Overexpression of DUF538 from Wild Arachis Enhances Plant Resistance to Meloidogyne spp.
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization and Cloning of AsDUF538
2.2. Nematode Inoculum
2.3. AsDUF538 Transcripts Distribution in Arachis spp. Roots Inoculated with Meloidogyne arenaria
2.4. Peanut and Soybean Hairy Roots Inoculation with Meloidogyne arenaria and Meloidogyne javanica
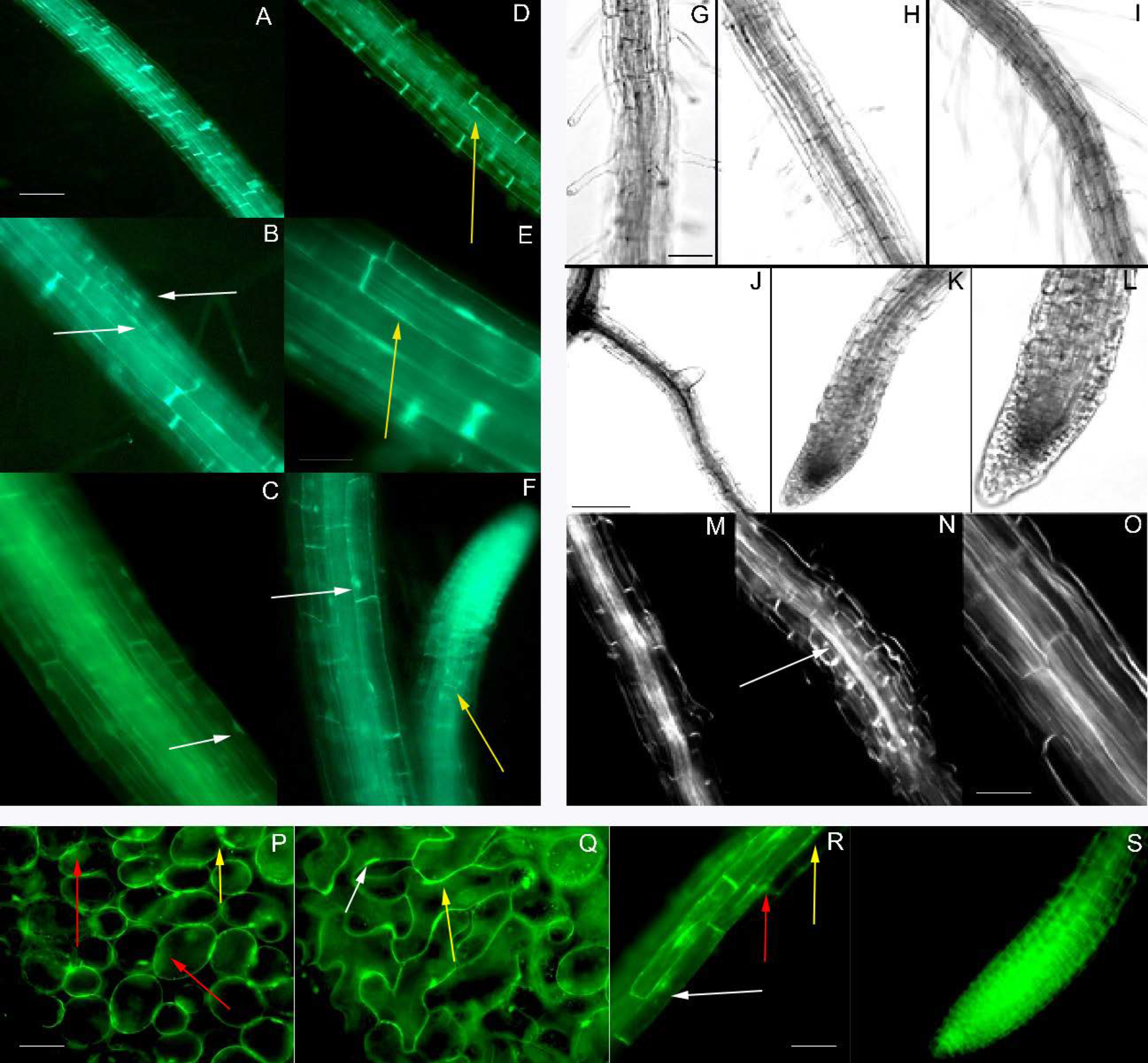
2.5. Arabidopsis Transformation and OE Lines Selection
2.6. Morphological Analysis of Arabidopsis OE Lines
2.7. Arabidopsis OE Lines Inoculation with Meloidogyne incognita
2.8. Gene Expression Analysis of Arabidopsis OE Lines
3. Results
3.1. Molecular Characterization and Cloning of AsDUF538
3.2. AsDUF538 Transcripts in Arachis Roots Inoculated with Meloidogyne arenaria
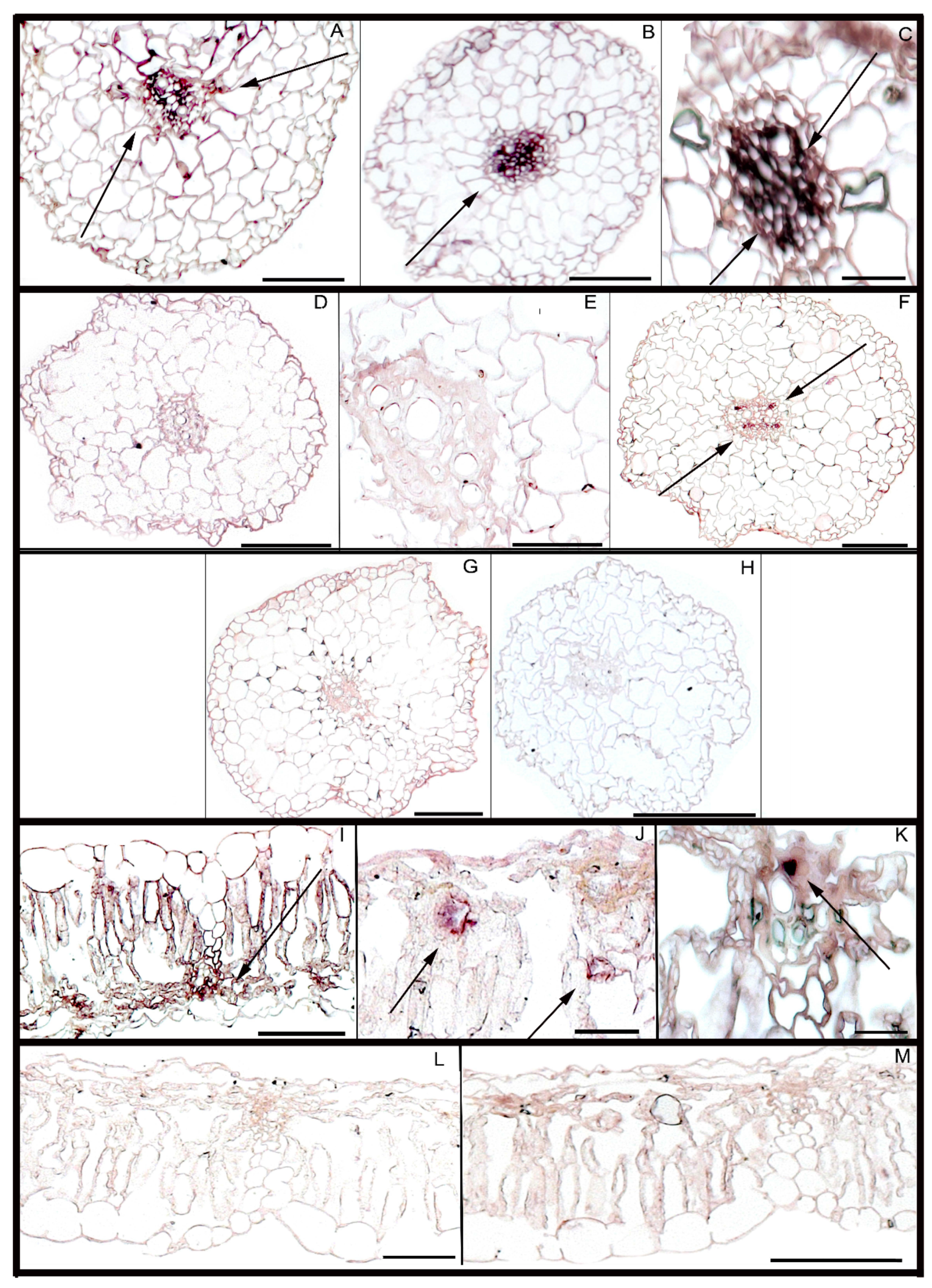
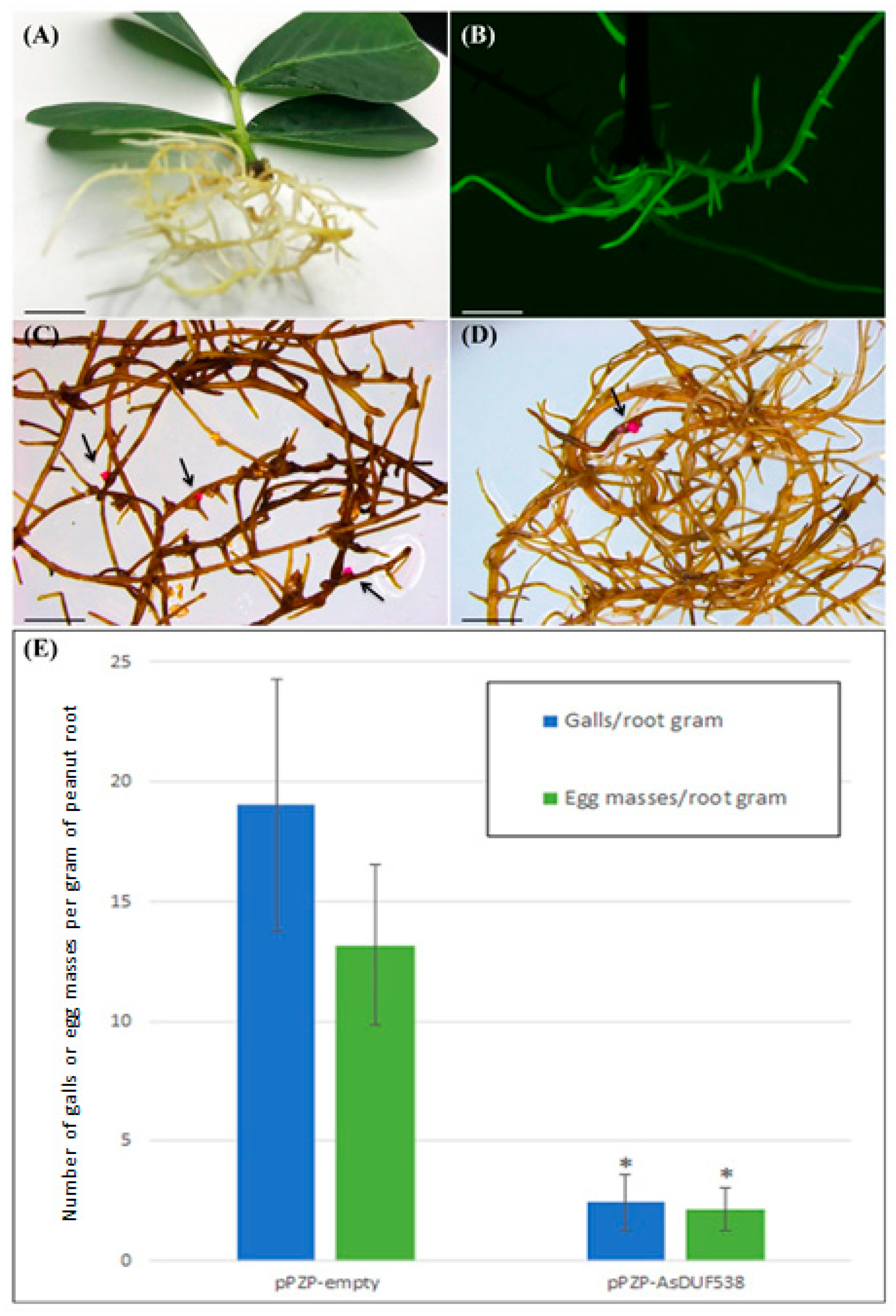
3.3. Meloidogyne arenaria Infection in Peanut Hairy Roots
3.4. Meloidogyne javanica Infection in Soybean Hairy Roots
3.5. AsDUF538 Overexpression in Transgenic Arabidopsis
3.6. Expression of Stress Related-Marker Genes in Arabidopsis OE Lines
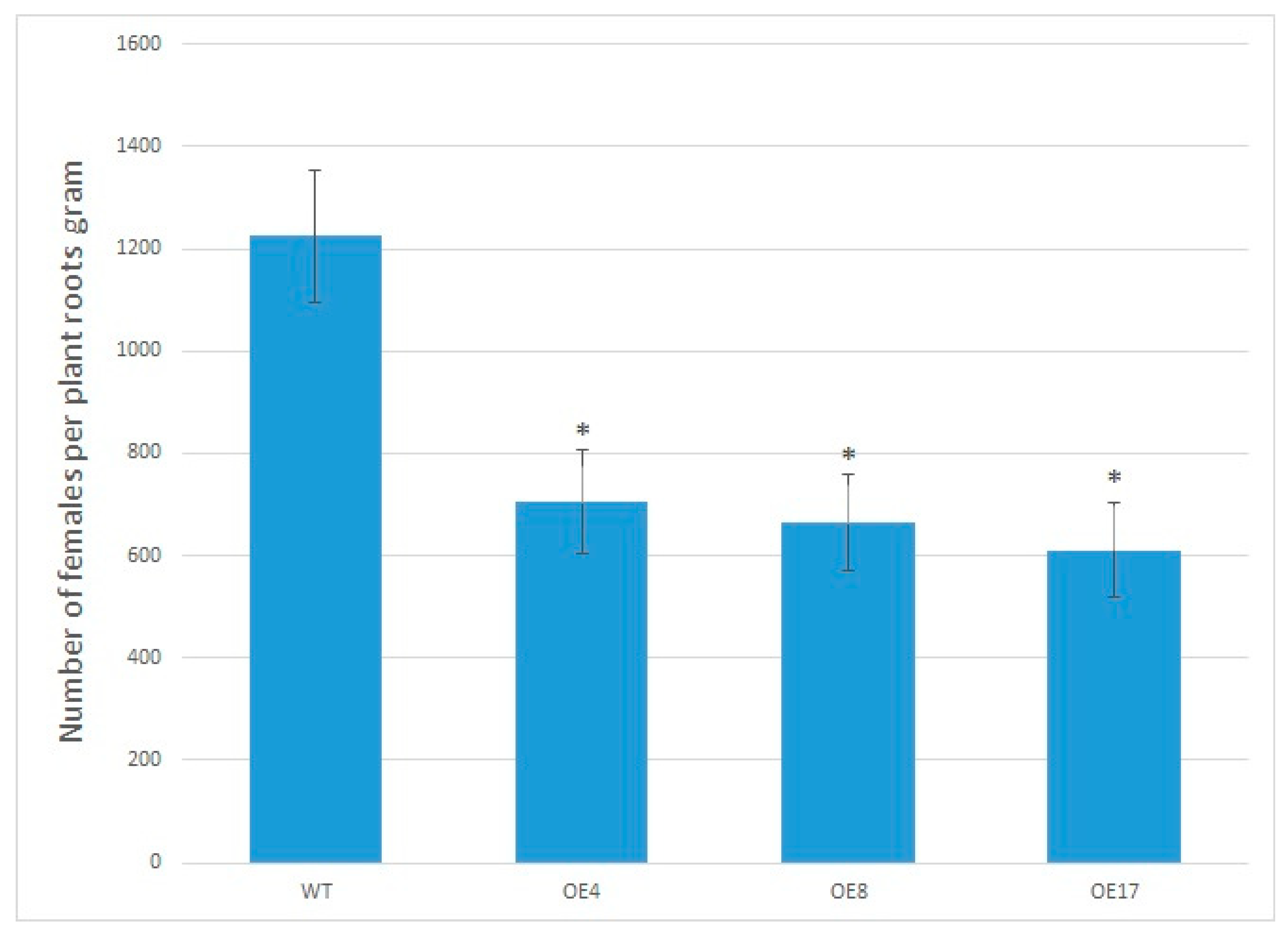
3.7. Arabidopsis OE Lines Infected with Meloidogyne incognita
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DAI | Days after inoculation; |
| DUF | Domain of unknown function; |
| ET | Ethylene; |
| J2 | Second-stage juveniles; |
| JA | Jasmonic acid; |
| OE lines | Overexpressing lines; |
| qRT-PCR | Quantitative reverse transcription-polymerase chain reaction; |
| RKN | Root-knot nematode; |
| ROS | Reactive oxygen species; |
| RT-PCR | Reverse transcription-polymerase chain reaction; |
| WT | Wild-type. |
References
- Li, L.; Xie, C.; Ye, T.; Xu, J.; Chen, R.; Gao, X.; Zhu, J.; Xu, Z. Molecular characterization, expression pattern and function analysis of the rice OsDUF866 family. Biotechnol. Biotechnol. Equip. 2017, 31, 243–249. [Google Scholar] [CrossRef]
- Li, L.-H.; Lv, M.-M.; Li, X.; Ye, T.-Z.; He, X.; Rong, S.-H.; Dong, Y.-L.; Guan, Y.; Gao, X.-L.; Zhu, J.-Q.; et al. The Rice OsDUF810 Family: OsDUF810.7 May be Involved in the Tolerance to Salt and Drought. Mol. Biol. 2018, 52, 489–496. [Google Scholar] [CrossRef]
- Nabi, R.B.S.; Tayade, R.; Imran, Q.M.; Hussain, A.; Shahid, M.; Yun, B.-W. Functional Insight of Nitric-Oxide Induced DUF Genes in Arabidopsis thaliana. Front. Plant. Sci. 2020, 11, 1041. [Google Scholar] [CrossRef]
