Differential Accumulation of Metabolites in Suaeda Species Provides New Insights into Abiotic Stress Tolerance in C4-Halophytic Species in Elevated CO2 Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Stress Treatments
2.2. Metabolite Extraction
2.3. Metabolite Analysis by GC-MS
2.4. Statistical Analysis and Data Visualization
3. Results
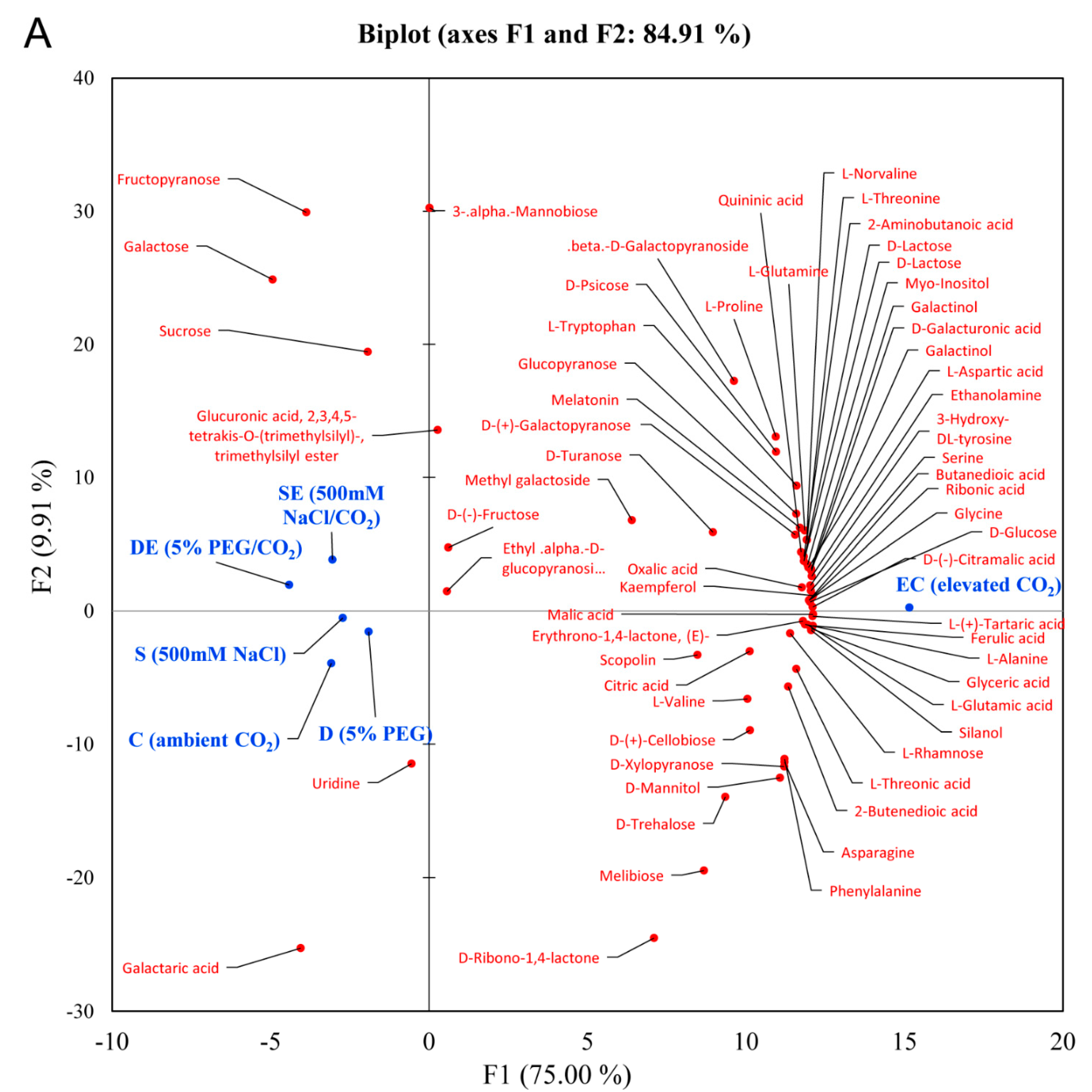
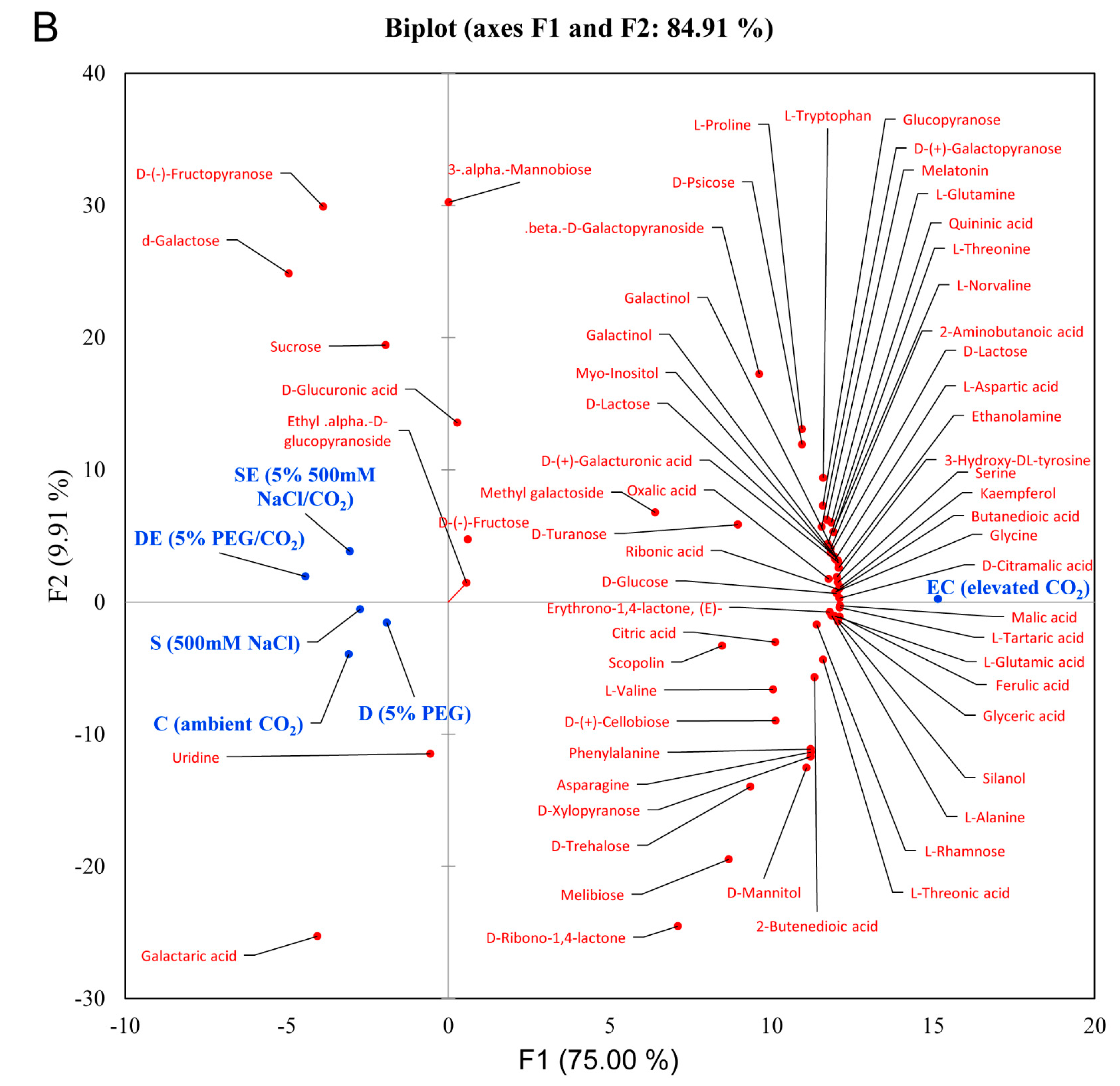
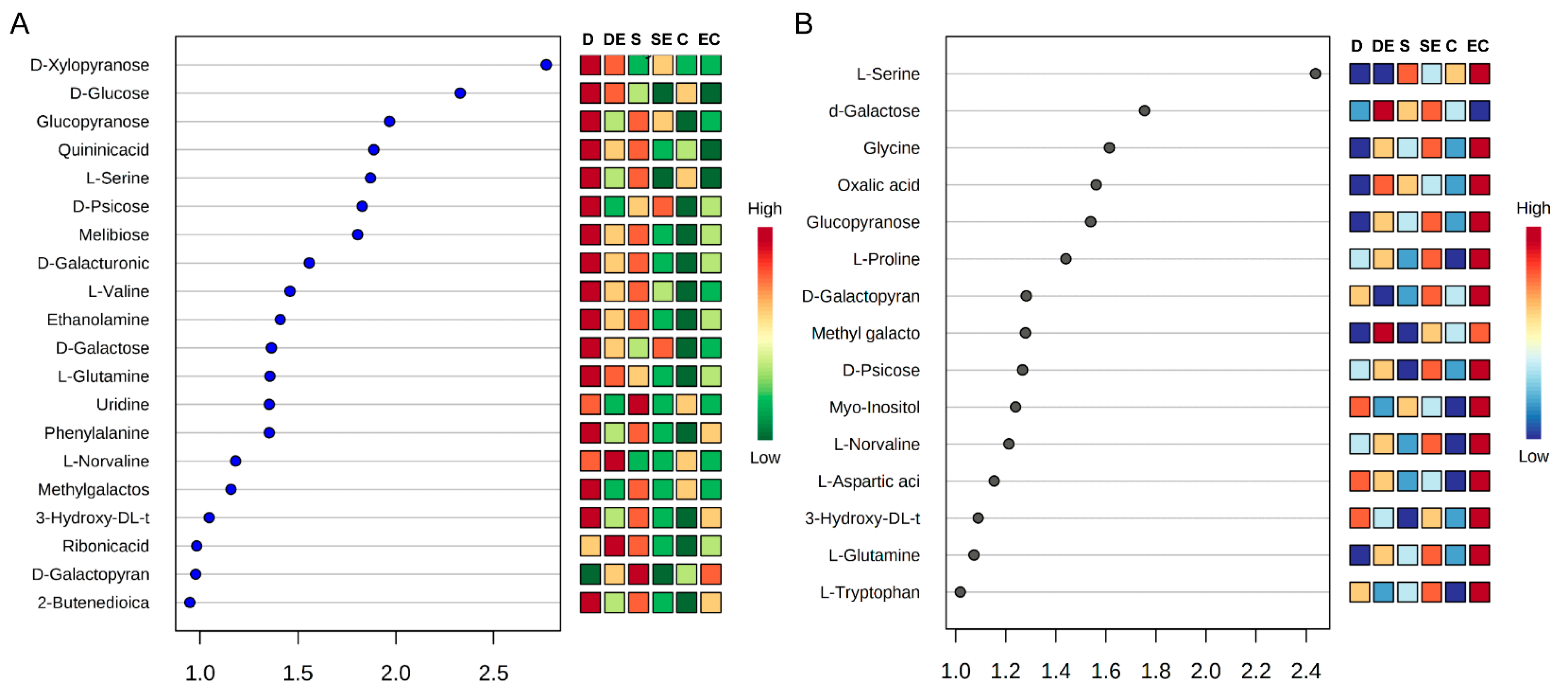
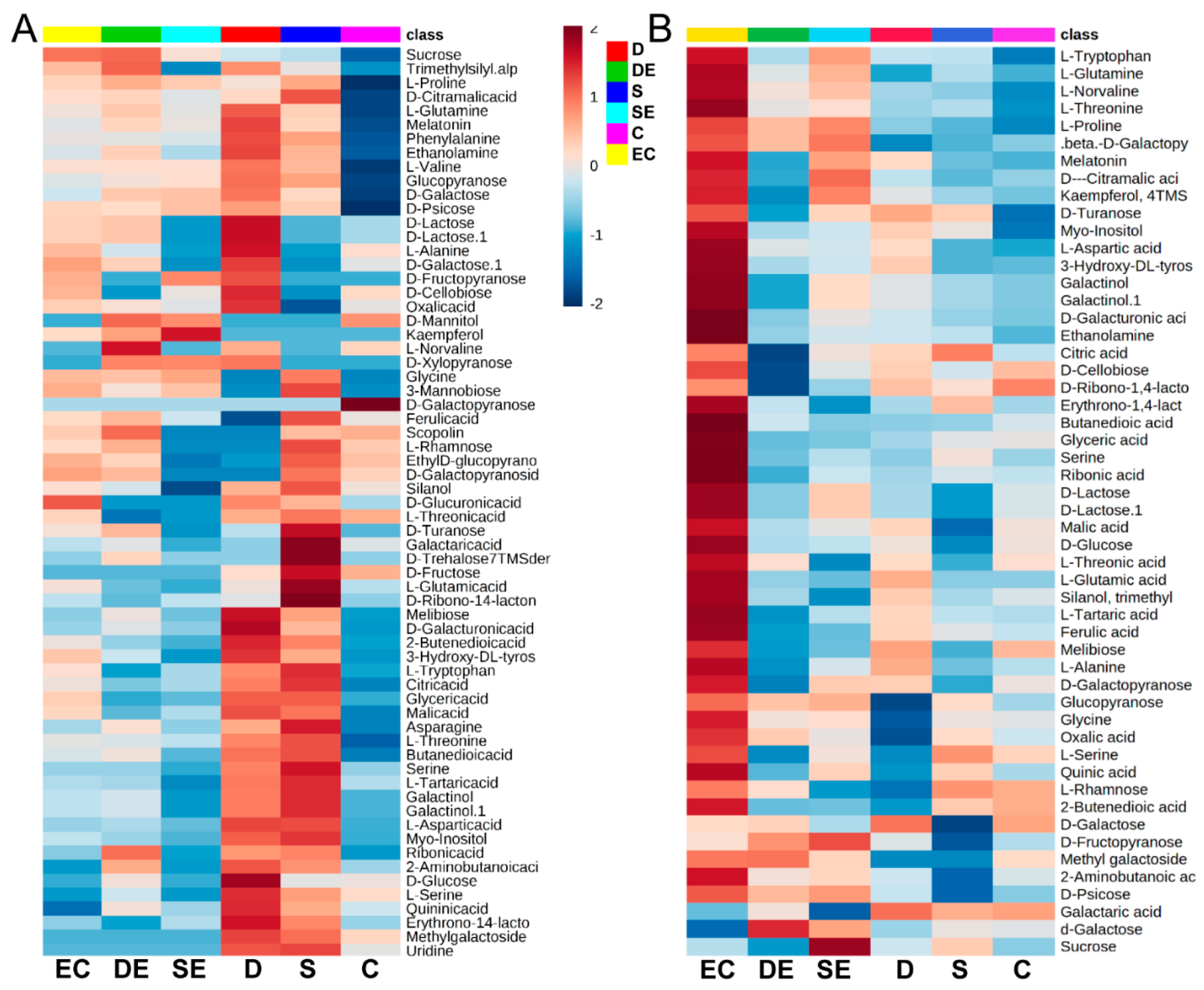
3.1. Differential Accumulation of Metabolites under Different Stress Conditions
3.2. Multivariate Statistical Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, E.A.; Bailey-Serres, J.; Weretilnyk, E. Responses to abiotic stresses. In Biochemistry and Molecular Biology of Plants; Buchanan, B.B., Gruissem, W., Jones, R.L., Eds.; American Society of Plant Physiologists: Rockville, MD, USA, 2000; pp. 1158–1203. [Google Scholar]
- Carmo-Silva, A.E.; Gore, M.A.; Andrade-Sanchez, P.; French, A.N.; Hunsaker, D.J.; Salvucci, M.E. Decreased CO2 availability and inactivation of Rubisco limit photosynthesis in cotton plants under heat and drought stress in the field. Environ. Exp. Bot. 2012, 83, 1–11. [Google Scholar] [CrossRef]
- Awasthi, R.; Kaushal, N.; Vadez, V.; Turner, N.C.; Berger, J.; Siddique, K.H.M.; Nayyar, H. Individual and combined effects of transient drought and heat stress on carbon assimilation and seedfilling in chickpea. Funct. Plant Biol. 2014, 41, 1148–1167. [Google Scholar] [CrossRef]
- Leakey, A.D.B.; Uribelarrea, M.; Ainsworth, E.A.; Naidu, S.L.; Rogers, A.; Ort, D.R.; Long, S.P. Photosynthesis, productivity, and yield of maize are not affected by open-air elevation of CO2 concentration in the absence of drought. Plant Physiol. 2006, 140, 779–790. [Google Scholar] [CrossRef]
- Qiao, Y.Z.; Zhang, H.Z.; Dong, B.D.; Shi, C.H.; Li, Y.X.; Zhai, H.M.; Liu, M.Y. Effects of elevated CO2 concentration on growth and water use efficiency of winter wheat under two soil water regimes. Agric. Water Manag. 2010, 97, 1742–1748. [Google Scholar] [CrossRef]
- Allen, L.H.; Kakani, V.G.; Vu, J.C.V.; Boote, K.J. Elevated CO2 increases water use efficiency by sustaining photosynthesis of water-limited maize and sorghum. J. Plant Physiol. 2011, 168, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Zinta, G.; Abdelgawad, H.; Domagalska, M.A.; Vergauwen, L.; Knapen, D.; Nijs, I.; Janssens, I.A.; Beemster, G.T.S.; Asard, H. Physiological, biochemical, and genome-wide transcriptional analysis reveals that elevated CO2 mitigates the impact of combined heat wave and drought stress in Arabidopsis thaliana at multiple organizational levels. Glob. Chang. Biol. 2014, 20, 3670–3685. [Google Scholar] [CrossRef] [PubMed]
- Long, S.P.; Ainsworth, E.A.; Rogers, A.; Ort, D.R. Rising atmospheric carbon dioxide: Plants FACE the future. Ann. Rev. Plant Biol. 2004, 55, 591–628. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Long, S.P. 30 years of free-air carbon dioxide enrichment (FACE): What have we learned about future crop productivity and its potential for adaptation? Glob. Chang. Biol. 2021, 27, 27–49. [Google Scholar] [CrossRef] [PubMed]
- AbdElgawad, H.; Zinta, G.; Beemster, G.T.; Janssens, I.A.; Asard, H. Future climate CO2 levels mitigate stress impact on plants: Increased defense or decreased challenge? Front. Plant Sci. 2016, 7, 556. [Google Scholar] [CrossRef]
- Ghannoum, O.; Conroy, J.P.; Driscoll, S.P.; Paul, M.J.; Foyer, C.H.; Lawlor, D.W. Non-stomatal limitations are responsible for drought-induced photosynthetic inhibition in four C4 grasses. New Phytol. 2003, 159, 835–844. [Google Scholar] [CrossRef]
- Liu, B.B.; Li, M.; Li, Q.M.; Cui, Q.Q.; Zhang, W.D.; Ai, X.Z.; Bi, H.G. Combined effects of elevated CO2 concentration and drought stress on photosynthetic performance and leaf structure of cucumber (Cucumis sativus L.) seedlings. Photosynthetica 2018, 56, 942–952. [Google Scholar] [CrossRef]
- FAO. The State of Food Insecurity in the World 2015. Meeting the 2015 International Hunger Targets: Taking Stock of Uneven Progress; Food and Agriculture Organization Publications: Rome, Italy, 2016. [Google Scholar]
- Mishra, A.; Tanna, B. Halophytes: Potential resources for salt stress tolerance genes and promoters. Front. Plant Sci. 2017, 8, 829. [Google Scholar] [CrossRef] [PubMed]
