Stabilizing Grain Yield and Nutrition Quality in Purple Rice Varieties by Management of Planting Elevation and Storage Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Effects of Planting Elevation on Yield and Its Grain Nutritional Quality
2.2. Effects of Storage Condition on Anthocyanin Concentration and Antioxidant Capacity
2.3. Anthocyanin Concentration Analysis
2.4. Antioxidant Capacity Analysis
2.5. Statistical Analysis
3. Results
3.1. Effects of Planting Elevation on Yield and Grain Nutritional Quality
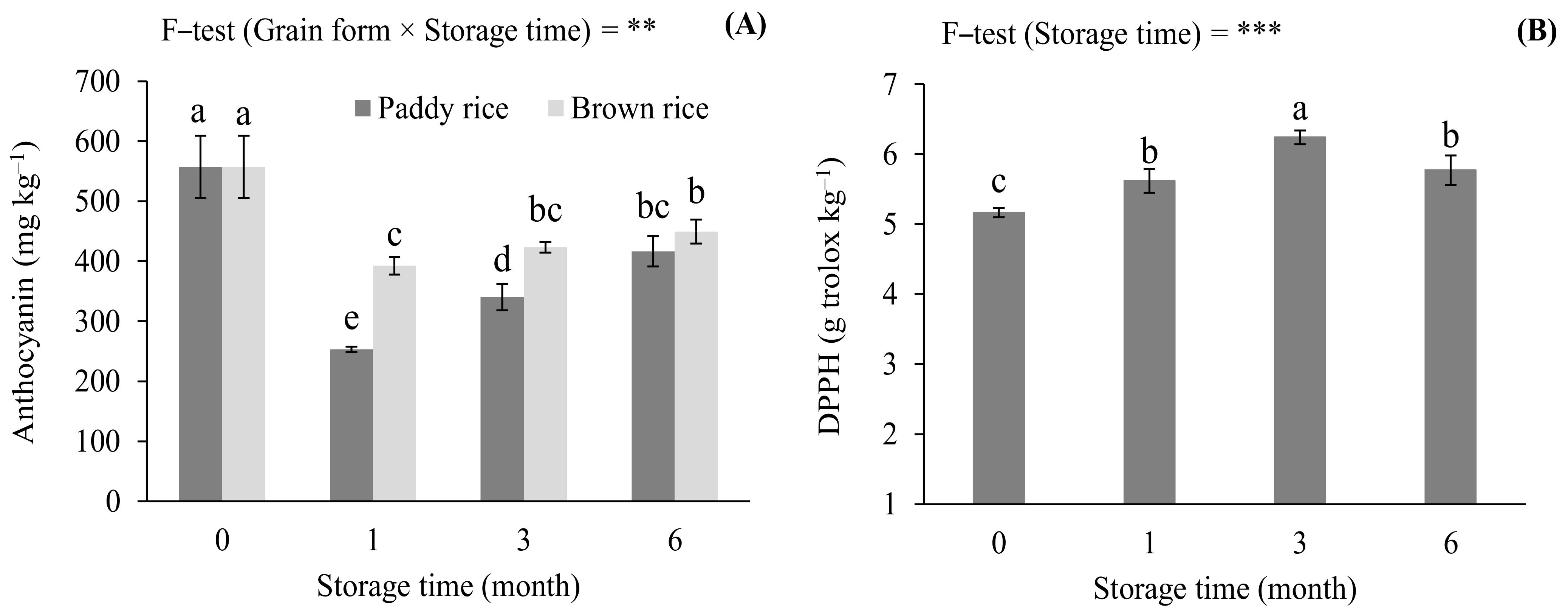
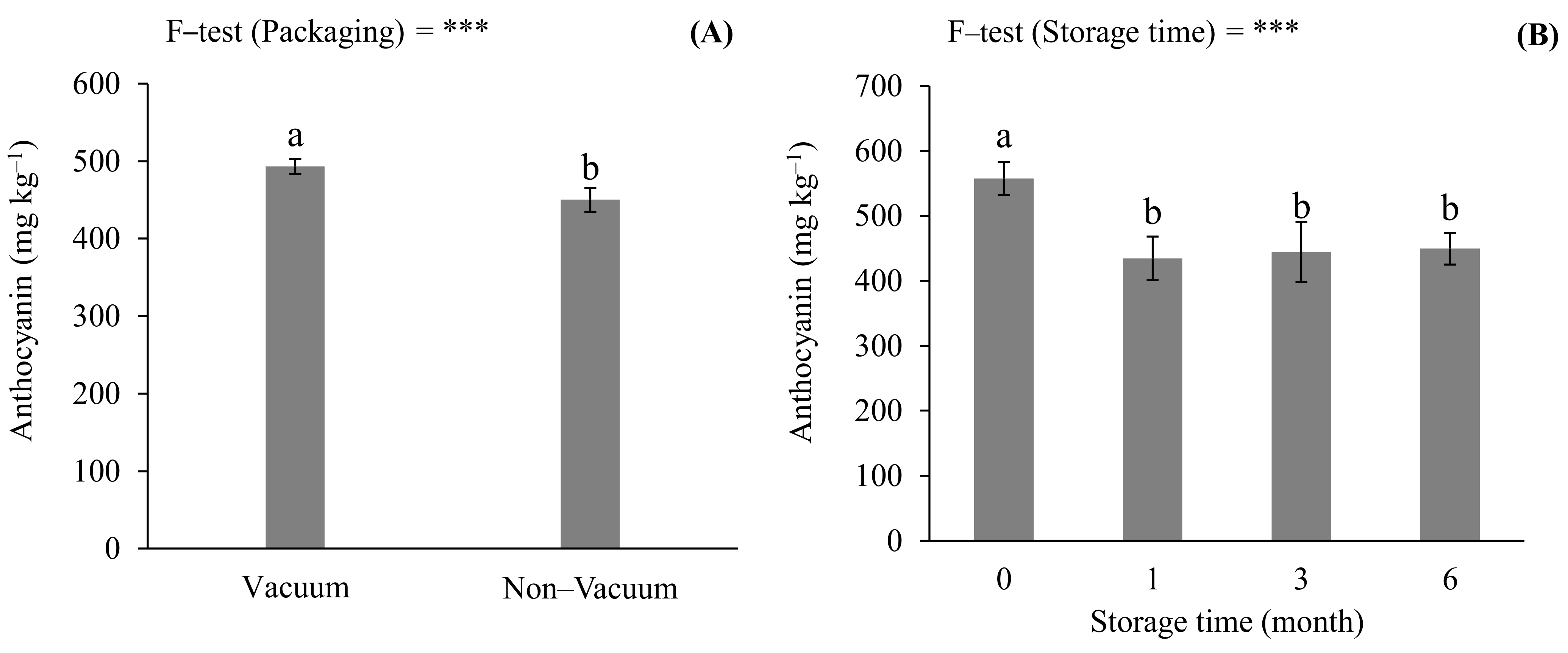
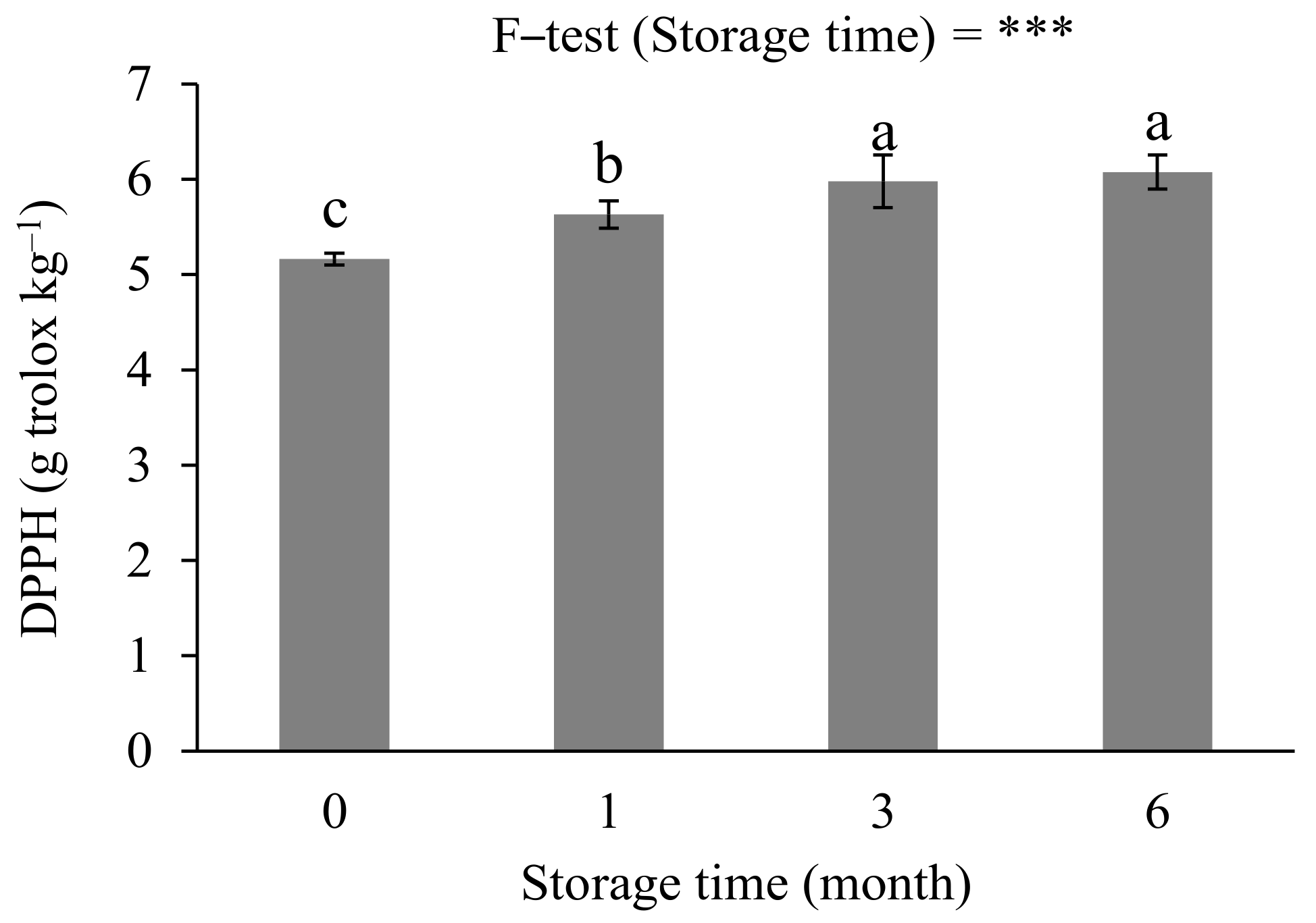
3.2. Effects of Storage Conditions on Anthocyanin Concentration and Antioxidant Capacity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, G.F.; Xu, X.R.; Zhang, Y.; Li, D.; Gan, R.Y.; Li, H.B. Phenolic compounds and bioactivities of pigmented rice. Crit. Rev. Food Sci. Nutr. 2013, 53, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Jamjod, S.; Yimyam, N.; Lordkaew, S.; Rerkasem, B. Characterization of on‒farm rice germplasm in an area of the crop’s center of diversity. CMUJ Nat. Sci. 2017, 16, 85–98. [Google Scholar] [CrossRef][Green Version]
- Boonsit, P.; Pongpiachan, P.; Julsrigival, S.; Karladee, D. Gamma oryzanol content in glutinous purple rice landrace varieties. CMUJ Nat. Sci. 2010, 9, 151–158. [Google Scholar]
- Rerkasem, B.; Jumrus, S.; Yimyam, N. Variation of grain nutritional quality among Thai purple rice genotypes grown at two different altitudes. ScienceAsia 2015, 41, 377–385. [Google Scholar] [CrossRef]
- Jaksomsak, P.; Rerkasem, B.; Prom-u-Thai, C. Variation in nutritional quality of pigmented rice varieties under different water regimes. Plant Prod. Sci. 2020, 1–12. [Google Scholar] [CrossRef]
