Dissecting the Genotypic and Environmental Factors Underpinning the Quantitative Trait Variation in a Set of Wild Tomato (Solanum habrochaites LA1777) Introgression Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Field Trials and Phenotypic Characterization
2.3. Data Analyses
3. Results
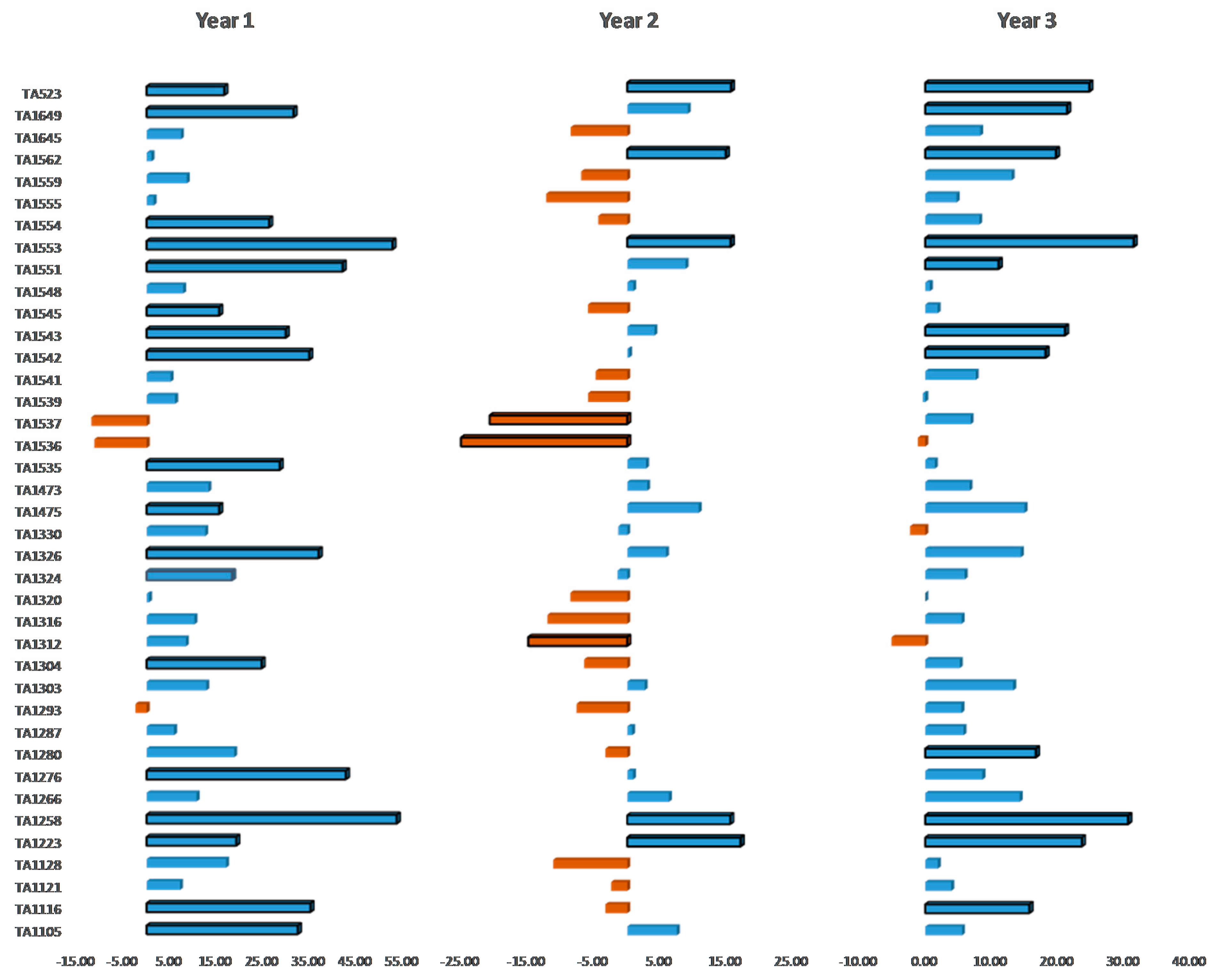
3.1. Phenotypic Variation and Trait Performances of SL and SH ILs
3.2. Genotypic and Environmental Components Underlying Trait Variation
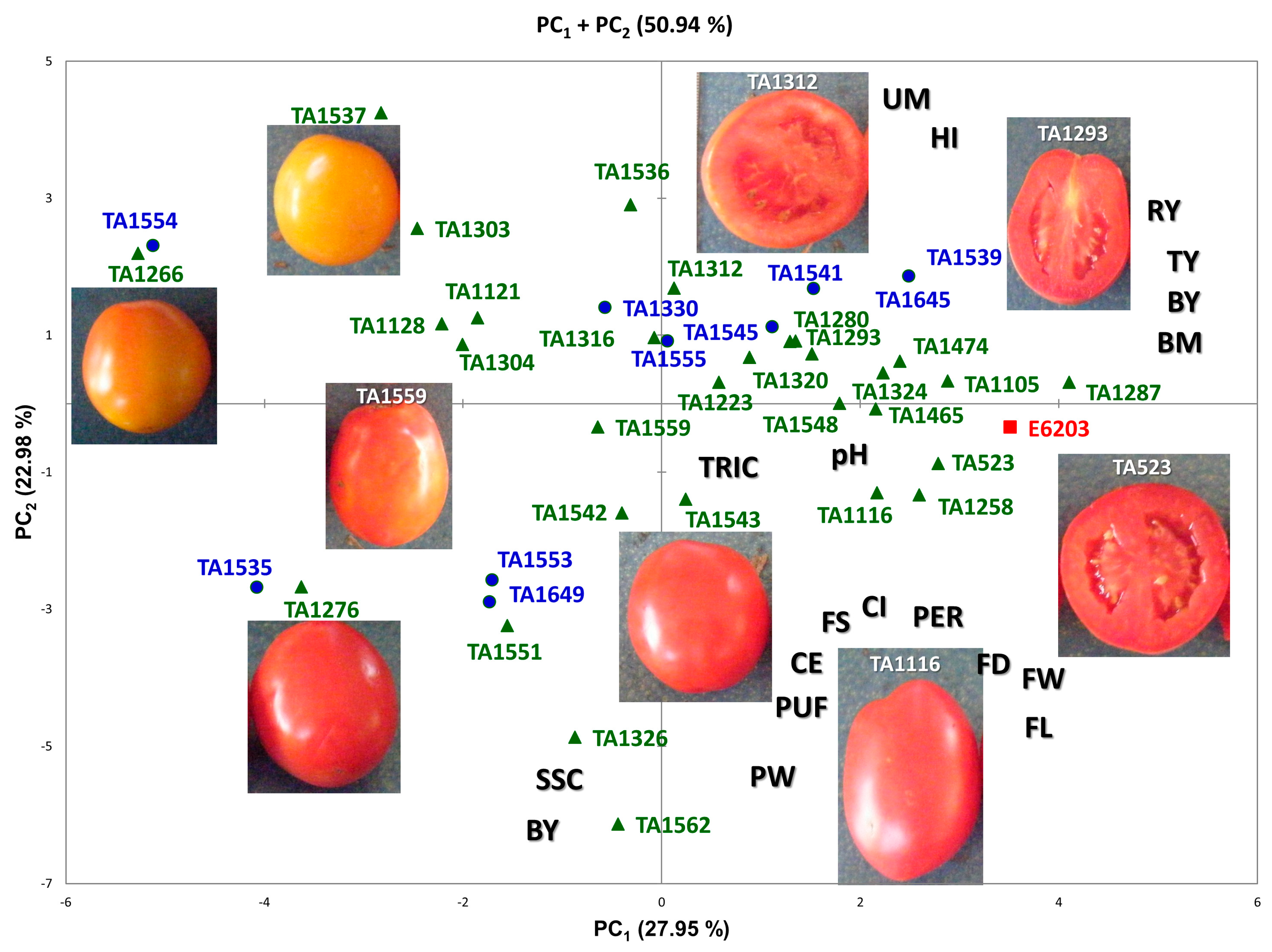
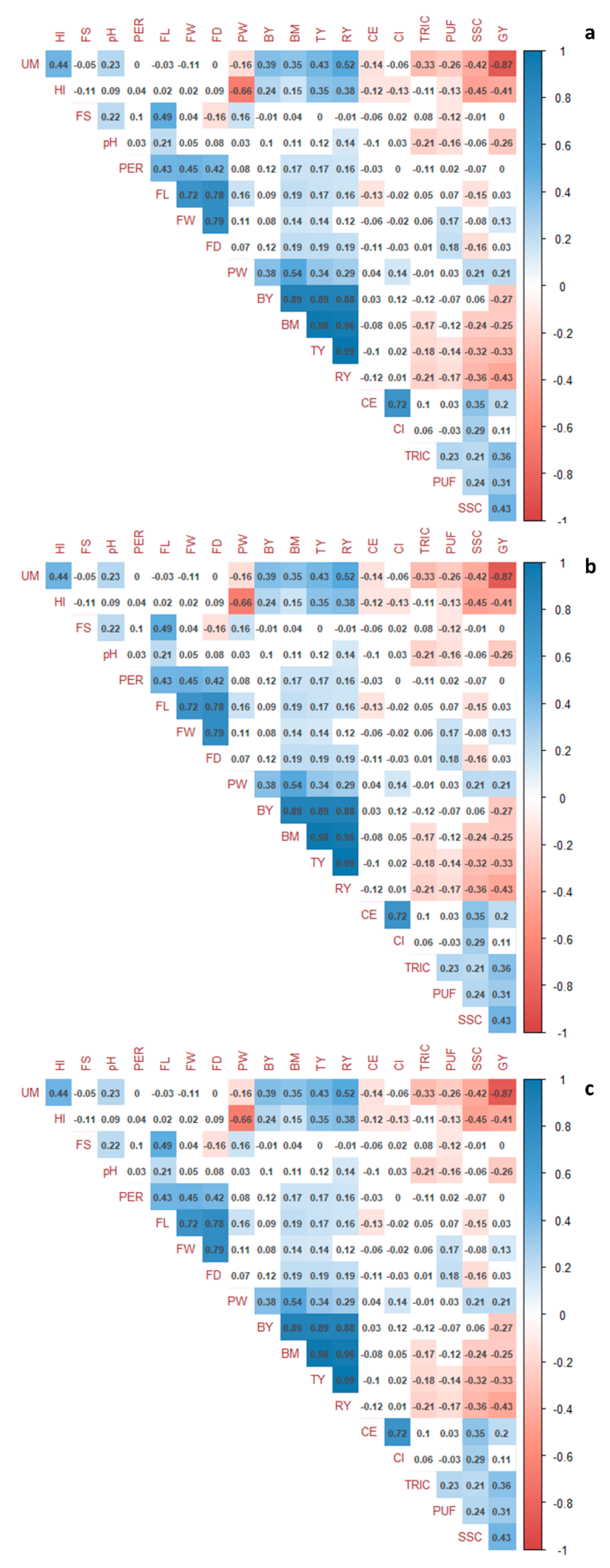