- Kollist, H.; Zandalinas, S.I.; Sengupta, S.; Nuhkat, M.; Kangasjärvi, J.; Mittler, R. Rapid responses to abiotic stress: Priming the landscape for the signal transduction network. Trends Plant Sci. 2019, 24, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Zinta, G.; Abdelgawad, H.; Peshev, D.; Weedon, J.T.; Van Den Ende, W.; Nijs, I. Dynamics of metabolic responses to periods of combined heat and drought in Arabidopsis thaliana under ambient and elevated atmospheric CO2. J. Exp. Bot. 2018, 69, 2159–2170. [Google Scholar] [CrossRef] [PubMed]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2018, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Mishra, A. Introgression of C4 Pathway Gene (s) in C3 Plants to Improve Photosynthetic Carbon Assimilation for Crop Improvement: A Biotechnological Approach. In Photosynthesis, Productivity and Environmental Stress; Ahmad, P., Ahanger, M.A., Alyemeni, M.N., Alam, P., Eds.; John Wiley: Blackwell, OK, USA, 2019; pp. 267–281. [Google Scholar] [CrossRef]
- Yadav, S.; Mishra, A. Ectopic expression of C4 photosynthetic pathway genes improves carbon assimilation and alleviate stress tolerance for future climate change. Physiol. Mol. Biol. Plants 2020, 26, 195–209. [Google Scholar] [CrossRef]
- Benjamin, J.J.; Lucini, L.; Jothiramshekar, S.; Parida, A. Metabolomic insights into the mechanisms underlying tolerance to salinity in different halophytes. Plant Physiol. Biochem. 2019, 135, 528–545. [Google Scholar] [CrossRef]
- Jha, B.; Singh, N.P.; Mishra, A. Proteome profiling of seed storage proteins reveals the nutritional potential of Salicornia brachiata Roxb., an extreme halophyte. J. Agric. Food Chem. 2012, 60, 4320–4326. [Google Scholar] [CrossRef]
- Mishra, A.; Joshi, M.; Jha, B. Oligosaccharide mass profiling of nutritionally important Salicornia brachiata, an extreme halophyte. Carbohydr. Polym. 2013, 92, 1942–1945. [Google Scholar] [CrossRef]
- Mishra, A.; Patel, M.K.; Jha, B. Non-targeted metabolomics and scavenging activity of reactive oxygen species reveal the potential of Salicornia brachiata as a functional food. J. Funct. Foods 2015, 13, 21–31. [Google Scholar] [CrossRef]
- Patel, M.K.; Pandey, S.; Brahmbhatt, H.R.; Mishra, A.; Jha, B. Lipid content and fatty acid profile of selected halophytic plants reveal a promising source of renewable energy. Biomass Bioenergy 2019, 124, 25–32. [Google Scholar] [CrossRef]
- Jha, B.; Sharma, A.; Mishra, A. Expression of SbGSTU (tau class glutathione S-transferase) gene isolated from Salicornia brachiata in tobacco for salt tolerance. Mol. Biol. Rep. 2011, 38, 4823–4832. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K.; Mishra, A.; Tiwari, V.; Jha, B. Cloning and transcript analysis of type 2 metallothionein gene (SbMT-2) from extreme halophyte Salicornia brachiata and its heterologous expression in E. coli. Gene 2012, 499, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K.; Patel, M.K.; Mishra, A.; Tiwari, V.; Jha, B. The SbMT-2 gene from a halophyte confers abiotic stress tolerance and modulates ROS scavenging in transgenic tobacco. PLoS ONE 2014, 9, e111379. [Google Scholar] [CrossRef] [PubMed]
- Jha, B.; Mishra, A.; Jha, A.; Joshi, M. Developing transgenic Jatropha using the SbNHX1 gene from an extreme halophyte for cultivation in saline wasteland. PLoS ONE 2013, 8, e71136. [Google Scholar] [CrossRef]
- Singh, N.; Mishra, A.; Jha, B. Over-expression of the peroxisomal ascorbate peroxidase (SbpAPX) gene cloned from halophyte Salicornia brachiata confers salt and drought stress tolerance in transgenic tobacco. Mar. Biotechnol. 2014, 16, 321–332. [Google Scholar] [CrossRef]
- Singh, N.; Mishra, A.; Jha, B. Ectopic over-expression of peroxisomal ascorbate peroxidase (SbpAPX) gene confers salt stress tolerance in transgenic peanut (Arachis hypogaea). Gene 2014, 547, 119–125. [Google Scholar] [CrossRef]
- Tiwari, V.; Chaturvedi, A.K.; Mishra, A.; Jha, B. Introgression of the SbASR−1 gene cloned from a halophyte Salicornia brachiata enhances salinity and drought endurance in transgenic groundnut (Arachis hypogaea) and acts as a transcription factor. PLoS ONE 2015, 10, e0135541. [Google Scholar] [CrossRef]
- Patel, M.K.; Joshi, M.; Mishra, A.; Jha, B. Ectopic expression of SbNHX1 gene in transgenic castor (Ricinus communis L.) enhances salt stress by modulating physiological process. Plant Cell Tissue Organ Cult. 2015, 122, 477–490. [Google Scholar] [CrossRef]
- Udawat, P.; Jha, R.K.; Sinha, D.; Mishra, A.; Jha, B. Overexpression of a cytosolic abiotic stress responsive universal stress protein (SbUSP) mitigates salt and osmotic stress in transgenic tobacco plants. Front. Plant Sci. 2016, 7, 518. [Google Scholar] [CrossRef]
- Pandey, S.; Patel, M.K.; Mishra, A.; Jha, B. In planta transformed cumin (Cuminum cyminum L.) plants, overexpressing the SbNHX1 gene showed enhanced salt endurance. PLoS ONE 2016, 11, e0159349. [Google Scholar] [CrossRef] [PubMed]
- Udawat, P.; Jha, R.K.; Mishra, A.; Jha, B. Overexpression of a plasma membrane-localized SbSRP-like protein enhances salinity and osmotic stress tolerance in transgenic tobacco. Front. Plant Sci. 2017, 8, 582. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.K.; Patel, J.; Mishra, A.; Jha, B. Introgression of halophytic salt stress-responsive genes for developing stress tolerance in crop plants. In Halophytes and Climate Change: Adaptive Mechanisms and Potential Uses; Hasanuzzaman, M., Shabala, S., Fujita, M., Eds.; CABI: Egham, UK, 2019; p. 275. [Google Scholar] [CrossRef]