- Zaidi, S.H.R.; Zakari, S.A.; Zhao, Q.; Khan, A.R.; Shah, J.M.; Cheng, F. Anthocyanin accumulation in black kernel mutant rice and its contribution to ROS detoxification in response to high temperature at the filling stage. Antioxidants 2019, 8, 510. [Google Scholar] [CrossRef]
- Hinojosa-Gómez, J.; Martín-Hernández, C.S.; Heredia, J.B.; Osuna-Enciso, T.; Muy-Rangel, M.D. Anthocyanin Induction by Drought Stress in the Calyx of Roselle Cultivars. Molecules 2020, 25, 1555. [Google Scholar] [CrossRef]
- Yamuangmorn, S.; Dell, B.; Rerkasem, B.; Prom-u-thai, C. Stability of anthocyanin content and antioxidant capacity among local Thai purple rice genotypes in different storage conditions. Chiang Mai J. Sci. 2018, 45, 927–936. [Google Scholar]
- Santos, N.C.; Silva, W.P.; Barros, S.L.; Araújo, A.D.; Gomes, J.P.; Almeida, R.L.; Nascimento, A.P.; Almeida, R.D.; Silva, C.E.; Queiroz, A.J.; et al. Study on drying of black rice (Oryza sativa L.) grains: Physical‒chemical and bioactive quality. J. Agric. Sci. 2019, 11, 203–212. [Google Scholar] [CrossRef]
- Norkaew, O.; Boontakham, P.; Dumri, K.; Noenplab, A.N.L.; Sookwong, P.; Mahatheeranont, S. Effect of post‒harvest treatment on bioactive phytochemicals of Thai black rice. Food Chem. 2017, 217, 98–105. [Google Scholar] [CrossRef]
- Tananuwong, K.; Lertsiri, S. Changes in volatile aroma compounds of organic fragrant rice during storage under different conditions. J. Sci. Food Agric. 2010, 90, 1590–1596. [Google Scholar] [CrossRef] [PubMed]
- Htwe, N.; Srilaong, V.; Tanprasert, K.; Photchanachai, S.; Kanlayanarat, S.; Uthairatanakij, A. Low oxygen concentrations affecting antioxidant activity and bioactive compounds in coloured rice. Asian J. Agric. Food Sci. 2010, 3, 269–281. [Google Scholar]