3.3. Multivariate Analysis and Correlations between Traits
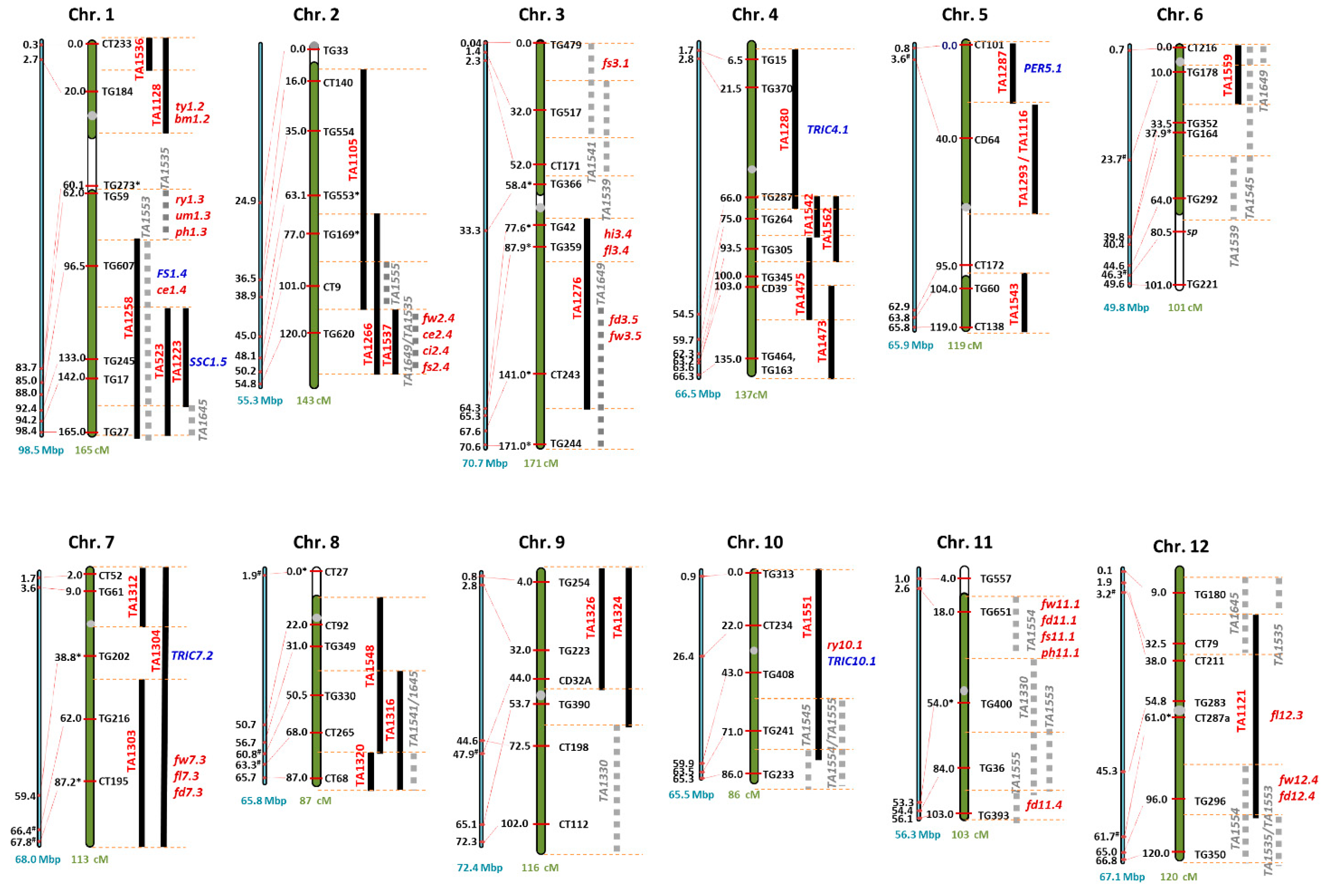
3.4. Genetic Regions Underlying the Phenotypic Variations
3.4.1. Yield Traits
3.4.2. Fruit Traits
3.4.3. Chemical Traits
3.4.4. Trichomes
4. Discussion
4.1. Performance of S. habrochaites Introgression Lines
4.2. QTL Mapping
4.3. Future Prospects for Using S. habrochaites ILs in Tomato Breeding
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Available online: http://www.fao.org/faostat/ (accessed on 15 November 2020).
