Effect of Calcium on the Growth of Djulis (Chenopodium formosanum Koidz.) Sprouts
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Plant Material and Growth
2.3. Calcium Treatment
2.4. Physiological Parameter Assays
2.5. Analysis of Antioxidant Enzyme Activity
2.6. Proline Assay
2.7. Assay of Na+ and K+
2.8. Statistical Analysis
3. Results
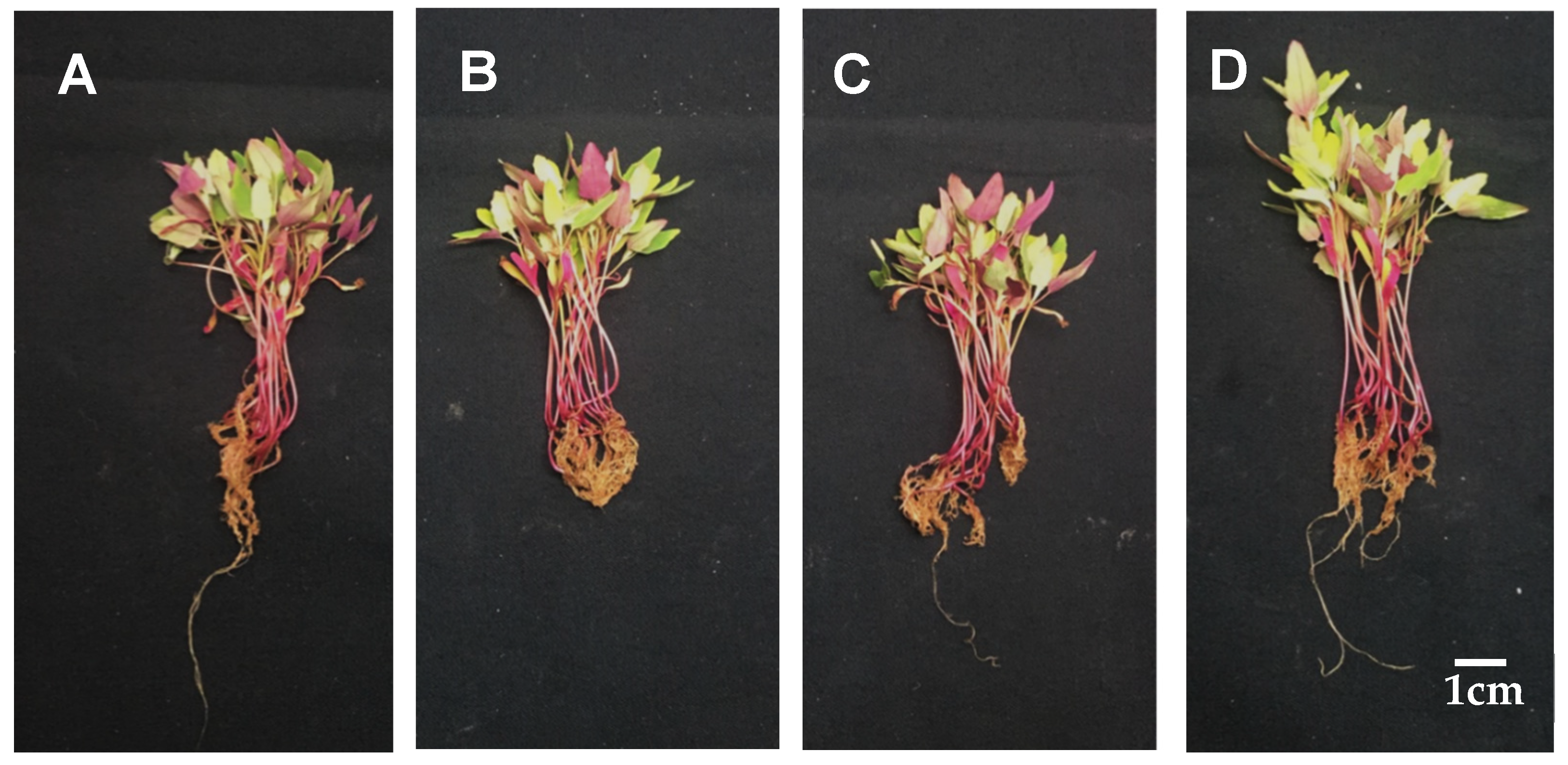
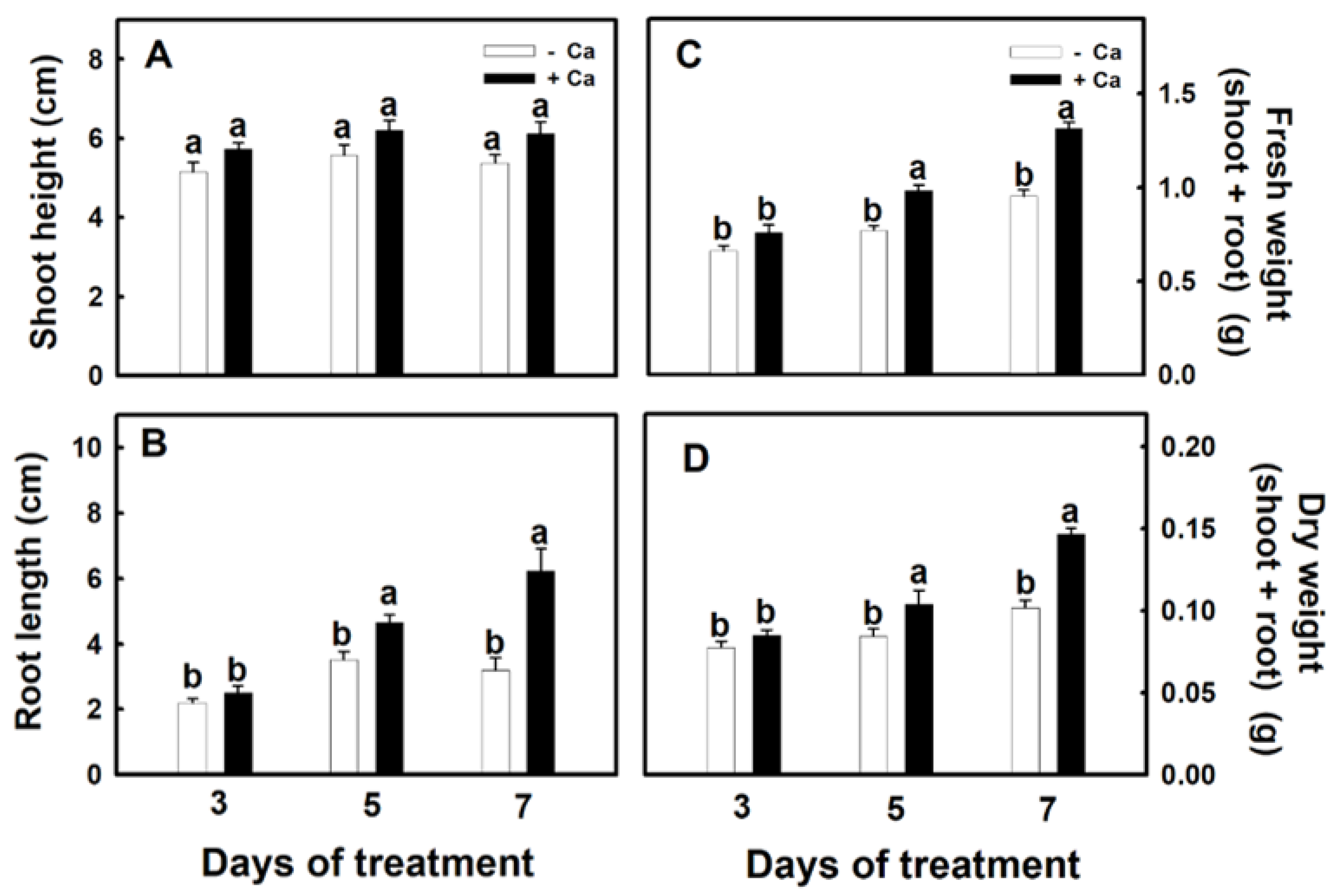
3.1. Establishment of Conditions for the Treatment of Djulis Sprouts with Calcium
3.2. Effect of Calcium on Physiology of Djulis Sprouts
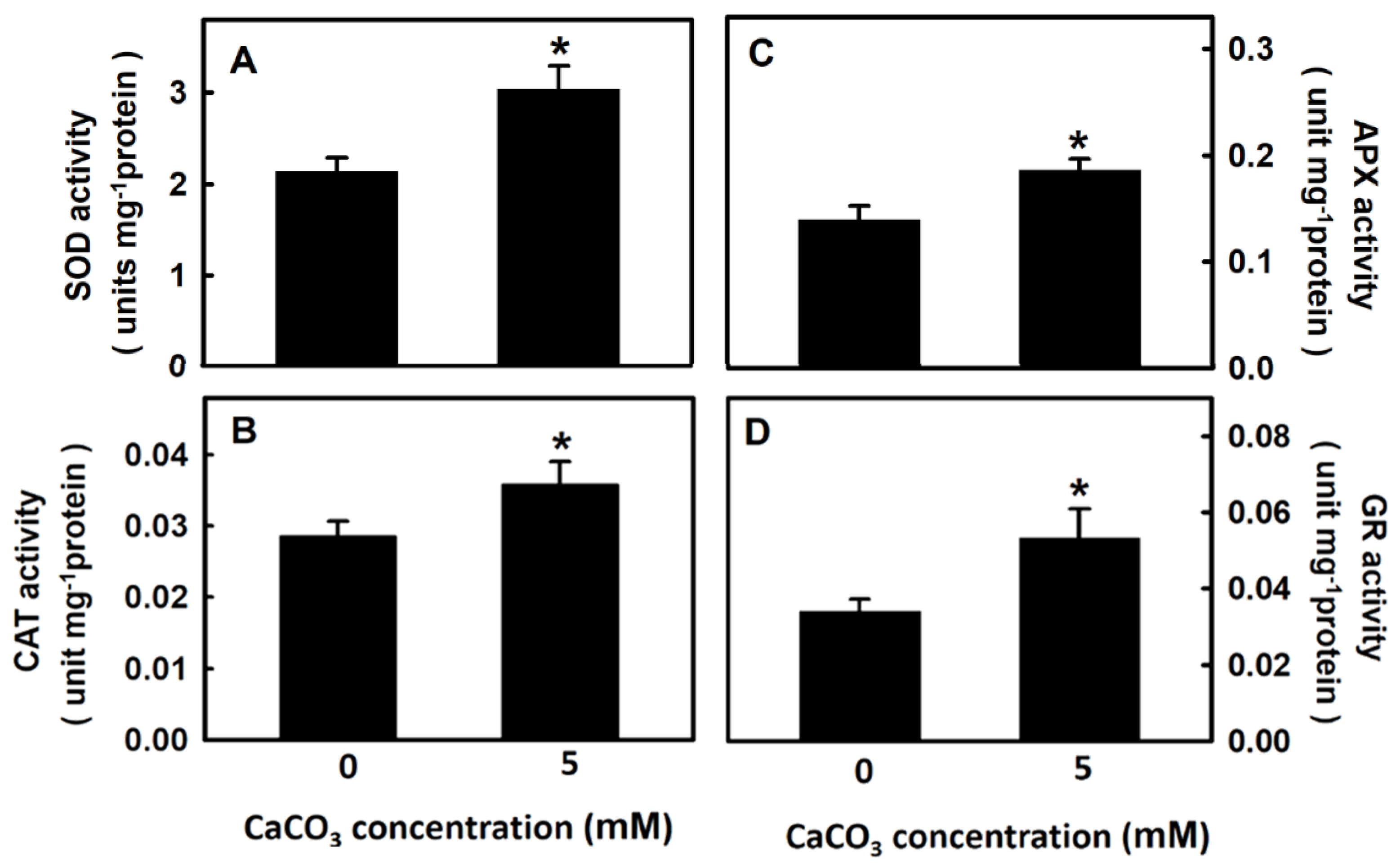
3.3. Effect of Calcium on Antioxidant Enzyme Activity of Djulis Sprouts
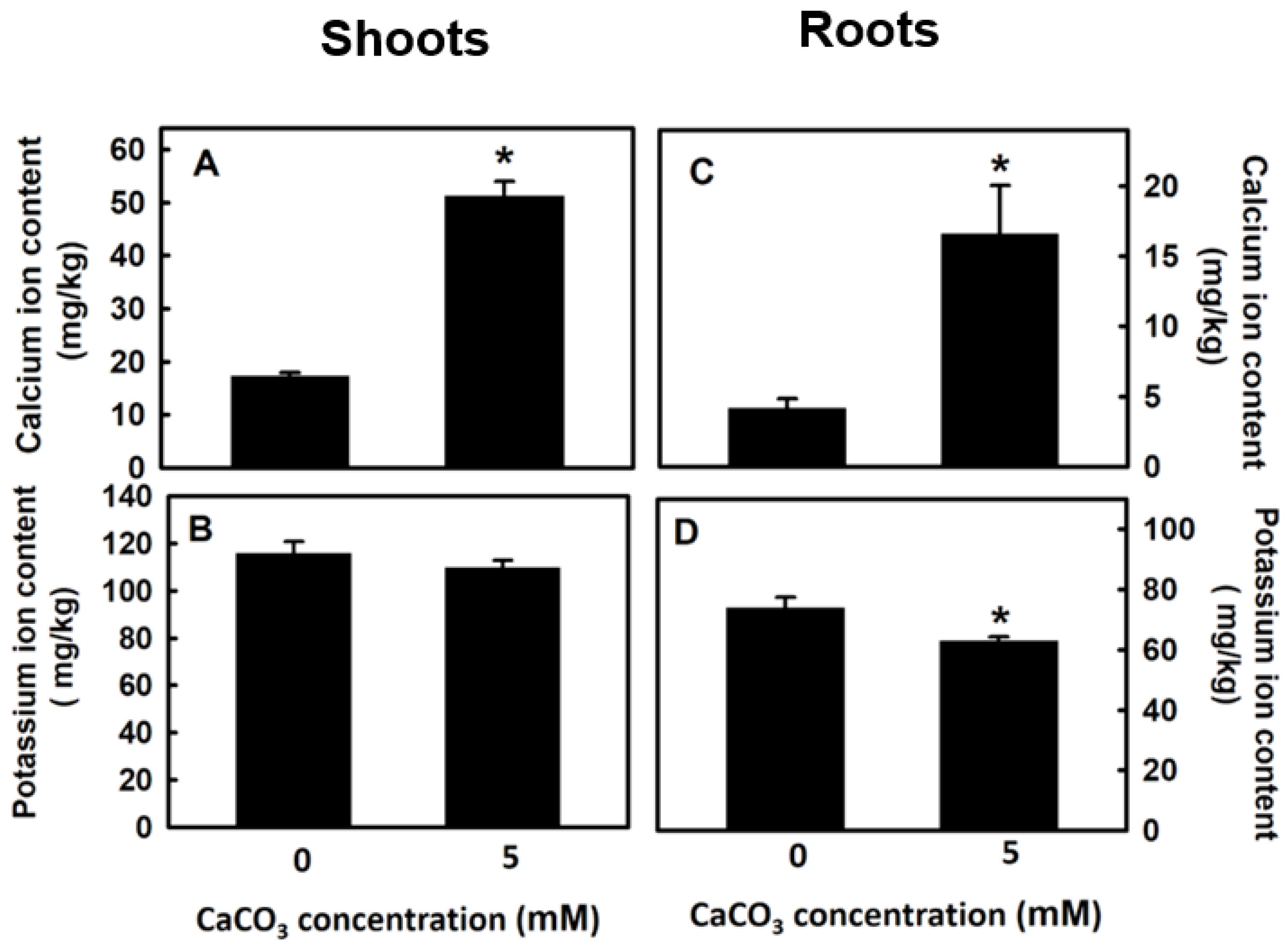
3.4. Effect of Calcium on Potassium Content of Djulis Sprouts
3.5. Effect of Calcium on Proline Content of Djulis Sprouts
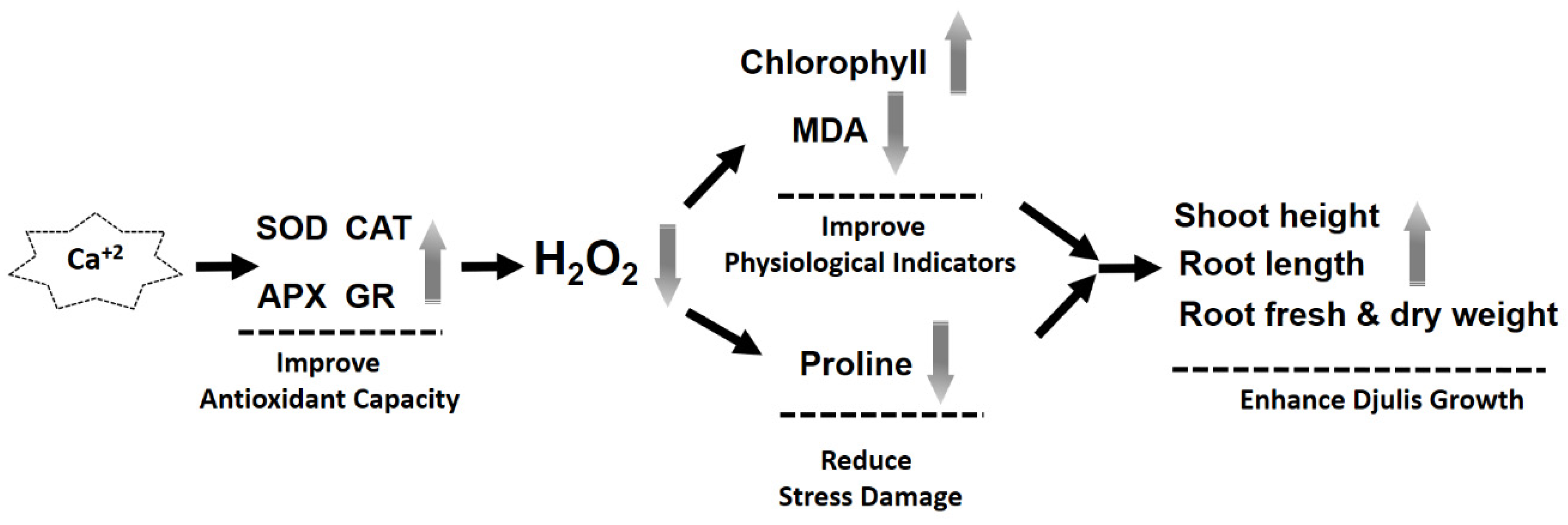
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsai, P.-J.; Chen, Y.-S.; Sheu, C.-H.; Chen, C.-Y. Effect of nanogrinding on the pigment and bioactivity of djulis (Chenopodium formosanum Koidz.). J. Agric. Food Chem. 2011, 59, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, A.; Shukla, S.; Rajan, S.; Ohri, D. Genetic diversity for morphological and quality traits in quinoa (Chenopodium quinoa Willd.) germplasm. Genetic Resour. Crop Evol. 2006, 54, 167–173. [Google Scholar] [CrossRef]