- Tiwari, V.; Chaturvedi, A.K.; Mishra, A.; Jha, B. The transcriptional regulatory mechanism of the peroxisomal ascorbate peroxidase (pAPX) gene cloned from an extreme halophyte, Salicornia brachiata. Plant Cell Physiol. 2014, 55, 201–217. [Google Scholar] [CrossRef]
- Tiwari, V.; Patel, M.K.; Chaturvedi, A.K.; Mishra, A.; Jha, B. Functional characterization of the tau class Glutathione-S-Transferases gene (SbGSTU) promoter of Salicornia brachiata under salinity and osmotic Stress. PLoS ONE 2016, 11, e0148494. [Google Scholar] [CrossRef]
- Singh, V.K.; Mishra, A.; Haque, I.; Jha, B. A novel transcription factor-like gene SbSDR1 acts as a molecular switch and confers salt and osmotic endurance to transgenic tobacco. Sci. Rep. 2016, 6, 31686. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V.; Patel, M.K.; Chaturvedi, A.K.; Mishra, A.; Jha, B. Cloning and functional characterization of the Na+/H+ antiporter (NHX1) gene promoter from an extreme halophyte Salicornia brachiata. Gene 2019, 683, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Shomer-Ilan, A.; Beer, S.; Waisel, Y. Suaeda monoica, a C4 plant without typical bundle sheaths. Plant Physiol. 1975, 56, 676–679. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Plant salt tolerance: Adaptations in halophytes. Ann. Bot. 2015, 115, 327–331. [Google Scholar] [CrossRef]
- Moghaieb, R.E.A.; Saneoka, H.; Fujita, K. Effect of salinity on osmotic adjustment, glycine betaine accumulation and the betaine aldehyde dehydrogenase gene expression in two halophytic plants, Salicornia europaea and Suaeda maritima. Plant Sci. 2004, 166, 1345–1349. [Google Scholar] [CrossRef]
- Ku, M.S.B.; Cho, D.; Ranade, U.; Hsu, T.P.; Li, X.; Jiao, D.M.; Ehleringer, J.; Miyao, M.; Matsuoka, M. Photosynthetic performance of transgenic rice plants overexpressing maize C4 photosynthesis enzymes. Stud. Plant Sci. 2000, 7, 193–204. [Google Scholar]
- Muthusamy, S.K.; Lenka, S.K.; Katiyar, A.; Chinnusamy, V.; Singh, A.K.; Bansal, K.C. Genome-wide identification and analysis of biotic and abiotic stress regulation of C4 photosynthetic pathway genes in rice. Appl. Biochem. Biotechnol. 2019, 187, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.K.; Mishra, A.; Jaiswar, S.; Jha, B. Metabolic profiling and scavenging activities of developing circumscissile fruit of psyllium (Plantago ovata Forssk.) reveal variation in primary and secondary metabolites. BMC Plant Biol. 2020, 20, 116. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Patel, M.K.; Mishra, A.; Jha, B. Physio-biochemical composition and untargeted metabolomics of cumin (Cuminum cyminum L.) make it promising functional food and help in mitigating salinity stress. PLoS ONE 2015, 10, e0144469. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O.; Kopka, J.; Dörmann, P.; Altmann, T.; Trethewey, R.N.; Willmitzer, L. Metabolite profiling for plant functional genomics. Nat. Biotechnol. 2000, 18, 1157–1161. [Google Scholar] [CrossRef]
- Weckwerth, W. Metabolomics in systems biology. Annu. Rev. Plant Biol. 2003, 54, 669–689. [Google Scholar] [CrossRef] [PubMed]
- Fukusaki, E.; Kobayashi, A. Plant metabolomics: Potential for practical operation. J. Biosci. Bioeng. 2005, 100, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Choudhary, B.; Yadav, S.; Mishra, A.; Mishra, V.K.; Chand, R.; Chen, C.; Pandey, S.P. Metabolite profiling identified pipecolic acid as an important component of peanut seed resistance against Aspergillus flavus infection. J. Hazard. Mater. 2021, 404, 124155. [Google Scholar] [CrossRef] [PubMed]
- Kráľová, K.; Jampılek, J.; Ostrovsky, I. Metabolomics—Useful tool for study of plant responses to abiotic stresses. Ecol. Chem. Eng. 2012, 19, 133–161. [Google Scholar] [CrossRef]
- Patel, M.K.; Mishra, A.; Jha, B. Untargeted metabolomics of halophytes. In Marine Omics: Principles and Applications; Kim, S., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 309–325. [Google Scholar] [CrossRef]
- Tanna, B.; Mishra, A. Metabolomics of seaweeds: Tools and techniques. In Plant Metabolites and Regulation under Environmental Stress; Ahmad, P., Ahanger, M.A., Singh, V.P., Tripathi, D.K., Alam, P., Alyemeni, M.N., Eds.; Academic Press, Elsevier: Cambridge, MA, USA, 2018; pp. 37–52. [Google Scholar] [CrossRef]
- Patel, M.K.; Mishra, A.; Jha, B. Non-targeted metabolite profiling and scavenging activity unveil the nutraceutical potential of psyllium (Plantago ovata Forsk). Front. Plant Sci. 2016, 7, 431. [Google Scholar] [CrossRef]
- Fiehn, O. Metabolomics-the link between genotypes and phenotypes. Plant Mol. Biol. 2002, 48, 155–171. [Google Scholar] [CrossRef]