- Grandillo, S.; Chetelat, R.; Knapp, S.; Spooner, D.; Peralta, I.; Cammareri, M.; Perez, O.; Termolino, P.; Tripodi, P.; Chiusano, M.; et al. Solanum sect. Lycopersicon. In Wild Crop Relatives and Breeding Resources: Vegetables; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 129–215. [Google Scholar]
- Foolad, M.R.; Panthee, D.R. Marker-assisted selection in tomato breeding. Crit. Rev. Plant Sci. 2012, 31, 93–123. [Google Scholar] [CrossRef]
- Zamir, D. Improving plant breeding with exotic genetic libraries. Nat. Rev. Genet. 2001, 2, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, P.; Ficcadenti, N.; Rotino, G.L.; Festa, G.; Bertone, A.; Pepe, A.; Caramanico, R.; Migliori, C.A.; Spadafora, D.; Schiavi, M.; et al. Genotypic and environmental effects on the agronomic, health-related compounds and antioxidant properties of chilli peppers for diverse market destinations. J. Sci. Food Agric. 2019, 99, 4550–4560. [Google Scholar] [CrossRef] [PubMed]
- Gur, A.; Zamir, D. Unused natural variation can lift yield barriers in plant breeding. PLoS Biol. 2004, 2, 1610–1615. [Google Scholar] [CrossRef]
- D’Agostino, N.; Tripodi, P. NGS-based genotyping, high throughput phenotyping and genome-wide association studies laid the foundations for next-generation breeding in horticultural crops. Diversity 2017, 9, 38. [Google Scholar] [CrossRef]
- Eshed, Y.; Zamir, D. An introgression line population of Lycopersicon pennellii in the cultivated tomato enables the identification and fine mapping of yield-associated QTL. Genetics 1995, 141, 1147–1162. [Google Scholar]
- Monforte, A.J.; Tanksley, S.D. Development of a set of near isogenic and backcross recombinant inbred lines containing most of the Lycopersicon hirsutum genome in a L. esculentum genetic background: A tool for gene mapping and gene discovery. Genome 2000, 43, 803–813. [Google Scholar] [CrossRef]
- Monforte, A.J.; Tanksley, S.D. Fine mapping of a quantitative trait locus (QTL) from Lycopersicon hirsutum chromosome 1 affecting fruit characteristics and agronomic traits: Breaking linkage among QTLs affecting different traits and dissection of heterosis for yield. Theor. Appl. Genet. 2000, 100, 471–479. [Google Scholar] [CrossRef]
- Monforte, A.J.; Friedman, E.; Zamir, D.; Tanksley, S.D. Comparison of a set of allelic QTL-NILs for chromosome 4 of tomato: Deductions about natural variation and implications for germplasm utilization. Theor. Appl. Genet. 2001, 102, 572–590. [Google Scholar] [CrossRef]
- Finkers, R.; van Heusden, A.W.; Meijer-Dekens, F.; van Kan, J.A.L.; Maris, P.; Lindhout, P. The construction of a Solanum habrochaites LYC4 introgression line population and the identification of QTLs for resistance to Botrytis cinerea. Theor. Appl. Genet. 2007, 114, 1071–1080. [Google Scholar] [CrossRef]
- Chetelat, R.T.; Meglic, V. Molecular mapping of chromosome segments introgressed from Solanum lycopersicoides into cultivated tomato (Lycopersicon esculentum). Theor. Appl. Genet. 2000, 100, 232–241. [Google Scholar] [CrossRef]
- Canady, M.A.; Meglic, V.; Chetelat, R.T. A library of Solanum lycopersicoides introgression lines in cultivated tomato. Genome 2005, 48, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Barrantes, W.; López-Casado, G.; García-Martínez, S.; Alonso, A.; Rubio, F.; Ruiz, J.J.; Fernández-Muñoz, R.; Granell, A.; Monforte, A.J. Exploring New Alleles Involved in Tomato Fruit Quality in an Introgression Line Library of Solanum pimpinellifolium. Front. Plant Sci. 2016, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chetelat, R.T.; Qin, X.; Tan, M.; Burkart-Waco, D.; Moritama, Y.; Huo, X.; Wills, T.; Pertuzé, R. Introgression lines of Solanum sitiens; a wild nightshade of the Atacama Desert; in the genome of cultivated tomato. Plant J. 2019, 100, 836–850. [Google Scholar] [CrossRef]
- Stevens, R.; Buret, M.; Duffé, P.; Garchery, C.; Baldet, P.; Rothan, C.; Causse, M. Candidate genes and Quantitative Trait Loci affecting fruit ascorbic acid content in three tomato populations. Plant Physiol. 2007, 143, 1943–1953. [Google Scholar] [CrossRef]
- Goodstal, J.F.; Kohler, G.; Randall, L.; Bloom, A.; Clair, D.S. A major QTL introgressed from wild Lycopersicon hirsutum confers chilling tolerance to cultivated tomato (Lycopersicon esculentum). Theor. Appl. Genet. 2005, 111, 898–905. [Google Scholar] [CrossRef]
- Easlon, H.M.; Asensio, J.S.; St Clair, D.A.; Bloom, A.J. Chilling-induced water stress: Variation in shoot turgor maintenance among wild tomato species from diverse habitats. Am. J. Bot. 2013, 100, 1991–1999. [Google Scholar] [CrossRef]
