci facilitate the potential for gene flow between cultivated and weedy rice in the US. PLoS ONE 2014, 9, e86647. [Google Scholar] [CrossRef] [Green Version]
- Messeguer, J.; Marfà, V.; Català, M.M.; Guiderdoni, E.; Melè, E. A field study of pollen-mediated gene flow from Mediterranean GM rice to conventional rice and the red rice weed. Mol. Breed. 2004, 13, 103–112. [Google Scholar] [CrossRef]
- Andres, A.; Fogliatto, S.; Ferrero, A.; Vidotto, F. Susceptibility to imazamox in Italian weedy rice populations and Clearfield® rice varieties. Weed Res. 2014, 54, 492–500. [Google Scholar] [CrossRef]
- Song, X.; Wang, Z.; Qiang, S. Agronomic performance of F1, F2 and F3 hybrids between weedy rice and transgenic glufosinate-resistant rice. Pest Manag. Sci. 2011, 67, 921–931. [Google Scholar] [CrossRef]
- Oard, J.; Cohn, M.A.; Linscombe; Gealy, D. Field evaluation of seed production, shattering, and dormancy in hybrid populations of transgenic rice (Oryza sativa) and the weed, red rice (O. sativa). Plant Sci. 2000, 157, 13–22. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Lin, S.Y.; Sasaki, T.; Yano, M. Fine linkage mapping enables dissection of closely linked quantitative trait loci for seed dormancy and heading in rice. Theor. Appl. Genet. 2003, 107, 1174–1180. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.-Y.; Pipatpongpinyo, W.; Zhang, L.; Zhou, Y.; Ye, H.; Feng, J. Two Contrasting patterns and underlying genes for coadaptation of seed dormancy and flowering time in rice. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zainudin, H.; Azmi, M.; Othman, A.S. Morphological study of the relationships between weedy rice accessions (Oryza sativa complex) and commercial rice varieties in Penang’s rice granary area. Trop. Life Sci. Res. 2010, 21, 47–62. [Google Scholar]
- Labrada, R. Present trends in weed management. In Weed Management for Developing Countries -FAO Plant Production and Protection Paper; R. Labrada: Rome, Italy, 2003; Volume 120, p. 227. [Google Scholar]
- Cai, H.-W.; Morishima, H. Genomic regions affecting seed shattering and seed dormancy in rice. Theor. Appl. Genet. 2000, 100, 840–846. [Google Scholar] [CrossRef]
- Gu, X.Y.; Kianian, S.F.; Hareland, G.A.; Hoffer, B.L.; Foley, M.E. Genetic analysis of adaptive syndromes interrelated with seed dormancy in weedy rice (Oryza sativa). Theor. Appl. Genet. 2005, 110, 1108–1118. [Google Scholar] [CrossRef]
- Thurber, C.S.; Hepler, P.K.; Caicedo, A.L. Timing is everything: Early degradation of abscission layer is associated with increased seed shattering in U.S. weedy rice. BMC Plant Biol. 2011, 11, 14. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, S.; Stallworth, S.; Tseng, T.-M. Weedy Rice: Competitive Ability, Evolution, and Diversity. In Integrated View of Population Genetics; Trindade Maia, R., de Araújo Campos, M., Eds.; IntechOpen: London, UK, 2019; ISBN 978-1-78985-777-1. [Google Scholar]
- Qasem, J.R. Weed Seed Dormancy: The Ecophysiology and Survival Strategies. In Seed Dormancy and Germination; Jimenez-Lopez, J., Ed.; IntechOpen: London, UK, 2019; ISBN 978-1-78985-777-1. [Google Scholar]
- Xie, K.; Jiang, L.; Lu, B.; Yang, C.; Li, L.; Liu, X.; Zhang, L.; Zhao, Z.; Wan, J. Identification of QTLs for seed dormancy in rice (Oryza sativa L.). Plant Breed. 2011, 130, 328–332. [Google Scholar] [CrossRef]
- Sun, J.; Qian, Q.; Ma, D.-R.; Xu, Z.-J.; Liu, D.; Du, H.-B.; Chen, W.-F. Introgression and selection shaping the genome and adaptive loci of weedy rice in northern China. New Phytol. 2013, 197, 290–299. [Google Scholar] [CrossRef]
