Effect of Opuntia ficus-indica Mucilage Edible Coating on Quality, Nutraceutical, and Sensorial Parameters of Minimally Processed Cactus Pear Fruits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cactus Pear Fruit Samples
2.2. Fresh Mucilage Extraction and Application
2.3. Quality Parameters: Firmness, Soluble Solid Content, Titratable Acidity, Color, and Weight Loss
2.4. Headspace Gas Composition
2.5. Nutraceutical Attributes
2.5.1. Fruit Extract Preparation
2.5.2. Quantitation of Betalains in Fruit Extracts
2.5.3. DPPH Assay
2.5.4. Ascorbic Acid Content
2.6. Sensory Analysis and Visual Score
2.7. Microbiological Analyses
2.8. Statistical Analyses
3. Results
3.1. Quality Parameters: Firmness, Soluble Solids Content, Titratable Acidity, Color, and Weight Loss
3.2. Headspace Gas Composition
3.3. Bioactive Compounds and Radical Scavenging Activity
3.4. Evolution of Microbiological Parameters
3.5. Sensory Analysis and Visual Score
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liguori, G.; Inglese, P. Cactus pear (o. Ficus-indica (l.) Mill.) Fruit production: Ecophysiology, orchard and fresh-cut fruit management. Acta Hortic. 2015, 1067, 247–252. [Google Scholar] [CrossRef]
- Piga, A. Cactus pear: A fruit of nutraceutical and functional importance. J. Prof. Assoc. Cactus. Develop. 2004, 6, 9–22. [Google Scholar]
- Gentile, C.; Tesoriere, L.; Allegra, M.; Livrea, M.; D’Alessio, P. Antioxidant Betalains from Cactus Pear (Opuntia ficus-indica) Inhibit Endothelial ICAM-1 Expression. Ann. New York Acad. Sci. 2004, 1028, 481–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cefola, M.; Renna, M.; Pace, B. Marketability of ready-to-eat cactus pear as affected by temperature and modified atmosphere. J. Food Sci. Technol. 2014, 51, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Palma, A.; Continella, A.; La Malfa, S.; D’Aquino, S. Changes in physiological and some nutritional, nutraceuticals, chemical–physical, microbiological and sensory quality of minimally processed cactus pears cvs ‘Bianca’, ‘Gialla’ and ‘Rossa’ stored under passive modified atmosphere. J. Sci. Food Agric. 2018, 98, 1839–1849. [Google Scholar] [CrossRef] [PubMed]
- Piga, A.; D’Aquino, S.; Agabbio, M.; Emonti, G.; Farris, G. Influence of Storage Temperature on Shelf-life of Minimally Processed Cactus Pear Fruits. LWT 2000, 33, 15–20. [Google Scholar] [CrossRef]
- Liguori, G.; Gaglio, R.; Settanni, L.; Inglese, P.; D’Anna, F.; Miceli, A. Effect of Opuntia ficus-indica Mucilage Edible Coating in Combination with Ascorbic Acid, on Strawberry Fruit Quality during Cold Storage. J. Food Qual. 2021, 2021, 1–8. [Google Scholar] [CrossRef]
- Pushpendra, K.; Shruti, S. Edible coating for fresh fruit: A review. Int. J. Current Microbiol. Appl. Sci. 2018, 7, 2619–2626. [Google Scholar]
- Tapia, M.; Rojas-Graü, M.; Carmona, A.; Rodríguez, F.; Soliva-Fortuny, R.; Martin-Belloso, O. Use of alginate- and gellan-based coatings for improving barrier, texture and nutritional properties of fresh-cut papaya. Food Hydrocoll. 2008, 22, 1493–1503. [Google Scholar] [CrossRef]
- Allegra, A.; Inglese, P.; Sortino, G.; Settanni, L.; Todaro, A.; Liguori, G. The influence of Opuntia ficus-indica mucilage edible coating on the quality of ‘Hayward’ kiwifruit slices. Postharvest Biol. Technol. 2016, 120, 45–51. [Google Scholar] [CrossRef]