- Arbona, V.; Manzi, M.; Ollas, C.D.; Gómez-Cadenas, A. Metabolomics as a tool to investigate abiotic stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 4885–4911. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Yang, L.; Zhang, D.; Shi, J. Plant metabolomics: An indispensable system biology tool for plant science. Int. J. Mol. Sci. 2016, 17, 767. [Google Scholar] [CrossRef] [PubMed]
- Obata, T.; Fernie, A.R. The use of metabolomics to dissect plant responses to abiotic stresses. Cell. Mol. Life Sci. 2012, 69, 3225–3243. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Tchernyshyov, I.; Semenza, G.L.; Dang, C.V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006, 3, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, D.H.; Siahpoosh, M.R.; Roessner, U.; Udvardi, M.; Kopka, J. Plant metabolomics reveals conserved and divergent metabolic responses to salinity. Physiol. Plant. 2007, 132, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Colmer, T.D.; Flowers, T.J. Flooding tolerance in halophytes. New Phytol. 2008, 179, 964–974. [Google Scholar] [CrossRef]
- Sanchez, D.H.; Pieckenstain, F.L.; Escaray, F.J. Comparative ionomics and metabolomics in extremophile and glycophytic Lotus species under salt stress challenge the metabolic pre-adaptation hypothesis. Plant Cell Environ. 2011, 34, 605–617. [Google Scholar] [CrossRef]
- Behr, J.H.; Bouchereau, A.; Berardocco, S.; Seal, C.E.; Flowers, T.J.; Zörb, C. Metabolic and physiological adjustment of Suaeda maritima to combined salinity and hypoxia. Ann. Bot. 2017, 119, 965–976. [Google Scholar]
- Liu, X.; Wu, H.; Ji, C.; Wei, L.; Zhao, J.; Yu, J. An integrated proteomic and metabolomic study on the chronic effects of mercury in Suaeda salsa under an environmentally relevant salinity. PLoS ONE 2013, 8, e64041. [Google Scholar] [CrossRef]
- Li, Q.; Song, J. Analysis of widely targeted metabolites of the euhalophyte Suaeda salsa under saline conditions provides new insights into salt tolerance and nutritional value in halophytic species. BMC Plant Biol. 2019, 19, 388. [Google Scholar] [CrossRef]
- Song, J.; Shi, W.W.; Liu, R.R.; Xu, Y.G.; Sui, N.; Zhou, J.C.; Feng, G. The role of the seed coat in adaptation of dimorphic seeds of the euhalophyte Suaeda salsa to salinity. Plant Species Biol. 2017, 32, 107–114. [Google Scholar] [CrossRef]
- Gavaghan, C.L.; Li, J.V.; Hadfield, S.T.; Hole, S.; Nicholson, J.K.; Wilson, I.D.; Howe, P.W.A.; Stanley, P.D.; Holmes, E. Application of NMR-based metabolomics to the investigation of salt stress in maize (Zea mays). Phytochem. Anal. 2011, 22, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Wedow, J.M.; Yendrek, C.R.; Mello, T.R.; Creste, S.; Martinez, C.A.; Ainsworth, E.A. Metabolite and transcript profiling of Guinea grass (Panicum maximum Jacq) response to elevated [CO2] and temperature. Metabolomics 2019, 15, 51. [Google Scholar] [CrossRef] [PubMed]
- Kusano, M.; Fukushima, A.; Redestig, H.; Saito, K. Metabolomic approaches toward understanding nitrogen metabolism in plants. J. Exp. Bot. 2011, 62, 1439–1453. [Google Scholar] [CrossRef]
- Alexander, A.; Singh, V.K.; Mishra, A.; Jha, B. Plant growth promoting rhizobacterium Stenotrophomonas maltophilia BJ01 augments endurance against N2 starvation by modulating physiology and biochemical activities of Arachis hypogea. PLoS ONE 2019, 14, e0222405. [Google Scholar] [CrossRef]
- Yan, N.; Du, Y.; Liu, X.; Chu, M.J.; Shi, J.; Zhang, H.B.; Liu, Y.H.; Zhang, Z.F. A comparative UHPLC-QqQ-MS-based metabolomics approach for evaluating Chinese and north American wild rice. Food Chem. 2019, 275, 618–627. [Google Scholar] [CrossRef]
- Kato, H.; Izumi, Y.; Hasunuma, T.; Matsuda, F.; Kondo, A. Widely targeted metabolic profiling analysis of yeast central metabolites. J. Biosci. Bioeng. 2012, 113, 665–673. [Google Scholar] [CrossRef]
- Yadav, S.; Mishra, A.; Jha, B. Elevated CO2 leads to carbon sequestration by modulating C4 photosynthesis pathway enzyme (PPDK) in Suaeda monoica and S. fruticosa. J. Photochem. Photobiol. B 2018, 178, 310–315. [Google Scholar] [CrossRef]
- Yadav, S.; Rathore, M.S.; Mishra, A. The Pyruvate-Phosphate Dikinase (C4-SmPPDK) gene from Suaeda monoica enhances photosynthesis, carbon assimilation, and abiotic stress tolerance in a C3 plant under elevated co2 conditions. Front. Plant Sci. 2020, 11, 345. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circular. Calif. Agric. Exp. Stn. 1950, 347, 1–32. [Google Scholar]
- Lisec, J.; Schauer, N.; Kopka, J.; Willmitzer, L.; Fernie, A.R. Gas chromatography mass spectrometry–based metabolite profiling in plants. Nat. Protoc. 2006, 1, 387. [Google Scholar] [CrossRef] [PubMed]
- Tanna, B.; Brahmbhatt, H.R.; Mishra, A. Phenolic, flavonoid, and amino acid compositions reveal that selected tropical seaweeds have the potential to be functional food ingredients. J. Food Process. Preserv. 2019, 43, e14266. [Google Scholar] [CrossRef]