- Navruz-Varli, S.; Sanlier, N. Nutritional and health benefits of quinoa (Chenopodium quinoa Willd.). J. Cereal Sci. 2016, 69, 371–376. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Miranda, M.; Vergara, J.; Uribe, E.; Puente, L. Nutrition facts and functional potential of quinoa (Chenopodium quinoa willd.), an ancient Andean grain: A review. J. Sci. Food Agric. 2010, 90, 2541–2547. [Google Scholar] [CrossRef]
- González Martín, M.I.; Wells Moncada, G.; Fischer, S.; Escuredo, O. Chemical characteristics and mineral composition of quinoa by near-infrared spectroscopy. J. Sci. Food Agric. 2014, 94, 876–881. [Google Scholar] [CrossRef]
- Cooper, R. Re-discovering ancient wheat varieties as functional foods. J. Tradit. Complementary Med. 2015, 5, 138–143. [Google Scholar] [CrossRef]
- Chao, Y.-Y.; Hsueh, I.-E. Insights into Physiological Mechanisms of Salt Stress Tolerance in Djulis (Chenopodium formosanum Koidz.) Sprouts. J. Plant. Biol. 2019, 62, 263–273. [Google Scholar] [CrossRef]
- Thor, K. Calcium -nutrient and messenger. Front. Plant Sci. 2019, 10, 440. [Google Scholar] [CrossRef]
- Domingues, L.D.S.; Ribeiro, N.D.; Andriolo, J.L.; Possobom, M.T.D.F.; Zemolin, A.E.M. Growth, grain yield and calcium, potassium and magnesium accumulation in common bean plants as related to calcium nutrition. Acta Sci. Agron. 2016, 38, 207–217. [Google Scholar] [CrossRef]