- Takahashi, S.; Yoshikawa, M.; Kamada, A.; Ohtsuki, T.; Uchida, A.; Nakayama, K.; Satoh, H. The photoconvertible water-soluble chlorophyll-binding protein of Chenopodium album is a member of DUF538, a superfamily that distributes in Embryophyta. J. Plant. Physiol. 2013, 170, 1549–1552. [Google Scholar] [CrossRef] [PubMed]
- Francin-Allami, M.; Merah, K.; Albenne, C.; Rogniaux, H.; Pavlović, M.; Lollier, V.; Sibout, R.; Guillon, F.; Jamet, E.; Larré, C. Cell wall proteomic of Brachypodium distachyon grains: A focus on cell wall remodeling proteins. Proteomics 2015, 15, 2296–2306. [Google Scholar] [CrossRef]
- Nguyen-Kim, H.; Clemente, H.S.; Balliau, T.; Zivy, M.; Dunand, C.; Albenne, C.; Jamet, E. Arabidopsis thaliana root cell wall proteomics: Increasing the proteome coverage using a combinatorial peptide ligand library and description of unexpected Hyp in peroxidase amino acid sequences. Proteomics 2016, 16, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, C.; Virginie, L.; Audrey, G.; Axelle, B.; Colette, L.; Hélène, R.; Elisabeth, J.; Fabienne, G.; Mathilde, F.-A. Cell Wall Proteome of Wheat Grain Endosperm and Outer Layers at Two Key Stages of Early Development. Int. J. Mol. Sci. 2019, 21, 239. [Google Scholar] [CrossRef]
- Gholizadeh, A.; Kohnehrouz, B.B. Identification of DUF538 cDNA clone from Celosia cristata expressed sequences of nonstressed and stressed leaves. Russ. J. Plant. Physiol. 2010, 57, 247–252. [Google Scholar] [CrossRef]
- Li, Y.F.; Wang, Y.; Tang, Y.; Kakani, V.G.; Mahalingam, R. Transcriptome analysis of heat stress response in switchgrass (Panicum virgatum L.). BMC Plant. Biol. 2013, 13, 1–12. [Google Scholar] [CrossRef]
- Ajambang, W.; Volkaert, H.; Sudarsono, S. Carbohydrate deprivation upsurges the expression of genes responsible for programmed cell death in inflorescence tissues of oil palm (Elaeis guineensis Jacq.). Turk. J. Boil. 2016, 40, 1320–1327. [Google Scholar] [CrossRef]
- Ding, C.; Chang, Z.; Wang, Y.; You, S.; Wang, S.; Ding, Y. Proteomic Analysis Reveals That Developing Leaves are More Sensitive to Nitrogen Fertilizer Than Mature Leaves. J. Plant. Growth Regul. 2017, 37, 426–437. [Google Scholar] [CrossRef]