- Lang, G.H.; Lindemann, I.D.S.; Ferreira, C.D.; Hoffmann, J.F.; Vanier, N.L.; de Oliveira, M. Effects of drying temperature and long-term storage conditions on black rice phenolic compounds. Food Chem. 2019, 287, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, E.S.M.; Hucl, P. A Rapid method for quantifying total anthocyanins in blue aleurone and purple pericarp wheats. Cereal Chem. 1999, 76, 350–354. [Google Scholar] [CrossRef]
- Amarowicz, R.; Pegg, R.B.; Rahimi-Moghaddam, P.; Barl, B.; Weil, J.A. Free‒radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem. 2004, 84, 551–562. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, Y.; Xiang, J.; Uphoff, N.T.; Pan, X.; Zhu, D. Effects of low temperature stress on spikelet-related parameters during anthesis in indica-japonica hybrid rice. Front. Plant Sci. 2017, 8, 1350. [Google Scholar] [CrossRef]
- Tisarum, R.; Theerawitaya, C.; Samphumphuang, T.; Cha-Um, S. Regulation of anthocyanin accumulation in rice (Oryza sativa L. subsp. indica) using MgSO4 spraying and low temperature. Arch. Agron. Soil Sci. 2018, 64, 1663–1677. [Google Scholar] [CrossRef]
- Purwanto, E.; Hidayati, W. The yield and quality of black rice varieties in different altitude. IOP Conf. Ser. Earth Environ. Sci. 2018, 142, 012037. [Google Scholar] [CrossRef]
- Piero, A.; Puglisi, I.; Rapisarda, P.; Petrone, G. Anthocyanins accumulation and related gene expression in red orange fruit induced by low temperature storage. J. Agric. Food Chem. 2005, 53, 9083–9088. [Google Scholar] [CrossRef]
- He, Q.; Ren, Y.; Zhao, W.; Li, R.; Zhang, L. Low temperature promotes anthocyanin biosynthesis and related gene expression in the seedlings of purple head Chinese cabbage (Brassica rapa L.). Genes 2020, 11, 81. [Google Scholar] [CrossRef]