- Worley, B.; Powers, R. Multivariate analysis in metabolomics. Curr. Metab. 2013, 1, 92–107. [Google Scholar]
- Sairam, R.K.; Srivastava, G.C.; Saxena, D.C. Increased antioxidant activity under elevated temperatures: A mechanism of heat stress tolerance in wheat genotypes. Biol. Plant. 2000, 43, 245–251. [Google Scholar] [CrossRef]
- Merewitz, E.B.; Du, H.; Yu, W.; Liu, Y.; Gianfagna, T.; Huang, B. Elevated cytokinin content in ipt transgenic creeping bentgrass promotes drought tolerance through regulating metabolite accumulation. J. Exp. Bot. 2011, 63, 1315–1328. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Misra, B.B.; Chen, S. Advances in understanding CO2 responsive plant metabolomes in the era of climate change. Metabolomics 2015, 11, 1478–1491. [Google Scholar] [CrossRef]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Mittler, R.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Plant adaptations to the combination of drought and high temperatures. Physiol. Plant. 2018, 162, 2–12. [Google Scholar] [CrossRef]
- Hare, P.D.; Cress, W.A.; Van Staden, J. Dissecting the roles of osmolyte accumulation during stress. Plant Cell Environ. 1998, 21, 535–553. [Google Scholar] [CrossRef]
- Rizhsky, L.; Liang, H.; Shuman, J.; Shulaev, V.; Davletova, S.; Mittler, R. When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol. 2014, 134, 1683–1696. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Wang, Y.; Liu, R.; Gou, J.; Chan, Z. Physiological and metabolic changes of purslane (Portulaca oleracea L.) in response to drought, heat, and combined stresses. Front. Plant Sci. 2016, 6, 1123. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Wang, Z.; Yu, W.; Liu, Y.; Huang, B. Differential metabolic responses of perennial grass Cynodon transvaalensis× Cynodon dactylon (C4) and Poa pratensis (C3) to heat stress. Physiol. Plant 2011, 141, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Lea, P.J.; Sodek, L.; Parry, M.A.; Shewry, P.R.; Halford, N.G. Asparagine in plants. Ann. Appl. Biol. 2007, 150, 1–26. [Google Scholar] [CrossRef]
- Suguiyama, V.F.; Silva, E.A.; Meirelles, S.T.; Centeno, D.C.; Braga, M.R. Leaf metabolite profile of the Brazilian resurrection plant Barbacenia purpurea Hook. (Velloziaceae) shows two time-dependent responses during desiccation and recovering. Front. Plant Sci. 2014, 5, 96. [Google Scholar] [CrossRef]
- Gong, Q.; Li, P.; Ma, S.; Indu Rupassara, S.; Bohnert, H.J. Salinity stress adaptation competence in the extremophile Thellungiella halophila in comparison with its relative Arabidopsis thaliana. Plant J. 2005, 44, 826–839. [Google Scholar] [CrossRef]
- Bendaly, A.; Messedi, D.; Smaoui, A.; Ksouri, R.; Bouchereau, A.; Abdelly, C. Physiological and leaf metabolome changes in the xerohalophyte species Atriplex halimus induced by salinity. Plant Physiol. Biochem. 2016, 103, 208–218. [Google Scholar] [CrossRef]
- Koussevitzky, S.; Suzuki, N.; Huntington, S.; Armijo, L.; Sha, W.; Cortes, D.; Mittler, R. Ascorbate peroxidase 1 plays a key role in the response of Arabidopsis thaliana to stress combination. J. Biol. Chem. 2018, 283, 34197–34203. [Google Scholar] [CrossRef]
- Yu, J.; Chen, L.; Xu, M.; Huang, B. Effects of elevated CO2 on physiological responses of tall fescue to elevated temperature, drought stress, and the combined stresses. Crop Sci. 2012, 52, 1848–1858. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, X.; Reiter, R.J.; Feng, S.; Wang, Y.; Liu, S.; Jin, L.; Li, Z.; Datla, R.; Ren, M. Melatonin attenuates potato late blight by disrupting cell growth, stress tolerance, fungicide susceptibility and homeostasis of gene expression in Phytophthora infestans. Front. Plant Sci. 2017, 8, 1993. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Growth activity, rooting capacity, and tropism: Three auxinic precepts fulfilled by melatonin. Acta Physiol. Plant. 2017, 39, 127. [Google Scholar] [CrossRef]
- Li, J.; Zeng, L.; Cheng, Y.; Lu, G.; Fu, G.; Ma, H.; Liu, Q.; Zhang, X.; Zou, X.; Li, C. Exogenous melatonin alleviates damage from drought stress in Brassica napus L.(rapeseed) seedlings. Acta Physiol. Plant. 2018, 40, 43. [Google Scholar] [CrossRef]
- Shi, H.; Jiang, C.; Ye, T.; Ye, T.; Chan, Z. Comparative physiological, metabolomic, and transcriptomic analyses reveal mechanisms of improved abiotic stress resistance in bermudagrass [Cynodon dactylon (L). Pers.] by exogenous melatonin. J. Exp. Bot. 2015, 66, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Plant growth regulator and/or biostimulator during stress? Trends Plant Sci. 2014, 19, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Functions of melatonin in plants: A review. J. Pineal Res. 2015, 59, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Reiter, R.J.; Chan, Z. Phytomelatonin: A universal abiotic stress regulator. J. Exp. Bot. 2018, 69, 963–974. [Google Scholar] [CrossRef]