- Vidavski, F.; Czosnek, H.; Gazit, S.; Levy, D.; Lapidot, M. Pyramiding of genes conferring resistance to Tomato yellow leaf curl virus from different wild tomato species. Plant Breed. 2008, 127, 625–631. [Google Scholar] [CrossRef]
- Sifres, A.; Blanca, J.; Nuez, F. Pattern of genetic variability of Solanum habrochaites in its natural area of distribution. Genet. Resour. Crop Evol. 2011, 58, 347–360. [Google Scholar] [CrossRef]
- Momotaz, A.; Scott, J.W.; Schuster, D.J. Searching for silverleaf whitefly and begomovirus resistance genes from Lycopersicon hirsutum accession LA1777. Acta Hortic. (ISHS) 2004, 695, 417–422. [Google Scholar] [CrossRef]
- Thapa, S.P.; Miyao, E.M.; Davis, R.M.; Coaker, G. Identification of QTLs controlling resistance to Pseudomonas syringae pv. tomato race 1 strains from the wild tomato; Solanum habrochaites LA1777. Theor. Appl. Genet. 2015, 128, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Meng, F.; Strickler, S.R.; Dunham, D.M.; Munkvold, K.R.; Martin, G.B. Identification of a candidate gene in Solanum habrochaites for resistance to a race 1 strain of Pseudomonas syringae pv. tomato. Plant Genome 2015, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Avila, C.A.; Marconi, T.G.; Viloria, Z.; Kurpis, J.; Del Rio, S.Y. Bactericera cockerelli resistance in the wild tomato Solanum habrochaites is polygenic and influenced by the presence of Candidatus Liberibacter solanacearum. Sci. Rep. 2019, 9, 14031. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kang, K.; Gonzales-Vigil, E.; Shi, F.; Jones, A.D.; Barry, C.S.; Last, R.L. Striking Natural Diversity in Glandular Trichome Acylsugar Composition Is Shaped by Variation at the Acyltransferase2 Locus in the Wild Tomato Solanum habrochaites. Plant Physiol. 2012, 160, 1854–1870. [Google Scholar] [CrossRef] [PubMed]
- Zabel, S.; Brandt, W.; Porzel, A.; Athmer, B.; Kortbeek, R.; Bleeker, P.; Tissier, A. Two novel 7-epi-zingiberene derivatives with biological activity from Solanum habrochaites are produced by a single cytochrome P450 monooxygenase. Biorxiv 2020. [Google Scholar] [CrossRef]
- Dal Cin, V.; Kevany, B.; Fei, Z.; Klee, H.J. Identification of Solanum habrochaites loci that quantitatively influence tomato fruit ripening-associated ethylene emissions. Theor. Appl. Genet. 2009, 119, 1183–1192. [Google Scholar] [CrossRef]
- Bernacchi, D.; Beck-Bunn, T.; Emmatty, D.; Eshed, Y.; Inai, S.; Lopez, J.; Petiard, V.; Sayama, H.; Uhlig, J.; Zamir, D.; et al. Advanced backcross QTL analysis of tomato. II. Evaluation of near-isogenic lines carrying single-donor introgressions for desirable wild QTL-alleles derived from Lycopersicon hirsutum and L. pimpinellifolium. Theor. Appl. Genet. 1998, 97, 170–180. [Google Scholar] [CrossRef]
- Bernacchi, D.; Beck-Bunn, T.; Eshed, Y.; Lopez, J.; Petiard, V.; Uhlig, J.; Zamir, D.; Tanksley, S.D. Advanced backcross QTL analysis in tomato I. Identification of QTLs for traits of agronomic importance from Lycopersicon hirsutum. Theor. Appl. Genet. 1998, 97, 381–397. [Google Scholar] [CrossRef]
- Alseekh, S.; Ofner, I.; Pleban, T.; Tripodi, P.; Di Dato, F.; Cammareri, M.; Mohammad, A.; Grandillo, S.; Fernie, A.R.; Zamir, D. Resolution by recombination: Breaking up Solanum pennellii introgressions. Trends Plant Sci. 2013, 18, 536–538. [Google Scholar] [CrossRef]
- SOLGENOMICS. Available online: http://www.solegenomics.net (accessed on 15 November 2020).
- Frary, A.; Nesbitt, T.C.; Grandillo, S.; Knaap, E.; Cong, B.; Liu, J.; Meller, J.; Elber, R.; Alpert, K.B.; Tanksley, S.D. fw2.2: A quantitative trait locus key to the evolution of tomato fruit size. Science 2000, 289, 85–88. [Google Scholar] [CrossRef]
- Fridman, E.; Carrari, F.; Liu, Y.S.; Fernie, A.R.; Zamir, D. Zooming in on a quantitative trait for tomato yield using interspecific introgressions. Science 2004, 305, 1786–1789. [Google Scholar] [CrossRef] [PubMed]
- Hanson, P.M.; Sitathanim, K.; Sadashivam, A.T.; Yang, R.Y.; Grahamm, E.; Ledesma, D. Performance of Solanum habrochaites LA1777 introgression line hybrids for marketable tomato fruit yield in Asia. Euphytica 2007, 158, 167–178. [Google Scholar] [CrossRef]
- Tomato Genetic Resource Center. Available online: https//tgrc.ucdavis.edu (accessed on 15 November 2020).
- Ronga, D.; Parisi, M.; Pentangelo, A.; Mori, M.; Di Mola, I. Effects of nitrogen management on biomass production and dry matter distribution of processing tomato cropped in Southern Italy. Agronomy 2019, 9, 855. [Google Scholar] [CrossRef]
- Parisi, M.; Di Dato, F.; Ricci, S.; Mennella, G.; Cardi, T.; Tripodi, P. A multi-trait characterization of the ‘Friariello’ landrace: A Mediterranean resource for sweet pepper breeding. Plant Genet. Resour. 2017, 15, 165–176. [Google Scholar] [CrossRef]