- Li, L.; Du, Y.; He, C.; Dietrich, C.R.; Li, J.; Ma, X.; Wang, R.; Liu, Q.; Liu, S.; Wang, G. A novel maize gene, glossy6 involved in epicuticular wax deposition and drought tolerance. BioRxiv 2018. BioRxiv:378687. [Google Scholar] [CrossRef]
- Huang, Y.; Li, L.; Smith, K.P.; Muehlbauer, G.J. Differential transcriptomic responses to Fusarium graminearum infection in two barley quantitative trait loci associated with Fusarium head blight resistance. BMC Genom. 2016, 17, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Elagamey, E.; Sinha, A.; Narula, K.; Abdellatef, M.A.; Chakraborty, N.; Chakraborty, S. Molecular Dissection of Extracellular Matrix Proteome Reveals Discrete Mechanism RegulatingVerticillium DahliaeTriggered Vascular Wilt Disease in Potato. Proteomics 2017, 17, 1600373. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Weiberg, A.; Dellota Jr, E.; Yamane, D.; Jin, H. Botrytis small RNA Bc-siR37 suppresses plant defense genes by cross-kingdom RNAi. RNA Biol. 2017, 14, 421–428. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, Z.; Rocheleau, H.; Fauteux, F.; Wang, Y.; McCartney, C.; Ouellet, T. Transcriptome dynamics associated with resistance and susceptibility against fusarium head blight in four wheat genotypes. BMC Genom. 2018, 19, 1–26. [Google Scholar] [CrossRef]
- Gholizadeh, A.; Kohnehrouz, S.B. DUF538 Protein Super Family is Predicted to be the Potential Homologue of Bactericidal/Permeability-Increasing Protein in Plant System. Protein J. 2013, 32, 163–171. [Google Scholar] [CrossRef]
- Gholizadeh, A. Heterologous Expression of Stress-Responsive DUF538 Domain Containing Protein and its Morpho-Biochemical Consequences. Protein J. 2011, 30, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, A. DUF538 protein superfamily is predicted to be chlorophyll hydrolyzing enzymes in plants. Physiol. Mol. Biol. Plants 2016, 22, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Prezelj, N.; Covington, E.; Roitsch, T.; Gruden, K.; Fragner, L.; Weckwerth, W.; Chersicola, M.; Vodopivec, M.; Dermastia, M. Metabolic consequences of infection of grapevine (Vitis vinifera L.) cv.“Modra frankinja” with Flavescence Dorée phytoplasma. Front. Plant. Sci. 2016, 7, 711. [Google Scholar] [CrossRef] [PubMed]
- Proite, K.; Leal-Bertioli, S.C.M.; Bertioli, D.J.; Moretzsohn, M.C.; Da Silva, F.R.; Martins, N.F.; Guimarães, P.M. ESTs from a wild Arachis species for gene discovery and marker development. BMC Plant. Biol. 2007, 7, 1–10. [Google Scholar] [CrossRef]