- Li, X.L.; Lv, X.; Wang, X.; Wang, L.; Zhang, M.; Ren, M. Effects of abiotic stress on anthocyanin accumulation and grain weight in purple wheat. Crop. Pasture Sci. 2018, 69, 1208–1214. [Google Scholar] [CrossRef]
- Zhu, H.; Li, X.; Zhai, W.; Liu, Y.; Gao, Q.; Liu, J.; Ren, L.; Chen, H.; Zhu, Y. Effects of low light on photosynthetic properties, antioxidant enzyme activity, and anthocyanin accumulation in purple pak‒choi (Brassica campestris ssp. Chinensis Makino). PLoS ONE 2017, 12, e0179305. [Google Scholar] [CrossRef] [PubMed]
- Yamuangmorn, S.; Dell, B.; Rerkasem, B.; Prom-u-thai, C. Applying nitrogen fertilizer increased anthocyanin in vegetative shoots but not in grain of purple rice genotypes. J. Sci. Food Agric. 2018, 98, 4527–4532. [Google Scholar] [CrossRef] [PubMed]
- Zaupa, M.; Calani, L.; Del Rio, D.; Brighenti, F.; Pellegrini, N. Characterization of total antioxidant capacity and (poly)phenolic compounds of differently pigmented rice varieties and their changes during domestic cooking. Food Chem. 2015, 187, 338–347. [Google Scholar] [CrossRef]
- Fracassetti, D.; Pozzoli, C.; Vitalini, S.; Tirelli, A.; Iriti, M. Impact of cooking on bioactive compounds and antioxidant activity of pigmented rice cultivars. Foods 2020, 9, 967–979. [Google Scholar] [CrossRef]
- Zhang, Y.; Teng, B.; Wang, D. Discovery of a specific volatile substance from rice grain and its application in controlling stored-grain pests. Food Chem. 2020, 339, 1280142. [Google Scholar]
- Oren-Shamir, M. Does anthocyanin degradation play a significant role in determining pigment concentration in plants? Plant Sci. 2009, 177, 310–316. [Google Scholar] [CrossRef]
- Masutti, D.; Borgognone, A.; Setti, L. Production of enzymes from rice husks and wheat straw in solid state fermentation. Chem. Eng. Trans. 2012, 27, 133–138. [Google Scholar]