- Dingemanse, N.J.; Kazem, A.J.M.; Réalem, D.; Wright, J. Behavioural reaction norms, animal personality meets individual plasticity. Trends Ecol. Evol. 2010, 25, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Mantel, N.A. The detection of disease clustering and a generalized regression approach. Cancer Res. 1967, 27, 209–220. [Google Scholar] [PubMed]
- Dunnett, C.W. A multiple comparison procedure for comparing several treatments with a control. J. Am. Stat. Assoc. 1955, 50, 1096–1121. [Google Scholar] [CrossRef]
- Simmons, A.T.; Gurr, G.M. Trichomes of Lycopersicon species and their hybrids, effects on pests and natural enemies. Agric. For. Entomol. 2005, 7, 265–276. [Google Scholar] [CrossRef]
- Prohens, J.; Gramazio, P.; Plazas, M.; Dempewolf, H.; Kilian, B.; Díez, M.J.; Fita, A.; Herraiz, F.J.; Rodríguez-Burruezo, B.; Soler, S.; et al. Introgressiomics, a new approach for using crop wild relatives in breeding for adaptation to climate change. Euphytica 2017, 213, 158. [Google Scholar] [CrossRef]
- Magwaza, L.S.; Opara, U.L. Analytical methods for determination of sugars and sweetness of horticultural products—A review. Sci. Hortic. 2015, 184, 179–192. [Google Scholar] [CrossRef]
- Gautier, H.; Diakou-Verdin, V.; Bénard, C.; Reich, M.; Buret, M.; Bourgaud, F.; Poëssel, J.L.; Caris-Veyrat, C.; Génard, M. How does tomato quality (sugar.; acid.; and nutritional quality) vary with ripening stage.; temperature.; and irradiance? J. Agric. Food Chem. 2008, 56, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xiong, Y.; Huang, G.; Xu, X.; Huang, Q. Effects of water stress on processing tomatoes yield, quality and water use efficiency with plastic mulched drip irrigation in sandy soil of the Hetao Irrigation District. Agric. Water Manag. 2017, 179, 205–214. [Google Scholar] [CrossRef]
- Cammarano, D.; Ronga, D.; Di Mola, I.; Mori, M.; Parisi, M. Impact of climate change on water and nitrogen use efficiencies of processing tomato cultivated in Italy. Agric. Water Manag. 2020, 241, 106336. [Google Scholar] [CrossRef]
- Fulton, T.M.; Beck-Bunn, T.; Emmatty, D.; Eshed, Y.; Lopez, J.; Petiard, V.; Uhlig, J.; Zamir, D.; Tanksley, S.D. QTL analysis of an advanced backcross of Lycopersicon peruvianum to the cultivated tomato and comparisons with QTLs found in other wild species. Theor. Appl. Genet. 1997, 95, 881–894. [Google Scholar] [CrossRef]
- Fulton, T.M.; Grandillo, S.; Beck-Bunn, T.; Fridman, E.; Frampton, A.; Lopez, J.; Petiard, V.; Uhlig, J.; Zamir, D.; Tanksley, S.D. Advanced backcross QTL analysis of a Lycopersicon esculentum × Lycopersicon parviflorum cross. Theor. Appl. Genet. 2000, 100, 1025–1042. [Google Scholar] [CrossRef]
- Fulton, T.M.; Buchelim, E.; Voirolm, E.; Lopez, J.; Pétiard, V.; Tanksley, S.D. Quantitative trait loci (QTL) affecting sugars.; organic acids and other biochemical properties possibly contributing to flavor.; identified in four advanced backcross populations of tomato. Euphytica 2002, 127, 163–177. [Google Scholar] [CrossRef]
- Tanksley, S.D.; Grandillo, S.; Fulton, T.M.; Zamir, D.; Eshed, Y.; Petiard, V.; López, J.; Beck-Bunn, T. Advanced backcross QTL analysis in a cross between an elite processing line of tomato and its wild relative L. pimpinellifolium. Theor. Appl. Genet. 1996, 92, 213–224. [Google Scholar] [CrossRef]
- Liu, J.; Gur, A.; Ronen, G.; Causse, M.; Damidaux, R.; Buret, M.; Hirschberg, J.; Zamir, D. There is more to tomato fruit colour than candidate carotenoid genes. Plant Biotechnol. J. 2003, 1, 195–207. [Google Scholar] [CrossRef]
- Liabeuf, D.; Francis, D.M. The use of historical datasets to develop multi-trait selection models in processing tomato. Euphytica 2017, 213, 100. [Google Scholar] [CrossRef]
- Esposito, S.; Cardi, T.; Campanelli, G.; Sestili, S.; Díez, M.J.; Soler, S.; Prohens, J.; Tripodi, P. ddRAD sequencing-based genotyping for population structure analysis in cultivated tomato provides new insights into the genomic diversity of Mediterranean ‘da serbo’ type long shelf-life germplasm. Hortic. Res. 2020, 7, 134. [Google Scholar] [CrossRef]
- Colonna, V.; D’Agostino, N.; Garrison, E.; Albrechtsen, A.; Meisner, J.M.; Facchiano, A.; Cardi, T.; Tripodi, P. Genomic diversity and novel genome-wide association with fruit morphology in Capsicum, from 746k polymorphic sites. Sci. Rep. 2019, 9, 10067. [Google Scholar] [CrossRef] [PubMed]
| LA Pedigree TGRC a | TA IL Name b | Chromosomes Bearing Wild Segments c | N° of Donor Segments c | Introgression Size (cM) d | Total Percentage of Coverage e |
|---|---|---|---|---|---|
| LA3921 | TA1105 | 2 | 1 | 118.50 | 8.11 |
| LA3981 | TA1116 | 5 | 1 | 47.50 | 3.25 |