- Guimarães, P.M.; Brasileiro, A.C.M.; Proite, K.; De Araujo, A.C.G.; Leal-Bertioli, S.C.M.; Pic-Taylor, A.; Da Silva, F.R.; Morgante, C.V.; Ribeiro, S.D.G.; Bertioli, D.J. A Study of Gene Expression in the Nematode Resistant Wild Peanut Relative, Arachis stenosperma, in Response to Challenge with Meloidogyne arenaria. Trop. Plant. Biol. 2010, 3, 183–192. [Google Scholar] [CrossRef]
- Morgante, C.V.; Brasileiro, A.C.M.M.; Roberts, P.A.; Guimaraes, L.A.; Araujo, A.C.G.G.; Fonseca, L.N.; Leal-Bertioli, S.C.M.M.; Bertioli, D.J.; Guimaraes, P.M. A survey of genes involved in Arachis stenosperma resistance to Meloidogyne arenaria race 1. Funct. Plant. Biol. 2013, 40, 1298–1309. [Google Scholar] [CrossRef]
- Guimaraes, P.M.; Guimaraes, L.A.; Morgante, C.V.; Silva, O.B., Jr.; Araujo, A.C.G.; Martins, A.C.Q.; Saraiva, M.A.P.; Oliveira, T.N.; Togawa, R.C.; Leal-Bertioli, S.C.M.; et al. Root Transcriptome Analysis of Wild Peanut Reveals Candidate Genes for Nematode Resistance. PLoS ONE 2015, 10, e0140937. [Google Scholar] [CrossRef]
- Mota, A.P.Z.; Vidigal, B.; Danchin, E.G.J.; Togawa, R.C.; Leal-Bertioli, S.C.M.; Bertioli, D.J.; Araujo, A.C.G.; Brasileiro, A.C.M.; Guimaraes, P.M. Comparative root transcriptome of wild Arachis reveals NBS-LRR genes related to nematode resistance. BMC Plant. Biol. 2018, 18, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, A. Plants water soluble chlorophyll binding proteins act as enzyme-inhibitor pair. Russ. J. Plant. Physiol. 2017, 64, 91–99. [Google Scholar] [CrossRef]
- Gholizadeh, A. Chlorophyll Binding Ability of Non-chloroplastic DUF538 Protein Superfamily in Plants. Proc. Natl. Acad. Sci. USA India Sect. B Boil. Sci. 2018, 88, 967–976. [Google Scholar] [CrossRef]
- Gholizadeh, A. Pectin methylesterase activity of plant DUF538 protein superfamily. Physiol. Mol. Biol. Plants 2020, 26, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Proite, K.; Carneiro, R.; Falcao, R.; Gomes, A.; Leal-Bertioli, S.; Guimaraes, P.; Bertioli, D.; Falcão, R.; Gomes, A. Post-infection development and histopathology of Meloidogyne arenaria race 1 on Arachis spp. Plant. Pathol. 2008, 57, 974–980. [Google Scholar] [CrossRef]
- Guimaraes, P.M.; Brasileiro, A.C.M.; Mehta, A.; Araujo, A.C.G. Functional genomics in peanut wild relatives. In The Peanut Genome—Compendium of Plant Genomes; Varshney, R.K., Pandey, M.K., Puppala, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 149–164. [Google Scholar]