- Feagley, S.E.; Fenn, L.B. Using Soluble Calcium to Stimulate Plant Growth. Texas Agricultural Extension Service; Agricultural Communications, The Texas A&M University System: College Station, TX, USA, 1998. [Google Scholar]
- Tsantili, E.; Konstantinidis, K.; Athanasopoulos, P.E.; Pontikis, C. Effects of postharvest calcium treatments on respiration and quality attributes in lemon fruit during storage. J. Hortic. Sci. Biotechnol. 2002, 77, 479–484. [Google Scholar] [CrossRef]
- Kou, L.; Yang, T.; Luo, Y.; Liu, X.; Huang, L.; Codling, E. Pre-harvest calcium application increases biomass and delays senescence of broccoli microgreens. Postharvest Biol. Technol. 2014, 87, 70–78. [Google Scholar] [CrossRef]
- Madani, B.; Mahmud, T.M.M.; Awang, Y.; Kadir, J.; Patil, V.D. Effects of calcium treatment applied around the root zone on nutrient concentration and morphological traits of papaya seedlings (Carica papaya L. cv. Eksotika II). Aust. J. Crop Sci. 2013, 5, 568–572. [Google Scholar]
- White, P.J.; Broadley, M.R. Calcium in plants. Ann. Botony. 2003, 92, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Brestic, M.; Olsovska, K.; Yang, X. Photosynthesis is improved by exogenous calcium in heat-stressed tobacco plants. J. Plant Physiol. 2011, 168, 2063–2071. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Li, X.; Zhang, L. The effect of calcium chloride on growth, photosynthesis, and antioxidant responses of Zoysia japonica under drought conditions. PLoS ONE 2013, 8, e68214. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.S.; Hung, M.J.; Cheng, Y.I.; Cheng, L.J. Calcium- induced proline accumulation contributes to amelioration of NaCl injury and expression of glutamine synthetase in greater duckweed (Spirodela polyrhiza L.). Aquat. Toxicol. 2013, 144–145, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Sarwat, M.; Bhat, N.A.; Wani, M.R.; Kazi, A.G.; Tran, L.S. Alleviation of cadmium toxicity in Brassica juncea L. (Czern. & Coss.) by calcium application involves various physiological and biochemical strategies. PLoS ONE 2015, 10, e0114571. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Basalah, M.O. Interactive effect of calcium and gibberellin on nickel tolerance in relation to antioxidant systems in Triticum aestivum L. Protoplasma 2011, 248, 503–511. [Google Scholar] [CrossRef]
- Rahman, A.; Mostofa, M.G.; Nahar, K.; Alam, M.M.; Hasanuzzaman, M.; Fujita, M. Calcium mitigates arsenic toxicity in rice seedlings by reducing arsenic uptake and modulating the antioxidant defense and glyoxalase systems and stress markers. Biomed. Res. Int. 2015, 2015, 340812. [Google Scholar] [CrossRef]
- Yoshida, S.; Forno, D.A.; Cock, J.H.; Gomez, K.A. Laboratory Manual for Physiological Studies of Rice, 2nd ed.; International Rice Research Institute: Los Banos, Philippines, 1972; pp. 61–66. [Google Scholar]