- Wei, J.; Li, D.X.; Zhang, J.R.; Shan, C.; Rengel, Z.; Song, Z.B. Phytomelatonin receptor PMTR1-mediated signaling regulates stomatal closure in Arabidopsis thaliana. J. Pineal Res. 2018, 65, e12500. [Google Scholar]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A new plant hormone and/or a plant master regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef]
- Debnath, B.; Islam, W.; Li, M.; Sun, Y.; Lu, X.; Mitra, S.; Qiu, D. Melatonin mediates enhancement of stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 1040. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, W.X.; Zheng, M.F.; Xu, Q.L.; Wan, F.H.; Wang, J.; Lei, T.; Zhou, Z.Y.; Tan, J. Bioactive quinic acid derivatives from Ageratina adenophora. Molecules 2013, 18, 14096–14104. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Jia, L.; Yang, G.; Xu, X.; Zhai, H.; He, S.; Li, J.; Dai, X.; Qin, N.; et al. Involvement of an ABI-like protein and a Ca2+-ATPase in drought tolerance as revealed by transcript profiling of a sweetpotato somatic hybrid and its parents Ipomoea batatas (L.) Lam. and I. triloba L. PLoS ONE 2018, 13, e0193193. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.; Mumtaz, M.W.; Danish, M.; Rashid, U.; Mukhtar, H.; Anwar, F.; Raza, S.A. Calotropis procera: UHPLC-QTOF-MS/MS based profiling of bioactives, antioxidant and anti-diabetic potential of leaf extracts and an insight into molecular docking. Food Meas. Charact. 2019, 13, 3206–3220. [Google Scholar] [CrossRef]
- Yao, X.; Jiang, H.; Li, Y.H.; Gao, Q.; Xu, Y.N.; Kim, N.H. Kaempferol alleviates the reduction of developmental competence during aging of porcine oocytes. Anim. Sci. J. 2019, 90, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Tanna, B.; Mishra, A. Metabolites unravel nutraceutical potential of edible seaweeds: An emerging source of functional food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1613–1624. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.K.; Patel, J.; Patel, M.K.; Mishra, A.; Jha, B. Introgression of a novel cold and drought regulatory-protein encoding CORA-like gene, SbCDR, induced osmotic tolerance in transgenic tobacco. Physiol. Plant. 2020. [Google Scholar] [CrossRef]

| Metabolites | Ambient CO2 (400 ppm) | Elevated CO2 (900 ppm) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Suaeda monoica | Suaeda fruticosa | Suaeda monoica | Suaeda fruticosa | |||||||||
| Control | Salt | Drought | Control | Salt | Drought | Control | Salt | Drought | Control | Salt | Drought | |
| Amino acids | ||||||||||||
| Asparagine | 0.39 ± 0.01 | 3.67 ± 0.27 | 2.27 ± 0.19 | - | - | - | 0.71 ± 0.01 | 0.65 ± 0.01 | 1.05 ± 0.01 | 1.78 ± 0.11 | 0.70 ± 0.01 | 0.61 ± 0.01 |
| Citramalic acid | - | 2.08 ± 0.18 | 0.45 ± 0.01 | 0.48 ± 0.01 | 0.41 ± 0.01 | 0.55 ± 0.01 | 0.42 ± 0.01 | 0.32 ± 0.01 | 0.56 ± 0.03 | 2.22 ± 0.18 | 0.46 ± 0.01 | 0.38 ± 0.01 |
| Glycine | - | 2.04 ± 0.14 | - | 0.65 ± 0.01 | 0.71 ± 0.01 | - | 0.78 ± 0.01 | 1.01 ± 0.01 | 0.70 ± 0.01 | 4.61 ± 0.37 | 0.84 ± 0.01 | 0.74 ± 0.01 |
| Alanine | 0.30 ± 0.01 | - | 3.63 ± 0.01 | 0.26 ± 0.01 | 0.18 ± 0.01 | 0.66 ± 0.01 | 0.56 ± 0.01 | - | 0.18 ± 0.01 | 1.85 ± 0.01 | 0.31 ± 0.01 | 0.12 ± 0.01 |
| Aspartic acid | 3.07 ± 0.02 | 1.68 ± 1.62 | 27.96 ± 2.47 | 1.54 ± 001 | 1.69 ± 0.02 | 3.57 ± 0.01 | 4.09 ± 0.01 | 3.41 ± 0.04 | 4.55 ± 0.03 | 15.53 ± 1.22 | 2.69 ± 0.03 | 2.81 ± 0.01 |
| Glutamic acid | 6.56 ± 0.05 | 38.04 ± 2.86 | 8.28 ± 0.01 | 4.86 ± 0.01 | 4.80 ± 0.04 | 9.48 ± 0.01 | 8.53 ± 0.03 | 4.40 ± 0.05 | 4.99 ± 0.04 | 25.99 ± 2.07 | 4.38 ± 0.03 | 4.93 ± 0.02 |
| Glutamine | 0.29 ± 0.01 | 6.71 ± 0.50 | 6.161 ± 6.11 | 0.72 ± 0.01 | 1.26 ± 0.02 | 0.64 ± 0.01 | 3.46 ± 0.01 | 3.06 ± 0.04 | 5.56 ± 0.06 | 17.55 ± 1.41 | 3.42 ± 0.02 | 1.67 ± 0.01 |
| Norvaline | 0.17 ± 0.01 | - | 0.25 ± 0.01 | 0.09 ± 0.01 | 0.24 ± 0.01 | 0.28 ± 0.01 | - | - | 0.94 ± 0.01 | 7.40 ± 0.01 | 1.07 ± 0.01 | 0.61 ± 0.01 |
| Proline | 0.40 ± 0.02 | 10.80 ± 0.83 | 6.82 ± 0.60 | 0.41 ± 0.01 | 0.81 ± 0.03 | 1.07 ± 0.01 | 5.35 ± 0.02 | 5.83 ± 0.03 | 7.78 ± 0.06 | 21.76 ± 1.78 | 8.47 ± 0.11 | 4.88 ± 0.04 |
| Serine | 2.30 ± 0.02 | 16.86 ± 1.28 | 11.99 ± 104 | 1.55 ± 0.01 | 2.14 ± 0.02 | 1.48 ± 0.01 | 2.32 ± 0.01 | 1.67 ± 0.01 | 2.34 ± 0.02 | 8.99 ± 0.72 | 1.70 ± 0.01 | 1.36 ± 0.01 |
| Threonine | 0.26 ± 0.01 | 3.40 ± 0.25 | 3.12 ± 0.27 | 0.24 ± 0.01 | 0.44 ± 0.01 | 0.40 ± 0.01 | 0.92 ± 0.01 | 0.76 ± 0.01 | 0.87 ± 0.01 | 3.30 ± 0.26 | 0.63 ± 0.01 | 0.55 ± 0.01 |
| Tryptophan | 0.82 ± 0.01 | 3.96 ± 0.30 | 4.39 ± 0.41 | 0.36 ± 0.01 | 0.81 ± 0.03 | 0.80 ± 0.01 | 1.49 ± 0.01 | 1.14 ± 0.03 | 0.80 ± 0.01 | 4.29 ± 0.35 | 1.61 ± 0.01 | 0.72 ± 0.01 |