- Leal-Bertioli, S.C.M.; Moretzsohn, M.C.; Roberts, P.A.; Ballén-Taborda, C.; Borba, T.C.O.; Valdisser, P.A.; Vianello, R.P.; Araújo, A.C.G.; Guimarães, P.M.; Bertioli, D.J. Genetic Mapping of Resistance to Meloidogyne arenaria in Arachis stenosperma: A New Source of Nematode Resistance for Peanut. G3 Genes Genomes Genet. 2016, 6, 377–390. [Google Scholar] [CrossRef]
- Ballén-Taborda, C.; Chu, Y.; Ozias-Akins, P.; Timper, P.; Holbrook, C.C.; Jackson, S.A.; Bertioli, D.J.; Leal-Bertioli, S.C.M. A new source of root-knot nematode resistance from Arachis stenosperma incorporated into allotetraploid peanut (Arachis hypogaea). Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Martins, A.C.; Mehta, A.; Murad, A.M.; Mota, A.P.; Saraiva, M.A.P.; Araújo, A.C.; Miller, R.N.; Brasileiro, A.C.; Guimarães, P.M. Proteomics unravels new candidate genes for Meloidogyne resistance in wild Arachis. J. Proteom. 2020, 217, 103690. [Google Scholar] [CrossRef] [PubMed]
- Mota, A.P.Z.; Fernandez, D.; Arraes, F.B.M.; Petitot, A.-S.; De Melo, B.P.; De Sa, M.E.L.; Grynberg, P.; Saraiva, M.A.P.; Guimaraes, P.M.; Brasileiro, A.C.M.; et al. Evolutionarily conserved plant genes responsive to root-knot nematodes identified by comparative genomics. Mol. Genet. Genom. 2020, 295, 1063–1078. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Guimarães, L.A.; Wu, C.L.; Timper, P.; Holbrook, C.C.; Ozias-Akins, P. A Technique to Study Meloidogyne arenaria Resistance in Agrobacterium rhizogenes-Transformed Peanut. Plant. Dis. 2014, 98, 1292–1299. [Google Scholar] [CrossRef][Green Version]
- Pereira, B.M.; Guimaraes, L.A.; Souza, N.O.S.; Saraiva, M.A.P.; Guimaraes, P.M.; Brasileiro, A.C.M. Overexpression of Wild Arachis Lipocalin Enhances Root-Knot Nematode Resistance in Peanut Hairy Roots. Plant. Mol. Biol. Rep. 2019, 37, 74–86. [Google Scholar] [CrossRef]
- Guimaraes, L.A.; Pereira, B.M.; Araujo, A.C.G.; Guimaraes, P.M.; Brasileiro, A.C.M. Ex vitro hairy root induction in detached peanut leaves for plant-nematode interaction studies. Plant. Methods 2017, 13, 1–10. [Google Scholar] [CrossRef]