| LA3969 | TA1121 | 12 | 1 | 87.25 | 5.97 |
| LA3919 | TA1128 | 1 | 1 | 41.00 | 2.81 |
| LA3916 | TA1223 | 1 | 1 | 59.50 | 4.07 |
| LA3913 | TA1258 | 1 | 1 | 103.00 | 7.05 |
| LA3922 | TA1266 | 2 | 1 | 61.45 | 4.21 |
| LA3926 | TA1276 | 3 | 1 | 88.00 | 6.02 |
| LA3931 | TA1280 | 4 | 1 | 67.30 | 4.61 |
| LA3938 | TA1287 | 5 | 1 | 20.00 | 1.37 |
| LA3939 | TA1293 | 5 | 1 | 47.50 | 3.25 |
| LA3948 | TA1303 | 7 | 1 | 62.90 | 4.31 |
| LA3949 | TA1304 | 7 | 1 | 113.00 | 7.73 |
| LA3951 | TA1312 | 7 | 1 | 22.90 | 1.57 |
| LA3953 | TA1316 | 8 | 1 | 46.25 | 3.17 |
| LA3955 | TA1320 | 8 | 1 | 9.50 | 0.65 |
| LA3956 | TA1324 | 9 | 1 | 61.10 | 4.18 |
| LA3991 | TA1326 | 9 | 1 | 46.85 | 3.21 |
| LA3958 | TA1330 | 9, 11 | 1 | 45.90, 57.50 | 7.08 |
| LA3936 | TA1475 | 4 | 1 | 34.35 | 2.35 |
| LA3937 | TA1473 | 4 | 1 | 33.50 | 2.29 |
| LA3917 | TA1535 | 1, 2, 12 | 4 | 17.25, 21.00, 28.25 | 4.55 |
| LA3920 | TA1536 | 1 | 1 | 10.00 | 0.68 |
| LA3923 | TA1537 | 2 | 1 | 21.00 | 1.44 |
| LA3944 | TA1539 | 3, 6 | 2 | 39.20, 21.30 | 4.14 |
| LA3929 | TA1541 | 3, 8 | 2 | 42.00, 9.50 | 3.52 |
| LA3933 | TA1542 | 4 | 1 | 13.75 | 0.94 |
| LA3941 | TA1543 | 5 | 1 | 19.50 | 1.33 |
| LA3945 | TA1545 | 6, 10 | 2 | 50.95, 7.50 | 4.00 |
| LA3954 | TA1548 | 8 | 1 | 66.50 | 4.55 |
| LA3961 | TA1551 | 10 | 1 | 78.50 | 5.37 |
| LA3995 | TA1553 | 1, 11, 12 | 3 | 103.00, 24.50, 12.00 | 9.55 |
| LA3966 | TA1554 | 10, 11, 12 | 3 | 29.00, 31.00, 41.50 | 6.95 |
| LA3965 | TA1555 | 2, 10, 11 | 3 | 21.50, 29.00, 9.50 | 4.11 |
| LA3947 | TA1559 | 6 | 1 | 21.75 | 1.49 |
| LA4005 | TA1562 | 4 | 1 | 26.25 | 1.80 |
| LA4002 | TA1645 | 1, 8, 12 | 3 | 11.50, 9.50, 30.75 | 3.54 |
| LA4004 | TA1649 | 2, 3, 6 | 3 | 21.00, 56.55, 5.00 | 5.65 |
| LA3914 | TA523 | 1 | 1 | 50.25 | 3.44 |
| Location | Battipaglia | |||
| Geographical coordinates | 40°37′ N; 14°58′ E | |||
| M.a.s.l. * | 65 | |||
| Soil regions + | Cambisol, Regosol, Calcisol, Phaeozem, Luvisol | |||
| Year | Y1 (2014) | Y2 (2015) | Y3 (2016) | Average 50 Years # |
| Maximum temperature (°C) | 28.10 | 27.67 | 26.66 | 21.10 |
| Minimum temperature (°C) | 17.50 | 17.14 | 16.02 | 11.50 |
| Precipitation (mm) | 12.60 | 14.17 | 8.53 | 16.50 |
| Humidity (%) | 67.30 | 65.76 | 63.98 | 70.64 |
| Traits a | # | MS b | R b | F Value c | H2 b | Mean SL | Range SL | CV b | Mean IL | Range IL | CV b | Prob > F |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yield | ||||||||||||
| Total yield | TY | 12.13 | 0.22 | 8.46 ** | 0.89 | 4.11 | 4.7–3.3 | 31.69 | 3.41 | 4.16–2.76 | 38.58 | *** |
| Red yield | RY | 13.53 | 0.22 | 8.49 ** | 0.89 | 3.77 | 4.67–2.96 | 36.53 | 3.16 | 4.06–2.32 | 44.19 | *** |
| Green yield | GY | 1.47 | 0.18 | 5.36 ** | 0.84 | 0.50 | 0.82–0.03 | 95.77 | 0.56 | 0.99–0.24 | 102.67 | ns |
| Uniformity maturity | UM | 974.95 | 0.21 | 8.33 ** | 0.89 | 91.01 | 99.26–80.45 | 11.55 | 90.98 | 96.54–83.43 | 13.38 | ns |
| Plant weight | PW | 0.77 | 0.19 | 7.01 ** | 0.88 | 0.67 | 0.86–0.45 | 68.56 | 0.68 | 0.75–0.55 | 51.43 | ns |
| Biomass | BM | 13.68 | 0.19 | 7.37 ** | 0.88 | 4.79 | 5.45–3.75 | 33.43 | 4.08 | 4.89–3.31 | 35.88 | *** |
| Harvest index | HI | 0.06 | 0.27 | 11.50 ** | 0.92 | 0.86 | 0.88–0.84 | 6.93 | 0.83 | 0.85–0.81 | 10.66 | *** |
| Fruit | ||||||||||||
| Fruit weight | FW | 2029.75 | 0.25 | 10.28 ** | 0.91 | 74.27 | 83.52–67.85 | 20.16 | 60.06 | 62.96–57.08 | 25.76 | *** |
| Fruit length | FL | 3.05 | 0.07 | 21.02 ** | 0.95 | 5.68 | 6.01–5.52 | 7.17 | 5.09 | 5.13–5.04 | 9.04 | *** |
| Fruit diameter | FD | 1.52 | 0.24 | 15.92 ** | 0.94 | 4.94 | 5.23–4.79 | 7.37 | 4.52 | 4.57–4.45 | 7.83 | *** |
| Fruit shape | FS | 0.07 | 0.41 | 21.51 ** | 0.96 | 1.15 | 1.15–1.15 | 4.29 | 1.13 | 1.13–1.12 | 6.62 | ** |
| External color | CE | 2.57 | 0.49 | 18.77 ** | 0.94 | 2.92 | 3.75–2.44 | 20.61 | 2.90 | 3.27–2.67 | 19.23 | ns |
| Internal color | CI | 1.69 | 0.60 | 13.73 ** | 0.84 | 3.11 | 3.81–2.73 | 17.04 | 3.09 | 3.36–2.93 | 19.18 | ns |
| Pericarp | PER | 1.51 | 0.22 | 8.41 ** | 0.89 | 2.07 | 2.22–1.67 | 22.76 | 1.90 | 1.93–1.87 | 24.56 | *** |
| Puffiness | PUF | 2.52 | 0.27 | 11.20 ** | 0.92 | 1.68 | 2.16–1.24 | 35.18 | 1.57 | 1.76–1.33 | 34.48 | * |
| Chemical | ||||||||||||
| Soluble solids | SSC | 6.86 | 0.31 | 13.53 ** | 0.93 | 4.99 | 5.73–4.36 | 16.52 | 5.44 | 5.67–5.05 | 15.30 | *** |
| pH | pH | 1.44 | 0.36 | 36.05 ** | 0.94 | 4.52 | 4.6–4.41 | 4.21 | 4.40 | 4.47–4.34 | 4.57 | *** |
| SSC per plant | BY | 248.62 | 0.22 | 5.01 * | 0.89 | 18.23 | 19.9–15.09 | 36.54 | 16.73 | 19.96–12.87 | 41.99 | * |
| Stem | ||||||||||||
| Trichomes | TRIC | 2.99 | 0.17 | 6.37 ** | 0.86 | 1.00 | 1.00–1.00 | 0.00 | 1.30 | 3.00–1.00 | 31.89 | ns |