- Lakshmi, S.; Shashidhara, G.; Madhu, G.; Muthappa, R.; Hamse, V.; Prasad, M.N.N. Characterization of peroxidase enzyme and detoxification of phenols using peroxidase enzyme obtained from Zea mays L. waste. Appl. Water Sci. 2018, 8, 207. [Google Scholar] [CrossRef]
- Alasalvar, C.; Al-Farsi, M.; Quantick, P.; Shahidi, F.; Wiktorowicz, R. Effect of chill storage and modified atmosphere packing (MAP) on antioxidant activity, anthocyanins, carotenoids, phenolics and sensory quality of ready-to-eat shredded orange and purple carrots. Food Chem. 2005, 89, 69–76. [Google Scholar] [CrossRef]
- Oliveira, K.G.; Queiroz, V.A.; de Carlos, L.A.; de Cardoso, L.M.; Pinheiro-Sant’Ana, H.M.; Anunciação, P.C.; Menezes, C.B.; Silva, E.C.; Barros, F. Effect of the storage time and temperature on phenolic compounds of sorghum grain and flour. Food Chem. 2017, 216, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Ito, V.C.; Zielinski, A.A.F.; Demiate, I.M.; Spoto, M.; Nogueira, A.; Lacerda, L.G. Effects of gamma radiation on the stability and degradation kinetics of phenolic compounds and antioxidant activity during storage of (Oryza sativa L.) black rice flour. Braz. Arch. Biol. Technol. 2019, 62, e19180470. [Google Scholar] [CrossRef]

| Area | Grain Yield (ton ha−1) | Number of Tillers Plant−1 | Number of Panicles Plant−1 | Filled Grain (%) | 1000 Grain Weight | Number of Spiklets Plant−1 | Panicle Length | Culm Length | Harvest Index | Days to Flowering |
|---|---|---|---|---|---|---|---|---|---|---|
| High-grain anthocyanin PES variety | ||||||||||
| Lowland | 5.14 ± 0.46 a | 7.9 ± 0.2 | 7.6 ± 0.0 | 93.8 ± 0.2 a | 33.0 ± 0.57 | 114 ± 13 | 28.3 ± 0.3 | 83.4 ± 1.8 a | 0.37 ± 0.01 | 86 ± 1 b |