- Liu, K.; Baskin, J.M.; Baskin, C.C.; Bu, H.; Du, G.; Ma, M. Effect of diurnal fluctuating versus constant temperatures on germination of 445 species from the Eastern Tibet Plateau. PLoS ONE 2013, 8, e69364. [Google Scholar] [CrossRef] [Green Version]
- Fogliatto, S.; Vidotto, F.; Ferrero, A. Effects of winter flooding on weedy rice (Oryza sativa L.). Crop Prot. 2010, 29, 1232–1240. [Google Scholar] [CrossRef]
- Cohn, M.A.; Hughes, J.A. Seed dormancy in red rice (Oryza sativa) I. Effect of temperature on dry-afterripening. Weed Sci. 1981, 29, 402–404. [Google Scholar] [CrossRef]
- Bathiany, S.; Dakos, V.; Scheffer, M.; Lenton, T.M. Climate models predict increasing temperature variability in poor countries. Sci. Adv. 2018, 4, eaar5809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, N.-J.; Paek, N.-C. Photoblastism and ecophysiology of seed germination in weedy rice. Agron. J. 2003, 95, 184–190. [Google Scholar] [CrossRef]
- Andrew, I.K.S.; Storkey, J.; Sparkes, D.L. A review of the potential for competitive cereal cultivars as a tool in integrated weed management. Weed Res. 2015, 55, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.-B.; Xia, H.; Ellstrand, N.C.; Yang, C.; Lu, B.-R. Rapid evolutionary divergence and ecotypic diversification of germination behavior in weedy rice populations. New Phytol. 2011, 191, 1119–1127. [Google Scholar] [CrossRef]
- Streck, N.A.; Uhlmann, L.O.; Gabriel, L.F. Leaf development of cultivated rice and weedy red rice under elevated temperature scenarios. Rev. Bras. Eng. Agríc. E Ambient. 2013, 17, 190–199. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, C.; Agostinetto, D.; Langaro, A.C.; Garcia, J.R.; Lamego, F.P. Physiological and molecular responses in rice, weedy rice and barnyardgrass exposed to supra-optimal temperatures. Planta Daninha 2019, 37, e019182522. [Google Scholar] [CrossRef]
- Ramesh, K.; Matloob, A.; Aslam, F.; Florentine, S.K.; Chauhan, B.S. Weeds in a changing climate: Vulnerabilities, consequences, and implications for future weed management. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
- Lee, J.-S. Combined effect of elevated CO2 and temperature on the growth and phenology of two annual C3 and C4 weedy species. Agric. Ecosyst. Environ. 2011, 140, 484–491. [Google Scholar] [CrossRef]
- Bevilacqua, C.B.; Basu, S.; Pereira, A.; Tseng, T.-M.; Zimmer, P.D.; Burgos, N.R. Analysis of stress-responsive gene expression in cultivated and weedy rice differing in cold stress tolerance. PLoS ONE 2015, 10, e0132100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borjas, A.H.; De Leon, T.B.; Subudhi, P.K. Genetic analysis of germinating ability and seedling vigor under cold stress in US weedy rice. Euphytica 2016, 208, 251–264. [Google Scholar] [CrossRef]
- Han, B.; Ma, X.; Cui, D.; Wang, Y.; Geng, L.; Cao, G.; Zhang, H.; Han, L. comprehensive evaluation and analysis of the mechanism of cold tolerance based on the transcriptome of weedy rice seedlings. Rice 2020, 13. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Han, Y.; Li, X.; Dai, W.; Song, X.; Olsen, K.M.; Qiang, S. Climate-dependent variation in cold tolerance of weedy rice and rice mediated by OsICE1 promoter methylation. Mol. Ecol. 2020, 29, 121–137. [Google Scholar] [CrossRef]
- Baek, J.-S.; Chung, N.-J. Seed wintering and deterioration characteristics between weedy and cultivated rice. Rice 2012, 5, 21. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Mo, S.-D.; Kong, M.-Y.; Chao, J.; Chen, X.-F.; Yang, J.-L.; Yan, Y.-J.; Shi, Z.-H.; Qiang, S.; Song, X.-L.; et al. Better performance of germination in hyperosmotic solutions in conspecific weedy rice than cultivated rice. J. Syst. Evol. 2019, 57, 519–529. [Google Scholar] [CrossRef]