| Trait | Genotype (G) df = 39 | Year (Y) df = 2 | G × Y df = 78 | Total Error df = 1108 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MS | TSS% | F | MS | TSS% | F | MS | TSS% | F | ||
| Yield | ||||||||||
| TY | 11.60 | 45.49 | 11.57 ** | 151.98 | 30.57 | 151.72 ** | 3.05 | 23.94 | 3.04 ** | 1109.89 |
| RY | 12.34 | 38.77 | 13.07 ** | 234.09 | 37.72 | 248.08 ** | 3.74 | 23.51 | 3.97 ** | 1045.53 |
| GY | 0.56 | 12.35 | 3.92 ** | 94.32 | 65.21 | 663.08 ** | 0.46 | 22.44 | 3.21 ** | 125.60 |
| UM | 946.65 | 39.85 | 13.1 ** | 13,639.01 | 29.45 | 188.68 ** | 364.62 | 30.70 | 5.04 ** | 80,094.20 |
| PW | 0.76 | 58.29 | 7.97 ** | 4.28 | 16.74 | 44.61 ** | 0.16 | 24.97 | 1.71 ** | 106.19 |
| BM | 12.69 | 43.66 | 9.43 ** | 191.67 | 33.82 | 142.5 ** | 3.27 | 22.53 | 2.43 ** | 1490.35 |
| HI | 0.08 | 62.64 | 17.76 ** | 0.10 | 4.07 | 22.48 ** | 0.02 | 33.30 | 4.72 ** | 4.85 |
| Fruit | ||||||||||
| FW | 2110.45 | 76.39 | 11.4 ** | 3122.66 | 5.80 | 16.87 ** | 245.99 | 17.81 | 1.33 * | 205,111.16 |
| FL | 3.11 | 81.24 | 23.99 ** | 0.95 | 1.27 | 7.33 ** | 0.33 | 17.49 | 2.58 ** | 22,962.97 |
| FD | 1.59 | 78.27 | 18.65 ** | 1.60 | 4.03 | 18.59 ** | 0.19 | 17.70 | 2.10 ** | 15,829.19 |
| FS | 0.07 | 73.70 | 25.90 ** | 0.01 | 0.62 | 4.23 * | 0.01 | 25.68 | 4.51 ** | 2.87 |
| CE | 2.08 | 85.50 | 19.98 ** | 0.65 | 0.68 | 6.29 * | 0.33 | 13.80 | 3.23 ** | 110.28 |
| CI | 2.92 | 89.75 | 23.70 ** | 0.05 | 0.04 | 0.38 NS | 0.33 | 12.96 | 2.69 ** | 90.69 |
| PER | 1.49 | 62.66 | 9.39 ** | 0.61 | 1.31 | 3.84 * | 0.43 | 36.02 | 2.70 ** | 176.04 |
| PUF | 2.48 | 54.72 | 15.84 ** | 16.58 | 18.79 | 106.03 ** | 0.60 | 26.50 | 3.83 ** | 173.06 |
| Chemical | ||||||||||
| SSC | 6.43 | 58.74 | 17.97 ** | 37.24 | 17.43 | 104.04 ** | 1.30 | 23.83 | 3.65 ** | 395.49 |
| pH | 0.44 | 77.47 | 18.29 ** | 3.09 | 14.33 | 128.6 ** | 0.02 | 8.20 | 0.96 NS | 25.51 |
| BY | 307.53 | 46.61 | 10.40 ** | 3863.56 | 30.03 | 130.6 ** | 77.08 | 23.36 | 2.61 ** | 32,776.91 |
| Stem | ||||||||||
| TRIC | 3.13 | 90.08 | 12.68 ** | 32.15 | 1.01 | 3.25 NS | 2.49 | 8.91 | 10.08 * | 27.32 |
| Trait | ILs | Marker(s) | Chr | BIN n° | BIN Position (cM) | BIN Size (cM) | QTL Name * | PV (%) 2 Years | PV (%) 3 Years |
|---|---|---|---|---|---|---|---|---|---|
| TY | TA1128 | TG184 | 1 | 1.2 | 10–40.5 | 30.5 | ty1.2 | −39.15 | |
| TA1535 | TG59 | 1 | 1.3 | 61–79.25 | 18.3 | ty1.3 | −50.62 | ||
| TA1276-TA1649 | TG42, TG359, CT243 | 3 | 3.5 | 68.0–156.0 | 88.0 | ty3.5 | −52.35 | ||
| TA1551 | TG313, CT234, TG408 | 10 | 10.1 | 0.00–57.0 | 57 | ty10.1 | −45.21 | ||
| TA1554 | TG651 | 11 | 11.1 | 11.0–36.0 | 25 | ty11.1 | −44.51 | ||
| TA1121 | CT211, TG283, CT287a | 12 | 12.3 | 32.25–78.5 | 43.25 | ty12.3 | −33.51 | ||
| RY | TA1535 | TG59 | 1 | 1.3 | 61–79.25 | 18.3 | ry1.3 | −51.38 | |
| TA1276-TA1649 | TG42, TG359, CT243 | 3 | 3.5 | 68.0–156.0 | 88.0 | ry3.5 | −52.30 | ||
| TA1551 | TG313, CT234, TG408 | 10 | 10.1 | 0.00–57.0 | 57 | ry10.1 | −41.28 | ||
| UM | TA1535 | TG59 | 1 | 1.3 | 61–79.25 | 18.3 | um1.3 | −14.25 | |
| TA1280 | TG15, TG370, TG287 | 4 | 4.1 | 3.25–70.5 | 67.25 | um4.1 | −9.95 | ||
| PW | TA1649 | TG244 | 3 | 3.6 | 156.0–171 | 15.0 | PW3.6 | 73.92 | |
| BM | TA1128 | TG184 | 1 | 1.2 | 10–40.5 | 30.5 | bm1.2 | −40.24 | |
| TA1535 | TG59 | 1 | 1.3 | 61–79.25 | 18.3 | bm1.3 | −44.70 | ||
| TA1276 | TG42, TG359 | 3 | 3.4 | 68.0–114.45 | 46.45 | bm3.4 | −52.03 | ||
| TA1551 | TG313, CT234, TG408 | 10 | 10.1 | 0.00–57.0 | 57 | bm10.1 | −40.79 | ||
| TA1554 | TG651 | 11 | 11.1 | 11.0–36.0 | 25 | bm11.1 | −47.15 | ||
| HI | TA1535 | TG59 | 1 | 1.3 | 61–79.25 | 18.3 | hi1.3 | −12.14 | |
| TA1276 | TG42, TG359 | 3 | 3.4 | 68.0–114.45 | 46.45 | hi3.4 | −25.52 | ||
| TA1542-TA1562 | TG264 | 4 | 4.3 | 70.5–84.25 | 13.75 | hi4.3 | −16.43 | ||
| TA1649 | TG244 | 3 | 3.6 | 156.0–171 | 15.0 | hi3.6 | −17.14 | ||
| FW | TA1266-TA1537-TA1649-TA1535 | TG620 | 2 | 2.4 | 110.5–131.5 | 21.0 | fw2.4a | −30.49 | |
| TA1276-TA1649 | TG42, TG359, CT243 | 3 | 3.5 | 68.0–156.0 | 88.0 | fw3.5 | −27.91 | ||
| TA1303-TA1304 | TG216, CT195 | 7 | 7.3 | 50.4–113 | 62.6 | fw7.3 | −42.33 | ||
| TA1330 | CT198, CT112 | 9 | 9.3 | 63.1–109.0 | 45.9 | fw9.3 | −32.29 | ||
| TA1545- TA1554-TA1555 | TG233 | 10 | 10.3 | 78.5–86.0 | 7.5 | fw10.3 | −35.63 | ||
| TA1554 | TG651 | 11 | 11.1 | 11.0–36.0 | 25 | fw11.1 | −43.14 | ||
| TA1330-TA1553 | TG36 | 11 | 11.3 | 93.5–69.0 | 24.5 | fw11.3 | −32.01 | ||
| TA1554-TA1221 | TG296 | 12 | 12.4 | 78.5–108.0 | 29.5 | fw12.4 | −36.44 | ||
| FL | TA1541 | TG479 | 3 | 3.1 | 0.0–16.0 | 16.0 | fl3.1 | −13.82 | |
| TA1276 | TG42, TG359 | 3 | 3.4 | 68.0–114.45 | 46.45 | fl3.4 | −16.85 | ||
| TA1280 | TG15, TG370, TG287 | 4 | 4.1 | 3.25–70.5 | 67.25 | fl4.1 | −9.95 | ||