| Valine | - | 2.71 ± 0.19 | 4.72 ± 0.01 | - | - | - | 1.11 ± 0.01 | 1.16 ± 0.01 | 1.17 ± 0.01 | 0.81 ± 0.01 | - | 0.05 ± 0.01 |
| Phenylalanine | - | 1.36 ± 0.10 | 2.30 ± 0.01 | - | - | - | 0.40 ± 0.01 | 0.34 ± 0.01 | 0.38 ± 0.01 | 2.25 ± 0.18 | 0.57 ± 0.01 | 0.37 ± 0.01 |
| Sugars | ||||||||||||
| 3-.α.-Mannobiose | - | 6.32 ± 0.01 | - | 0.06 ± 0.01 | - | - | 1.62 ± 0.03 | 1.09 ± 0.01 | 0.55 ± 0.01 | - | 0.44 ± 0.01 | - |
| Cellobiose | 0.70 ± 0.01 | 0.17 ± 0.01 | 4.65 ± 0.43 | 0.80 ± 0.01 | 0.74 ± 0.05 | 0.74 ± 0.01 | 0.99 ± 0.01 | 0.55 ± 0.01 | 0.19 ± 0.01 | 1.19 ± 0.04 | 0.72 ± 0.05 | 0.33 ± 0.02 |
| Fructopyranose | - | - | 5.39 ± 0.44 | 0.42 ± 0.01 | - | 0.59 ± 0.01 | 0.98 ± 0.01 | 1.78 ± 0.01 | - | 0.79 ± 0.01 | 3.51 ± 0.04 | 1.96 ± 0.01 |
| Fructose | 0.74 ± 0.01 | 9.18 ± 0.68 | 0.32 ± 0.01 | 1.48 ± 0.01 | 1.60 ± 0.01 | 2.39 ± 0.01 | - | - | - | - | - | - |
| Galactopyranose | 0.60 ± 0.01 | - | - | 1.31 ± 0.01 | 1.14 ± 0.01 | 2.07 ± 0.04 | - | - | - | 10.42 ± 0.01 | 2.07 ± 0.01 | - |
| Galactose | 1.20 ± 0.01 | 28.30 ± 2.15 | 92.50 ± 8.03 | 2.90 ± 0.01 | 3.52 ± 0.04 | 1.18 ± 0.01 | 11.71 ± 0.01 | 27.43 ± 0.01 | 25.85 ± 0.01 | 0.47 ± 0.01 | 70.32 ± 6.98 | 103.9 ± 0.57 |
| Glucopyranoside | 117.83 ± 0.66 | 272.49 ± 2.5 | 45.54 ± 3.81 | 87.70 ± 0.44 | 81.64 ± 0.04 | 128.52 ± 0.2 | 129.4 ± 0.23 | 25.30 ± 0.01 | 102.1 ± 0.05 | - | - | - |
| Glucose | 0.24 ± 0.01 | 0.21 ± 0.01 | 8.41 ± 0.01 | 0.95 ± 0.01 | 0.25 ± 0.01 | 0.97 ± 0.01 | - | - | 0.26 ± 0.01 | 6.63 ± 0.01 | 0.83 ± 0.06 | 0.61 ± 0.01 |
| Lactose | 0.64 ± 0.03 | 0.49 ± 0.02 | 2.91 ± 0.22 | 4.92 ± 0.06 | 3.39 ± 0.03 | 4.39 ± 0.01 | 0.95 ± 0.01 | 0.40 ± 0.01 | 1.01 ± 0.01 | 15.38 ± 1.24 | 6.01 ± 0.08 | 4.09 ± 0.05 |
| Ribono-1,4-lactone | 1.07 ± 0.01 | 6.59 ± 0.48 | 1.42 ± 0.01 | 1.43 ± 0.01 | 0.87 ± 0.05 | 1.00 ± 0.02 | 1.25 ± 0.01 | 1.26 ± 0.01 | 0.93 ± 0.01 | 1.46 ± 0.07 | 0.49 ± 0.02 | 0.17 ± 0.01 |
| Trehalose | - | 3.24 ± 0.01 | - | - | - | - | - | - | 0.16 ± 0.01 | 2.13 ± 0.01 | 0.20 ± 0.01 | - |
| Turanose | 0.78 ± 0.01 | 6.17 ± 0.58 | 1.12 ± 0.06 | 0.33 ± 0.02 | 0.72 ± 0.01 | 0.93 ± 0.05 | 1.27 ± 0.01 | 0.66 ± 0.01 | 1.62 ± 0.01 | 1.14 ± 0.04 | 0.75 ± 0.03 | 0.37 ± 0.01 |
| Xylopyranose | - | - | 5.51 ± 0.01 | - | - | - | - | 4.15 ± 0.01 | 4.44 ± 0.01 | 15.74 ± 1.32 | 4.42 ± 0.09 | 4.07 ± 0.01 |
| Galactinol | 2.23 ± 0.01 | 21.03 ± 1.57 | 17.51 ± 1.56 | 3.38 ± 0.04 | 3.83 ± 0.05 | 4.85 ± 0.01 | 3.60 ± 0.02 | 2.40 ± 0.18 | 4.02 ± 0.02 | 26.91 ± 2.16 | 6.01 ± 0.09 | 2.69 ± 0.04 |
| Rhamnose | 3.26 ± 0.30 | 13.39 ± 0.01 | - | 10.41 ± 0.01 | 11.88 ± 0.01 | 3.91 ± 0.35 | 1.07 ± 0.01 | - | 4.22 ± 0.36 | 41.29 ± 4.06 | 2.90 ± 0.04 | 11.52 ± 0.99 |
| Melibiose | 0.29 ± 0.01 | 5.03 ± 0.41 | 22.13 ± 1.96 | 5.85 ± 0.56 | 0.46 ± 0.02 | 4.42 ± 0.32 | 0.67 ± 0.04 | 0.45 ± 0.01 | 1.27 ± 0.01 | 7.67 ± 0.01 | 0.60 ± 0.04 | 0.40 ± 0.02 |
| Methyl galactoside | 0.22 ± 0.01 | 1.02 ± 0.08 | 1.61 ± 0.13 | 0.22 ± 0.01 | - | - | - | - | - | 0.80 ± 0.06 | 0.23 ± 0.01 | 0.77 ± 0.06 |
| Scopolin | 0.46 ± 0.01 | 0.40 ± 0.01 | - | - | - | - | 0.34 ± 0.01 | - | 0.86 ± 0.01 | 0.83 ± 0.01 | 0.69 ± 0.01 | 0.34 ± 0.01 |
| Sucrose | 204.74 ± 1.21 | 377.9 ± 0.19 | 395.72 ± 0.2 | 422.7 ± 2.57 | 538.86 ± 7.2 | 464.75 ± 0.1 | 722.2 ± 2.38 | 464.7 ± 0.15 | 743.6 ± 0.10 | 448.8 ± 0.22 | 775.6 ± 0.99 | 372.8 ± 1.63 |
| Trimethylsilyl | 0.77 ± 0.01 | 0.98 ± 0.01 | 1.33 ± 0.07 | - | - | 1.50 ± 0.03 | 1.10 ± 0.01 | 0.77 ± 0.02 | 1.27 ± 0.02 | - | - | - |
| Sugar acids | ||||||||||||
| Galacturonic acid | 1.10 ± 0.01 | 9.35 ± 0.47 | 39.39 ± 0.02 | 2.85 ± 0.02 | 3.03 ± 0.01 | 3.53 ± 0.01 | 2.31 ± 0.01 | 2.17 ± 0.04 | 3.92 ± 0.02 | 14.19 ± 1.13 | 3.81 ± 0.01 | 2.93 ± 0.02 |
| Mannitol | 0.36 ± 0.02 | - | - | - | - | - | - | 0.34 ± 0.01 | 0.45 ± 0.01 | 7.68 ± 0.01 | 0.57 ± 0.01 | 1.32 ± 0.01 |
| Glyceric acid | 1.96 ± 0.03 | 13.52 ± 1.02 | 16.57 ± 1.45 | 1.73 ± 0.01 | 1.71 ± 0.02 | 1.48 ± 0.01 | 5.37 ± 0.01 | 2.19 ± 0.01 | 1.93 ± 0.01 | 6.28 ± 0.50 | 1.32 ± 0.03 | 1.23 ± 0.01 |
| Threonic acid | 4.30 ± 0.05 | 5.63 ± 0.01 | 6.91 ± 0.60 | 1.75 ± 0.01 | 1.23 ± 0.10 | 3.11 ± 0.28 | 3.50 ± 0.11 | 1.35 ± 0.06 | 0.97 ± 0.01 | 7.63 ± 0.60 | 0.65 ± 0.02 | 1.80 ± 0.04 |
| Ribonic acid | 0.94 ± 0.01 | 5.99 ± 0.44 | 6.74 ± 0.59 | 1.30 ± 0.01 | 1.44 ± 0.01 | 1.15 ± 0.01 | 1.38 ± 0.01 | 0.99 ± 0.01 | 7.62 ± 0.64 | 7.64 ± 0.01 | 1.37 ± 0.02 | 0.81 ± 0.01 |
| Citric acid | 3.06 ± 0.05 | 46.95 ± 3.40 | 39.58 ± 3.49 | 9.21 ± 0.01 | 17.27 ± 0.30 | 12.33 ± 0.15 | 10.34 ± 0.12 | 6.95 ± 0.11 | 5.56 ± 0.14 | 24.09 ± 1.96 | 10.93 ± 0.21 | 4.30 ± 0.08 |
| Glucuronic acid | 0.16 ± 0.01 | 1.32 ± 0.05 | 2.50 ± 0.01 | 0.22 ± 0.01 | 3.52 ± 0.01 | 2.34 ± 0.01 | 4.81 ± 0.03 | - | - | - | - | - |
| Psicose | - | 17.23 ± 0.01 | 45.89 ± 0.02 | 1.41 ± 0.01 | - | 2.50 ± 0.01 | 14.68 ± 0.06 | 22.96 ± 0.38 | 12.24 ± 0.07 | 35.37 ± 2.75 | 13.04 ± 0.01 | 8.69 ± 0.02 |