- Koziol, L.; Rieseberg, L.H.; Kane, N.; Bever, J.D. Reduced drought tolerance during domestication and the evolution of weediness results from tolerance–growth trade-offs. Evolution 2012, 66, 3803–3814. [Google Scholar] [CrossRef]
- Puteh, A.B.; Jali, N.; Ismail, M.R.; Juraimi, A.S.; Samsudin, N. Pollen and Seed Yield Components of Water-stressed Cultivated and Weedy Rice. Pertanika J. Trop. Agric. Sci. 2009, 32, 293–303. [Google Scholar]
- Puteh, A.B.; Saragih, A.A.; Ismail, M.R.; Mondal, M.M.A. Chlorophyll fluorescence parameters of cultivated (Oryza sativa L. ssp. indica) and weedy rice (Oryza sativa L. var. nivara) genotypes under water stress. Aust. J. Crop Sci. 2013, 7, 1277–1283. [Google Scholar]
- Jeong, J.-M.; Cho, Y.-C.; Jeong, J.-U.; Mo, Y.-J.; Kim, C.-S.; Kim, W.-J.; Baek, M.-K.; Kim, S.-M. QTL mapping and effect confirmation for anaerobic germination tolerance derived from the japonica weedy rice landrace PBR. Plant Breed. 2020, 139, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Magneschi, L.; Perata, P. Rice germination and seedling growth in the absence of oxygen. Ann. Bot. 2009, 103, 181–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, D.; Rathore, M.; Brahmachari, K.; Singh, R.; Kumar, B. Impact of burial and flooding depths on Indian weedy rice. Crop Prot. 2017, 100, 106–110. [Google Scholar] [CrossRef]
- Mohd Hanafiah, N.; Mispan, M.S.; Lim, P.E.; Baisakh, N.; Cheng, A. The 21st century agriculture: When rice research draws attention to climate variability and how weedy rice and underutilized grains come in handy. Plants 2020, 9, 365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velmurugan, A.; Swarnam, T.P.; Ambast, S.K.; Kumar, N. Managing waterlogging and soil salinity with a permanent raised bed and furrow system in coastal lowlands of humid tropics. Agric. Water Manag. 2016, 168, 56–67. [Google Scholar] [CrossRef]
- Akbari, M.; Najafi Alamdarlo, H.; Mosavi, S.H. The effects of climate change and groundwater salinity on farmers’ income risk. Ecol. Indic. 2020, 110, 105893. [Google Scholar] [CrossRef]
- Singh, A. Soil salinization and waterlogging: A threat to environment and agricultural sustainability. Ecol. Indic. 2015, 57, 128–130. [Google Scholar] [CrossRef]
- Bertazzini, M.; Sacchi, G.A.; Forlani, G. A differential tolerance to mild salt stress conditions among six Italian rice genotypes does not rely on Na+ exclusion from shoots. J. Plant Physiol. 2018, 226, 145–153. [Google Scholar] [CrossRef]
- Serra, F.; Fogliatto, S.; Vidotto, F. Effect of salinity on Echinochloa crus-galli germination as affected by herbicide resistance. Ital. J. Agron. 2018, 13, 221–228. [Google Scholar] [CrossRef]
- Hakim, M.A.; Juraimi, A.S.; Hanafi, M.M.; Ismail, M.R.; Rafii, M.Y.; Aslani, F.; Selamat, A. The effect of salinity on chlorophyll, proline and mineral nutrients in common weeds of coastal rice fields in Malaysia. J. Environ. Biol. 2014, 35, 855–864. [Google Scholar]
- Hakim, M.A.; Juraimi, A.S.; Hanafi, M.M.; Selamat, A.; Ismail, M.R.; Karim, S.M.R. Studies on seed germination and growth in weed species of rice field under salinity stress. J. Environ. Biol. 2011, 32, 529–536. [Google Scholar]
- Zhang, Y.; Fang, J.; Wu, X.; Dong, L. Na+/K+ balance and transport regulatory mechanisms in weedy and cultivated rice (Oryza sativa L.) under salt stress. BMC Plant Biol. 2018, 18, 375. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, R. Climate Change: Atmospheric Carbon Dioxide. Available online: https://www.climate.gov/news-features/understanding-climate/climate-change-atmospheric-carbon-dioxide (accessed on 19 May 2020).