- Wintermans, J.E.G.M.; De Mots, A. Spectrophotometric characteristics of chlorophylls a and b and their phenophytins in ethanol. Biochim. Biophys. Acta 1965, 109, 448–453. [Google Scholar] [CrossRef]
- Heath, L.R.; Packer, L. Photoperoxidation in isolated chloroplasts: I. kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Jana, S.; Choudhuri, M.A. Glycolate metabolism of three submersed aquatic angiosperms: Effect of heavy metals. Aquat. Bot. 1981, 11, 67–77. [Google Scholar] [CrossRef]
- Paoletti, F.; Aldinucci, D.; Mocali, A.; Capparini, A. A sensitive spectrophotometric method for the determination of superoxide dismutase activity in tissue extracts. Anal. Biochem. 1986, 154, 536–541. [Google Scholar] [CrossRef]
- Kato, M.; Shimizu, S. Chlorophyll metabolism in higher plants. VII. Chlorophyll degradation in senescing tobacco leaves: Phenolic-dependent peroxidative degradation. Can. J. Bot. 1987, 65, 729–735. [Google Scholar] [CrossRef]
- Nakano, Y.; Asad, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Foster, J.G.; Hess, J.L. Responses of superoxide dismutase and glutathione reductase activities in cotton leaf tissue exposed to an atmosphere enriched in oxygen. Plant Physiol. 1980, 66, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Olle, M.; Bender, I. Causes and control of calcium deficiency disorders in vegetables: A review. J. Hortic. Sci. Biotechnol. 2009, 84, 577–584. [Google Scholar] [CrossRef]