- Kuma, K.M.; Lopes-Caitar, V.S.; Romero, C.C.T.; Silva, S.M.H.; Kuwahara, M.K.; Carvalho, M.; Abdelnoor, R.V.; Dias, W.P.; Marcelino-Guimarães, F.C. A high efficient protocol for soybean root transformation by Agrobacterium rhizogenes and most stable reference genes for RT-qPCR analysis. Plant. Cell Rep. 2015, 34, 1987–2000. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method forAgrobacterium-mediated transformation ofArabidopsis thaliana. Plant. J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Vinson, C.C.; Mota, A.P.Z.; Porto, B.N.; Oliveira, T.N.; Sampaio, I.; Lacerda, A.L.; Danchin, E.G.J.; Guimaraes, P.M.; Williams, T.C.R.; Brasileiro, A.C.M. Characterization of raffinose metabolism genes uncovers a wild Arachis galactinol synthase conferring tolerance to abiotic stresses. Sci. Rep. 2020, 10, 1–19. [Google Scholar] [CrossRef]
- Mota, A.P.Z.; Oliveira, T.N.; Vinson, C.C.; Williams, T.C.R.; Costa, M.M.D.C.; Araujo, A.C.G.; Danchin, E.G.J.; Grossi-De-Sá, M.F.; Guimaraes, P.M.; Brasileiro, A.C.M. Contrasting Effects of Wild Arachis Dehydrin Under Abiotic and Biotic Stresses. Front. Plant. Sci. 2019, 10, 497. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Fernald, R.D. Comprehensive Algorithm for Quantitative Real-Time Polymerase Chain Reaction. J. Comput. Biol. 2005, 12, 1047–1064. [Google Scholar] [CrossRef]
- Simon, P. Q-Gene: Processing quantitative real-time RT-PCR data. Bioinform 2003, 19, 1439–1440. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, L.A.; Mota, A.P.Z.; Araujo, A.C.G.; Figueiredo, L.F.D.A.; Pereira, B.M.; Saraiva, M.A.D.P.; Silva, R.B.; Danchin, E.G.J.; Guimaraes, P.M.; Brasileiro, A.C.M. Genome-wide analysis of expansin superfamily in wild Arachis discloses a stress-responsive expansin-like B gene. Plant. Mol. Biol. 2017, 94, 79–96. [Google Scholar] [CrossRef]
- El-Gebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; Salazar, G.A.; Smart, A. The Pfam protein families database in 2019. Nucleic Acids Res. 2019, 47, D427–D432. [Google Scholar] [CrossRef] [PubMed]
- Bateman, A.; Coggill, P.; Finn, R.D. DUFs: Families in search of function. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2010, 66, 1148–1152. [Google Scholar] [CrossRef] [PubMed]
- Clevenger, J.P.; Chu, Y.; Scheffler, B.; Ozias-Akins, P. A Developmental Transcriptome Map for Allotetraploid Arachis hypogaea. Front. Plant. Sci. 2016, 7, 1446. [Google Scholar] [CrossRef]