| TA1543 | TG60 | 5 | 5.3 | 99.5–119.0 | 19.5 | fl5.3 | −9.86 | ||
| TA1545 | TG352, TG164 | 6 | 6.3 | 21.75–50.95 | 29.2 | fl6.3 | −11.38 | ||
| TA1303-TA1304 | TG216, CT195 | 7 | 7.3 | 50.4–113 | 62.6 | fl7.3 | −19.05 | ||
| TA1545-TA1554-TA1555 | TG233 | 10 | 10.3 | 78.5–86.0 | 7.5 | fl10.3 | −18.7 | ||
| TA1330-TA1553 | TG36 | 11 | 11.3 | 93.5–69.0 | 24.5 | fl11.3 | −15.92 | ||
| TA1121 | CT211, TG283, CT287a | 12 | 12.3 | 32.25–78.5 | 43.25 | fl12.3 | −14.83 | ||
| FD | TA1105 | CT140, TG554, TG553 | 2 | 2.1 | 8.0–70.15 | 62.2 | fd2.1 | −17.32 | |
| TA1276-TA1649 | TG42, TG359, CT243 | 3 | 3.5 | 68.0–156.0 | 88.0 | fd3.5 | −14.71 | ||
| TA1473 | TG464, TG163 | 4 | 4.7 | 119–135 | 16.0 | fd4.7 | −11.21 | ||
| TA1303-TA1304 | TG216, CT195 | 7 | 7.3 | 50.4–113 | 62.6 | fd7.3 | −19.03 | ||
| TA1554 | TG651 | 11 | 11.1 | 11.0–36.0 | 25 | fd11.1 | −16.82 | ||
| TA1555 | TG393 | 11 | 11.4 | 93.5–104.0 | 10.5 | fd11.4 | −15.53 | ||
| TA1554- TA1221 | TG296 | 12 | 12.4 | 78.5–108.0 | 29.5 | fd12.4 | −16.14 | ||
| FS | TA1258 | TG607 | 1 | 1.4 | 79.25–114–75 | 35.5 | FS1.4 | 7.28 | |
| TA1258-TA523-TA1223 | TG245, TG17 | 1 | 1.5 | 114.75–153.5 | 38.8 | FS1.5 | 7.42 | ||
| TA1266-TA1537-TA1649-TA1535 * | TG620 | 2 | 2.4 | 110.5–131.5 | 21.0 | fs2.4b | −11.89 | ||
| TA1541 | TG479 | 3 | 3.1 | 0.0–16.0 | 16.0 | fs3.1 | −5.96 | ||
| TA1554 | TG651 | 11 | 11.1 | 11.0–36.0 | 25 | fs11.1 | −12.73 | ||
| CE | TA1258 | TG607 | 1 | 1.4 | 79.25–114–75 | 35.5 | ec1.4 | −15.11 | |
| TA1266-TA1537-TA1649-TA1535 * | TG620 | 2 | 2.4 | 110.5–131.5 | 21.0 | ec2.4c | −38.56 | ||
| TA1473-TA1475 | CD39 | 4 | 4.6 | 101.5–119 | 17.5 | EC4.6 | 24.27 | ||
| TA1330 | CT198, CT112 | 9 | 9.3 | 63.1–109.0 | 45.9 | EC9.3 | 21.6 | ||
| TA1330-TA1553 | TG36 | 11 | 11.3 | 69.0–93.5 | 24.5 | EC11.3 | 18.97 | ||
| CI | TA1535 | TG59 | 1 | 1.3 | 61–79.25 | 18.3 | ic1.3 | −25.85 | |
| TA1258 | TG607 | 1 | 1.4 | 79.25–114–75 | 35.5 | ic1.4 | −25.19 | ||
| TA1266-TA1537-TA1649-TA1535 * | TG620 | 2 | 2.4 | 110.5–131.5 | 21.0 | ic2.4c | −53.95 | ||
| TA1475 | TG345 | 4 | 4.5 | 96.75–101.5 | 4.75 | IC4.5 | 25.08 | ||
| PER | TA1287 | CT101 | 5 | 5.1 | 0.0–20.0 | 20.0 | PER5.1 | 32.49 | |
| TA1554 | TG651 | 11 | 11.1 | 11.0–36.0 | 25 | per11.1 | −38.69 | ||
| SSC | TA1258-TA523-TA1223 | TG245, TG17 | 1 | 1.5 | 114.75–153.5 | 38.8 | SSC1.5 | 24.01 | |
| TA1649 | TG244 | 3 | 3.6 | 156.0–171 | 15.0 | SSC3.6 | 26.36 | ||
| TA1543 | TG60 | 5 | 5.3 | 99.5–119.0 | 19.5 | SSC5.3 | 25.37 | ||
| TA1551 | TG313, CT234, TG408 | 10 | 10.1 | 0.00–57.0 | 57 | SSC10.1 | 26.47 | ||
| PH | TA1535 | TG59 | 1 | 1.3 | 61–79.25 | 18.3 | ph1.3 | −8.91 | |
| TA1473 | TG464, TG163 | 4 | 4.7 | 119–135 | 16.0 | ph4.7 | −4.73 | ||
| TA1554 | TG651 | 11 | 11.1 | 11.0–36.0 | 25 | ph11.1 | −8.50 | ||
| TA1330 | CT198, CT112 | 9 | 9.3 | 63.1–109.0 | 45.9 | ph9.3 | −4.24 | ||
| BY | TA1128 | TG184 | 1 | 1.2 | 10–40.5 | 30.5 | by1.2 | −39.33 | |
| TA1535 | TG59 | 1 | 1.3 | 61–79.25 | 18.3 | by1.3 | −49.15 | ||
| TA1276 | TG42, TG359 | 3 | 3.4 | 68.0–114.45 | 46.45 | by3.4 | −57.73 | ||
| TRIC | TA1280 | TG15, TG370, TG287 | 4 | 4.1 | 3.25–70.5 | 67.25 | TRIC4.1 | 100 | |
| TA1304 | TG202 | 7 | 7.2 | 23.9–50.4 | 26.5 | TRIC7.2 | 125 | ||
| TA1551 | TG313, CT234, TG408 | 10 | 10.1 | 0.00–57.0 | 57 | TRIC10.1 | 200 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tripodi, P.; Vitiello, A.; D’Onofrio, B.; Parisi, M.; Cammareri, M. Dissecting the Genotypic and Environmental Factors Underpinning the Quantitative Trait Variation in a Set of Wild Tomato (Solanum habrochaites LA1777) Introgression Lines. Agronomy 2021, 11, 38. https://doi.org/10.3390/agronomy11010038
Tripodi P, Vitiello A, D’Onofrio B, Parisi M, Cammareri M. Dissecting the Genotypic and Environmental Factors Underpinning the Quantitative Trait Variation in a Set of Wild Tomato (Solanum habrochaites LA1777) Introgression Lines. Agronomy. 2021; 11(1):38. https://doi.org/10.3390/agronomy11010038
Chicago/Turabian StyleTripodi, Pasquale, Antonella Vitiello, Bruno D’Onofrio, Mario Parisi, and Maria Cammareri. 2021. "Dissecting the Genotypic and Environmental Factors Underpinning the Quantitative Trait Variation in a Set of Wild Tomato (Solanum habrochaites LA1777) Introgression Lines" Agronomy 11, no. 1: 38. https://doi.org/10.3390/agronomy11010038
APA StyleTripodi, P., Vitiello, A., D’Onofrio, B., Parisi, M., & Cammareri, M. (2021). Dissecting the Genotypic and Environmental Factors Underpinning the Quantitative Trait Variation in a Set of Wild Tomato (Solanum habrochaites LA1777) Introgression Lines. Agronomy, 11(1), 38. https://doi.org/10.3390/agronomy11010038