- Ahmed, A.A.M.; Zuhair, A.D.; Wisam, K.K. Role of Boron and Calcium on growth, flowering and yield of strawberry (Fragaria x ananassa Duch) var. Liberation D’Orleans. Middle East J. Agric. Res. 2020, 9, 130–133. [Google Scholar] [CrossRef]
- Kumara, K.H.C.H.; Wathugala, D.L.; Hafeel, R.F.; Kumarasinghe, H.K.M.S. Effect of Nano Calcite Foliar Fertilizer on the Growth and Yield of Rice (Oryza sativa). J. Agric. Sci. 2019, 14, 154–164. [Google Scholar] [CrossRef]
- Ismael, S.Z.; Khandaker, M.M.; Mat, N.; Boyce, A.N. Effects of hydrogen peroxide on growth, development and quality of fruits: A review. J. Agron. 2015, 14, 331–336. [Google Scholar] [CrossRef]
- Niu, L.; Liao, W. Hydrogen peroxide signaling in plant development and abiotic responses: Crosstalk with nitric oxide and calcium. Front. Plant Sci. 2016, 7, 230. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.H.; Yu, C.W.; Lin, C.H. Hydrogen peroxide functions as a stress signal in plants. Bot. Bullet Acad. Sin. 2005, 46, 1–10. [Google Scholar]
- Aguilar, P.C.; Cutipa, Z.; Machaca, E.; Lopez, M.; Jacobsen, S.-E. Variation of proline content of quinoa (Chenopodium quinoa Willd.) in high beds (waru waru). Food Rev. Int. 2003, 19, 121–127. [Google Scholar] [CrossRef]
- Elewa, T.A.; Sadak, M.S.; Saad, A.M. Proline treatment improves physiological responses in quinoa plants under drought stress. Biosci. Res. 2017, 14, 21–33. [Google Scholar]


| CaCO3 Concentration (mM) | Root Length (cm) | Shoot Height (cm) | Shoots | Roots | ||