- Camejo, D.; Guzmán-Cedeño, Á.; Moreno, A. Reactive oxygen species, essential molecules, during plant–pathogen interactions. Plant. Physiol. Biochem. 2016, 103, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-C.; Wang, P.-H.; Lin, W.-R. Changes of mycorrhizal fungal community occurring during the natural restoration after the chi-chi earthquake in Taiwan. Symbiosis 2018, 77, 177–184. [Google Scholar] [CrossRef]
- Subramanian, S.; Cho, U.H.; Keyes, C.; Yu, O. Distinct changes in soybean xylem sap proteome in response to pathogenic and symbiotic microbe interactions. BMC Plant Biol. 2009, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Notaguchi, M.; Okamoto, S. Dynamics of long-distance signaling via plant vascular tissues. Front. Plant. Sci. 2015, 6, 161. [Google Scholar] [CrossRef] [PubMed]
- Kuromori, T.; Seo, M.; Shinozaki, K. ABA Transport and Plant Water Stress Responses. Trends Plant. Sci. 2018, 23, 513–522. [Google Scholar] [CrossRef]
- Choi, W.-G.; Miller, G.; Wallace, I.; Harper, J.; Mittler, R.; Gilroy, S. Orchestrating rapid long-distance signaling in plants with Ca2+, ROS and electrical signals. Plant. J. 2017, 90, 698–707. [Google Scholar] [CrossRef]
- Xia, X.-J.; Zhou, Y.-H.; Shi, K.; Zhou, J.; Foyer, C.H.; Yu, J.-Q. Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J. Exp. Bot. 2015, 66, 2839–2856. [Google Scholar] [CrossRef]
- Stenzel, I.; Hause, B.; Miersch, O.; Kurz, T.; Maucher, H.; Weichert, H.; Ziegler, J.; Feussner, I.; Wasternack, C. Jasmonate biosynthesis and the allene oxide cyclase family of Arabidopsis thaliana. Plant. Mol. Biol. 2003, 51, 895–911. [Google Scholar] [CrossRef]
- Pauwels, L.; Goossens, A. The JAZ Proteins: A Crucial Interface in the Jasmonate Signaling Cascade. Plant. Cell 2011, 23, 3089–3100. [Google Scholar] [CrossRef]
- Dombrecht, B.; Xue, G.P.; Sprague, S.J.; Kirkegaard, J.A.; Ross, J.J.; Reid, J.B.; Fitt, G.P.; Sewelam, N.; Schenk, P.M.; Manners, J.M.; et al. MYC2 Differentially Modulates Diverse Jasmonate-Dependent Functions in Arabidopsis. Plant. Cell 2007, 19, 2225–2245. [Google Scholar] [CrossRef]
- Chao, Q.; Rothenberg, M.; Solano, R.; Roman, G.; Terzaghi, W.; Ecker†, J.R. Activation of the Ethylene Gas Response Pathway in Arabidopsis by the Nuclear Protein Ethylene-Insensitive3 and Related Proteins. Cell 1997, 89, 1133–1144. [Google Scholar] [CrossRef]
- Mur, L.A.J.; Prats, E.; Pierre, S.; Hall, M.A.; Hebelstrup, K.H. Integrating nitric oxide into salicylic acid and jasmonic acid/ ethylene plant defense pathways. Front. Plant. Sci. 2013, 4, 215. [Google Scholar] [CrossRef] [PubMed]