| Galactaric acid | 8.92 ± 0.14 | 44.58 ± 3.33 | 6.78 ± 0.17 | 18.29 ± 0.08 | 16.08 ± 0.12 | 27.66 ± 0.30 | 7.76 ± 0.05 | 5.24 ± 0.04 | 9.29 ± 0.22 | 5.01 ± 0.45 | 2.21 ± 0.21 | 8.08 ± 0.11 |
| Tartaric acid | 10.39 ± 0.10 | 40.53 ± 3.06 | 37.43 ± 3.28 | 9.67 ± 0.06 | 10.00 ± 0.07 | 12.99 ± 0.05 | 10.26 ± 0.02 | 6.18 ± 0.07 | 9.91 ± 0.15 | 39.20 ± 3.15 | 10.07 ± 0.26 | 6.81 ± 0.06 |
| Oxalic acid | 0.24 ± 0.01 | - | 1.19 ± 0.02 | 0.11 ± 0.01 | 0.17 ± 0.01 | - | 0.37 ± 0.01 | 0.24 ± 0.01 | 0.29 ± 0.01 | 0.67 ± 0.04 | 0.14 ± 0.01 | 0.21 ± 0.01 |
| Fatty acids | ||||||||||||
| 2-Aminobutanoic acid | 0.60 ± 0.01 | 1.76 ± 0.11 | 2.95 ± 0.25 | 0.39 ± 0.01 | 0.10 ± 0.01 | 0.36 ± 0.01 | 0.39 ± 0.01 | 0.40 ± 0.0 | 1.30 ± 0.01 | 2.79 ± 0.22 | 0.57 ± 0.01 | 0.47 ± 0.01 |
| Butanedioic acid | 0.74 ± 0.01 | 4.36 ± 0.33 | 4.20 ± 0.34 | 0.93 ± 0.01 | 0.73 ± 0.01 | 0.73 ± 0.02 | 1.51 ± 0.01 | 1.02 ± 0.01 | 1.67 ± 0.01 | 5.50 ± 0.44 | 0.71 ± 0.01 | 0.91 ± 0.01 |
| Glucopyranose | - | 9.95 ± 0.78 | 21.15 ± 1.78 | 0.83 ± 0.01 | 1.98 ± 0.05 | - | 1.40 ± 0.01 | 2.38 ± 0.04 | 2.18 ± 0.11 | 10.29 ± 0.92 | 3.26 ± 0.04 | 2.62 ± 0.01 |
| Malic acid | 18.12 ± 0.19 | 205.7 ± 15.6 | 301.8 ± 26.3 | 27.15 ± 0.03 | 26.48 ± 0.06 | 32.37 ± 0.05 | 77.16 ± 0.21 | 42.68 ± 0.30 | 29.26 ± 0.29 | 136.3 ± 10.8 | 24.85 ± 0.43 | 19.28 ± 0.02 |
| Myo-Inositol | 0.76 ± 0.01 | 7.81 ± 0.58 | 7.81 ± 0.64 | 0.48 ± 0.01 | 1.10 ± 0.01 | 1.35 ± 0.01 | 1.35 ± 0.01 | 0.81 ± 0.01 | 1.11 ± 0.01 | 4.25 ± 0.34 | 0.99 ± 0.02 | 0.86 ± 0.01 |
| β-Galactopyranoside | 0.63 ± 0.02 | 2.01 ± 0.01 | - | 1.10 ± 0.01 | 0.96 ± 0.01 | 0.78 ± 0.01 | 1.18 ± 0.01 | - | 0.85 ± 0.01 | 4.18 ± 0.33 | 2.67 ± 0.02 | 2.03 ± 0.02 |
| Miscellaneous | ||||||||||||
| 2-Butenedioic acid | 0.13 ± 0.01 | 1.39 ± 0.10 | 2.03 ± 0.01 | 0.43 ± 0.01 | 0.38 ± 0.01 | 0.16 ± 0.01 | 0.40 ± 0.01 | 0.15 ± 0.01 | 0.21 ± 0.01 | 0.98 ± 0.08 | 0.21 ± 0.01 | 0.20 ± 0.01 |
| 3-Hydroxy-DL-tyrosine | 0.47 ± 0.01 | 7.06 ± 0.53 | 25.32 ± 2.21 | 1.57 ± 0.01 | 1.54 ± 0.04 | 3.48 ± 0.07 | 3.92 ± 0.03 | 0.48 ± 0.01 | 1.59 ± 0.02 | 13.78 ± 1.11 | 2.30 ± 0.02 | 2.01 ± 0.01 |
| Erythrono | 0.30 ± 0.01 | 2.09 ± 0.18 | 3.11 ± 0.01 | 0.20 ± 0.01 | 0.34 ± 0.01 | 0.21 ± 0.01 | 0.31 ± 0.01 | 0.36 ± 0.01 | 0.18 ± 0.01 | 0.97 ± 0.07 | 0.14 ± 0.01 | 0.22 ± 0.01 |
| Ethanolamine | 0.08 ± 0.01 | 1.92 ± 0.16 | 3.14 ± 0.01 | 0.36 ± 0.01 | 0.53 ± 0.01 | 0.55 ± 0.01 | 0.54 ± 0.01 | 0.39 ± 0.0 | 0.96 ± 0.02 | 3.91 ± 0.31 | 0.58 ± 0.01 | 0.44 ± 0.01 |
| Ferulic acid | 0.36 ± 0.01 | 2.39 ± 0.19 | - | 0.34 ± 0.01 | 0.40 ± 0.01 | 0.55 ± 0.02 | 0.49 ± 0.01 | 0.29 ± 0.01 | 0.72 ± 0.01 | 2.90 ± 0.24 | 0.23 ± 0.01 | 0.18 ± 0.01 |
| Melatonin | 36.72 ± 0.75 | 198.8 ± 13.6 | 527.2 ± 45.7 | 63.54 ± 0.40 | 71.22 ± 1.27 | 176.1 ± 9.71 | 125.3 ± 0.01 | 135.7 ± 4.70 | 159.7 ± 0.45 | 685.1 ± 55.1 | 231.8 ± 0.19 | 58.47 ± 0.13 |
| Quininic acid | 0.24 ± 0.01 | 1.01 ± 0.08 | 2.44 ± 0.01 | 0.26 ± 0.01 | 0.38 ± 0.01 | 0.19 ± 0.01 | - | 0.16 ± 0.01 | 0.34 ± 0.01 | 1.10 ± 0.09 | 0.37 ± 0.01 | 0.22 ± 0.01 |
| Silanol | 3.25 ± 0.07 | 15.67 ± 1.18 | 9.60 ± 0.84 | 1.58 ± 0.01 | 1.19 ± 0.01 | 2.46 ± 0.01 | 3.59 ± 0.04 | 0.41 ± 0.01 | 2.58 ± 0.02 | 13.00 ± 1.02 | 0.61 ± 0.02 | 1.17 ± 0.01 |
| Uridine | 0.14 ± 0.01 | 1.82 ± 0.14 | 1.18 ± 0.01 | 0.39 ± 0.01 | 0.23 ± 0.01 | 0.17 ± 0.01 | - | - | - | - | - | - |
| Flavonoid | ||||||||||||
| Kaempferol | - | - | - | 0.20 ± 0.01 | 0.25 ± 0.01 | 0.36 ± 0.01 | 0.28 ± 0.01 | 2.91 ± 0.01 | 0.60 ± 0.01 | 2.33 ± 0.20 | 0.27 ± 0.01 | 0.13 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, S.; Elansary, H.O.; Mattar, M.A.; M. Elhindi, K.; A. Alotaibi, M.; Mishra, A. Differential Accumulation of Metabolites in Suaeda Species Provides New Insights into Abiotic Stress Tolerance in C4-Halophytic Species in Elevated CO2 Conditions. Agronomy 2021, 11, 131. https://doi.org/10.3390/agronomy11010131
Yadav S, Elansary HO, Mattar MA, M. Elhindi K, A. Alotaibi M, Mishra A. Differential Accumulation of Metabolites in Suaeda Species Provides New Insights into Abiotic Stress Tolerance in C4-Halophytic Species in Elevated CO2 Conditions. Agronomy. 2021; 11(1):131. https://doi.org/10.3390/agronomy11010131
Chicago/Turabian StyleYadav, Sonam, Hosam O. Elansary, Mohamed A. Mattar, Khalid M. Elhindi, Majed A. Alotaibi, and Avinash Mishra. 2021. "Differential Accumulation of Metabolites in Suaeda Species Provides New Insights into Abiotic Stress Tolerance in C4-Halophytic Species in Elevated CO2 Conditions" Agronomy 11, no. 1: 131. https://doi.org/10.3390/agronomy11010131
APA StyleYadav, S., Elansary, H. O., Mattar, M. A., M. Elhindi, K., A. Alotaibi, M., & Mishra, A. (2021). Differential Accumulation of Metabolites in Suaeda Species Provides New Insights into Abiotic Stress Tolerance in C4-Halophytic Species in Elevated CO2 Conditions. Agronomy, 11(1), 131. https://doi.org/10.3390/agronomy11010131