|---|---|---|---|---|---|---|
| Fresh Weigh (g) | Dry Weigh (g) | Fresh Weigh (g) | Dry Weigh (g) | |||
| 0 | 2.41 ± 0.11 b | 5.90 ± 0.04 b | 0.912 ± 0.01 b | 0.162 ± 0.002 b | 0.082 ± 0.024 a | 0.022 ± 0.001 a |
| 1.25 | 2.33 ± 0.37 b | 5.96 ± 0.3 b | 0.984 ± 0.11 a,b | 0.168 ± 0.019 b | 0.090 ± 0.008 a | 0.025 ± 0.002 a |
| 2.5 | 2.53 ± 0.08 b | 6.26 ± 0.03 a,b | 1.064 ± 0.11 a,b | 0.163 ± 0.023 b | 0.115 ± 0.011 a | 0.024 ± 0.003 a |
| 5 | 3.74± 0.24 a | 6.64 ± 0.1 a | 1.252 ± 0.05 a | 0.189 ± 0.014 a | 0.109 ± 0.033 a | 0.027 ± 0.006 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chao, Y.-Y.; Wang, W.-J.; Liu, Y.-T. Effect of Calcium on the Growth of Djulis (Chenopodium formosanum Koidz.) Sprouts. Agronomy 2021, 11, 82. https://doi.org/10.3390/agronomy11010082
Chao Y-Y, Wang W-J, Liu Y-T. Effect of Calcium on the Growth of Djulis (Chenopodium formosanum Koidz.) Sprouts. Agronomy. 2021; 11(1):82. https://doi.org/10.3390/agronomy11010082
Chicago/Turabian StyleChao, Yun-Yang, Wei-Jia Wang, and Yan-Ting Liu. 2021. "Effect of Calcium on the Growth of Djulis (Chenopodium formosanum Koidz.) Sprouts" Agronomy 11, no. 1: 82. https://doi.org/10.3390/agronomy11010082
APA StyleChao, Y.-Y., Wang, W.-J., & Liu, Y.-T. (2021). Effect of Calcium on the Growth of Djulis (Chenopodium formosanum Koidz.) Sprouts. Agronomy, 11(1), 82. https://doi.org/10.3390/agronomy11010082