- Nahar, K.; Kyndt, T.; De Vleesschauwer, D.; Höfte, M.; Gheysen, G. The Jasmonate Pathway Is a Key Player in Systemically Induced Defense against Root Knot Nematodes in Rice. Plant. Physiol. 2011, 157, 305–316. [Google Scholar] [CrossRef]
- Fudali, S.L.; Wang, C.; Williamson, V.M. Ethylene Signaling Pathway Modulates Attractiveness of Host Roots to the Root-Knot Nematode Meloidogyne hapla. Mol. Plant.-Microb. Interact. 2013, 26, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, A. Maltose-binding protein switches programmed cell death in Nicotiana glutinosa leaf cells. Cytol. Genet. 2014, 48, 85–91. [Google Scholar] [CrossRef][Green Version]
- Nakagami, H.; Sugiyama, N.; Mochida, K.; Daudi, A.; Yoshida, Y.; Toyoda, T.; Tomita, M.; Ishihama, Y.; Shirasu, K. Large-Scale Comparative Phosphoproteomics Identifies Conserved Phosphorylation Sites in Plants. Plant. Physiol. 2010, 153, 1161–1174. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araujo, A.C.G.; Guimaraes, P.M.; Mota, A.P.Z.; Guimaraes, L.A.; Pereira, B.M.; Vinson, C.C.; Lacerda, A.L.; Martins, A.C.Q.; Brasileiro, A.C.M. Overexpression of DUF538 from Wild Arachis Enhances Plant Resistance to Meloidogyne spp. Agronomy 2021, 11, 559. https://doi.org/10.3390/agronomy11030559
Araujo ACG, Guimaraes PM, Mota APZ, Guimaraes LA, Pereira BM, Vinson CC, Lacerda AL, Martins ACQ, Brasileiro ACM. Overexpression of DUF538 from Wild Arachis Enhances Plant Resistance to Meloidogyne spp. Agronomy. 2021; 11(3):559. https://doi.org/10.3390/agronomy11030559
Chicago/Turabian StyleAraujo, Ana Claudia Guerra, Patricia Messenberg Guimaraes, Ana Paula Zotta Mota, Larissa Arrais Guimaraes, Bruna Medeiros Pereira, Christina Cleo Vinson, Ana Luíza Lacerda, Andressa Cunha Quintana Martins, and Ana Cristina Miranda Brasileiro. 2021. "Overexpression of DUF538 from Wild Arachis Enhances Plant Resistance to Meloidogyne spp." Agronomy 11, no. 3: 559. https://doi.org/10.3390/agronomy11030559
APA StyleAraujo, A. C. G., Guimaraes, P. M., Mota, A. P. Z., Guimaraes, L. A., Pereira, B. M., Vinson, C. C., Lacerda, A. L., Martins, A. C. Q., & Brasileiro, A. C. M. (2021). Overexpression of DUF538 from Wild Arachis Enhances Plant Resistance to Meloidogyne spp. Agronomy, 11(3), 559. https://doi.org/10.3390/agronomy11030559