- Ziska, L.H.; Tomecek, M.B.; Gealy, D.R. Competitive interactions between cultivated and red rice as a function of recent and projected increases in atmospheric carbon dioxide. Agron. J. 2010, 102, 118–123. [Google Scholar] [CrossRef] [Green Version]
- Shah, K.; Chaturvedi, V.; Gupta, S. Climate Change and Abiotic Stress-Induced Oxidative Burst in Rice. In Advances in Rice Research for Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Nahar, K., Biswas, J.K., Eds.; Elsevier: Duxford, UK, 2019; pp. 505–535. ISBN 978-0-12-814332-2. [Google Scholar]
- Ziska, L.H.; Gealy, D.R.; Tomecek, M.B.; Jackson, A.K.; Black, H.L. Recent and projected increases in atmospheric CO2 concentration can enhance gene flow between wild and genetically altered rice (Oryza sativa). PLoS ONE 2012, 7, e37522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziska, L.H.; Tomecek, M.B.; Gealy, D.R. Assessment of cultivated and wild, weedy rice lines to concurrent changes in CO2 concentration and air temperature: Determining traits for enhanced seed yield with increasing atmospheric CO2. Funct. Plant Biol. 2014, 41, 236–243. [Google Scholar] [CrossRef]
- Durand-Morat, A.; Nalley, L.L. Economic benefits of controlling red rice: A case study of the United States. Agronomy 2019, 9, 422. [Google Scholar] [CrossRef] [Green Version]
- Milan, M.; Ferrero, A.; Fogliatto, S.; De Palo, F.; Vidotto, F. Imazamox dissipation in two rice management systems. J. Agric. Sci. 2017, 155, 431–443. [Google Scholar] [CrossRef] [Green Version]
- Ziska, L.H. The role of climate change and increasing atmospheric carbon dioxide on weed management: Herbicide efficacy. Agric. Ecosyst. Environ. 2016, 231, 304–309. [Google Scholar] [CrossRef] [Green Version]
- Varanasi, A.; Prasad, P.V.V.; Jugulam, M. Impact of climate change factors on weeds and herbicide efficacy. In Advances in Agronomy; Sparks, D.L., Ed.; Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2016; Volume 135, pp. 107–146. [Google Scholar] [CrossRef]
- Formentin, E.; Sudiro, C.; Perin, G.; Riccadonna, S.; Barizza, E.; Baldoni, E.; Lavezzo, E.; Stevanato, P.; Sacchi, G.A.; Fontana, P.; et al. Transcriptome and cell physiological analyses in different rice cultivars provide new insights into adaptive and salinity stress responses. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [Green Version]


| Weedy Rice Traits | Trait Range between Weedy Rice Populations |
|---|---|
| Awn presence | awnless, mucronate, awned |
| Awn color | straw, black, brown |
| Awn distribution (section of the panicle in which are present awned grains) | tip only; 1/4 upper only; upper half only; 3/4 total length; whole length |
| Awn length (mm) | 1.3–52.6 |
| Hull coloration | straw, black, brown |
| 1000 seed weight (g) | 22.8–41.3 |
| Number of seeds per panicle | 80.3–205.7 |
| Whole seed length (mm) | 6.7–9.8 |
| Whole seed width (mm) | 2.6–4.2 |
| Dehulled seed length (mm) | 5.0–9.2 |
| Dehulled seed width (mm) | 2.3–4.4 |
| Germination level at harvest (%) | 0–33.9 |
| Germination level at 10 days after harvest (%) | 0–48.2 |
| Germination level at 30 days after harvest (%) | 0–73.8 |
| Plant height (excluding panicle) (cm) | 54.6–97.5 |
| Flag leaf attitude of blade | erect, semi-erect, horizontal, recurved |
| Flag leaf length (cm) | 18.3–46.2 |
| Anthocyanin coloration of auricles and nodes | green, purple |
| Panicle attitude in relation to stem | Upright, semi-upright, slightly-drooping, drooping |
| Panicle attitude of branches | Erect, semi-erect, spreading |
| Panicle length (cm) | 16.6–25.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fogliatto, S.; Ferrero, A.; Vidotto, F. How Can Weedy Rice Stand against Abiotic Stresses? A Review. Agronomy 2020, 10, 1284. https://doi.org/10.3390/agronomy10091284
Fogliatto S, Ferrero A, Vidotto F. How Can Weedy Rice Stand against Abiotic Stresses? A Review. Agronomy. 2020; 10(9):1284. https://doi.org/10.3390/agronomy10091284
Chicago/Turabian StyleFogliatto, Silvia, Aldo Ferrero, and Francesco Vidotto. 2020. "How Can Weedy Rice Stand against Abiotic Stresses? A Review" Agronomy 10, no. 9: 1284. https://doi.org/10.3390/agronomy10091284
APA StyleFogliatto, S., Ferrero, A., & Vidotto, F. (2020). How Can Weedy Rice Stand against Abiotic Stresses? A Review. Agronomy, 10(9), 1284. https://doi.org/10.3390/agronomy10091284