| Highland | 2.85 ± 0.36 b | 6.8 ± 0.1 | 6.7 ± 0.1 | 91.9 ± 0.1 b | 33.0 ± 0.21 | 135 ± 4 | 27.5 ± 0.4 | 76.4 ± 1.2 b | 0.37 ± 0.03 | 100 ± 1 a |
| F-test | * | ns | ns | ** | ns | ns | ns | * | ns | *** |
| High-DPPH activity KAK variety | ||||||||||
| Lowland | 2.95 ± 0.10 | 7.5 ± 1.0 | 7.0 ± 0.8 | 84.8 ± 3.4 | 37.8 ± 1.1 | 86 ± 3.0 | 25.9 ± 1.5 | 66.5 ± 4.7 | 0.41 ± 0.03 | 87 ± 0 b |
| Highland | 2.99 ± 0.09 | 6.9 ± 0.6 | 6.8 ± 0.6 | 89.3 ± 5.5 | 37.9 ± 1.1 | 89 ± 0.6 | 24.9 ± 0.5 | 63.7 ± 1.3 | 0.37 ± 0.01 | 100 ± 1 a |
| F-test | ns | ns | ns | ns | ns | ns | ns | ns | ns | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamuangmorn, S.; Jumrus, S.; Jamjod, S.; Yimyam, N.; Prom-u-Thai, C. Stabilizing Grain Yield and Nutrition Quality in Purple Rice Varieties by Management of Planting Elevation and Storage Conditions. Agronomy 2021, 11, 83. https://doi.org/10.3390/agronomy11010083
Yamuangmorn S, Jumrus S, Jamjod S, Yimyam N, Prom-u-Thai C. Stabilizing Grain Yield and Nutrition Quality in Purple Rice Varieties by Management of Planting Elevation and Storage Conditions. Agronomy. 2021; 11(1):83. https://doi.org/10.3390/agronomy11010083
Chicago/Turabian StyleYamuangmorn, Supapohn, Suchada Jumrus, Sansanee Jamjod, Narit Yimyam, and Chanakan Prom-u-Thai. 2021. "Stabilizing Grain Yield and Nutrition Quality in Purple Rice Varieties by Management of Planting Elevation and Storage Conditions" Agronomy 11, no. 1: 83. https://doi.org/10.3390/agronomy11010083
APA StyleYamuangmorn, S., Jumrus, S., Jamjod, S., Yimyam, N., & Prom-u-Thai, C. (2021). Stabilizing Grain Yield and Nutrition Quality in Purple Rice Varieties by Management of Planting Elevation and Storage Conditions. Agronomy, 11(1), 83. https://doi.org/10.3390/agronomy11010083