- Sogvar, O.B.; Saba, M.K.; Emamifar, A. Aloe vera and ascorbic acid coatings maintain postharvest quality and re-duce microbial load of strawberry fruit. Postharv. Biol. Technol. 2016, 114, 29–35. [Google Scholar] [CrossRef]
- Del Nobile, M.; Conte, A.; Scrocco, C.; Brescia, I. New strategies for minimally processed cactus pear packaging. Innov. Food Sci. Emerg. Technol. 2009, 10, 356–362. [Google Scholar] [CrossRef]
- Allegra, A.; Sortino, G.; Inglese, P.; Settanni, L.; Todaro, A.; Gallotta, A. The effectiveness of Opuntia ficus-indica mucilage edible coating on post-harvest maintenance of ‘Dottato’ fig ( Ficus carica L.) fruit. Food Packag. Shelf Life 2017, 12, 135–141. [Google Scholar] [CrossRef]
- Del-Valle, V.; Hernández-Muñoz, P.; Guarda, A.; Galotto, M. Development of a cactus-mucilage edible coating (Opuntia ficus indica) and its application to extend strawberry (Fragaria ananassa) shelf-life. Food Chem. 2005, 91, 751–756. [Google Scholar] [CrossRef]
- Aquino, L.V.; Rodriguez, J.; Mendez, L.L.; Torres, K.F. Inhibicion del oscurecimiento con mucilago de nopal (Opuntia ficus-indica) en el secado se platano roatan. Informacion Tecnol. 2009, 20, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Riaz, S.; Sultan, M.T.; Sibt-E-Abass, M.; Imran, M.; Ahmad, R.S.; Hussain, M.B.; Shariati, M.A.; Kosenko, I.; Kleymenova, N.L.; Egorova, G.N. Extraction of Polysaccharides From Opuntia Cactus For Its Potential Application In Edible Coating to Improve The Shelf Life of Citrus (Kinnow Mandarin) Fruit. J. Microbiol. Biotechnol. Food Sci. 2018, 8, 745–750. [Google Scholar] [CrossRef] [Green Version]
- Sáenz, C.; Sepúlveda, E.; Matsuhiro, B. Opuntia spp mucilage’s: A functional component with industrial perspectives. J. Arid. Environ. 2004, 57, 275–290. [Google Scholar] [CrossRef]
- Du Toit, A.; De Wit, M. A Process for Extracting Mucilage from Opuntia Ficus-Indica and Aloe Barbadensis. South Africa Patent No. PA153178/P, 12 May 2011. [Google Scholar]
- McGuire, R.G. Reporting of Objective Color Measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef] [Green Version]
- Mannino, G.; Perrone, A.; Campobenedetto, C.; Schittone, A.; Bertea, C.M.; Gentile, C. Phytochemical profile and antioxidative properties of Plinia trunciflora fruits: A new source of nutraceuticals. Food Chem. 2020, 307, 125515. [Google Scholar] [CrossRef]
- Kanner, J.; Harel, S.; Granit, R. BetalainsA New Class of Dietary Cationized Antioxidants. J. Agric. Food Chem. 2001, 49, 5178–5185. [Google Scholar] [CrossRef]
- Schwartz, S.J.; von Elbe, J.H. Quantitative determination of individual betacyanin pigments by high-performance liquid chromatography. J. Agric. Food Chem. 1980, 28, 540–543. [Google Scholar] [CrossRef]
- Butera, D.; Tesoriere, L.; DI Gaudio, F.; Bongiorno, A.; Allegra, M.; Pintaudi, A.M.; Kohen, R.; Livrea, M.A. Antioxidant Activities of Sicilian Prickly Pear (Opuntia ficus indica) Fruit Extracts and Reducing Properties of Its Betalains: Betanin and Indicaxanthin. J. Agric. Food Chem. 2002, 50, 6895–6901. [Google Scholar] [CrossRef] [Green Version]
- Piattelli, M.; Minale, L.; Prota, G. Isolation, structure and absolute configuration of indicaxanthin. Tetrahedron 1964, 20, 2325–2329. [Google Scholar] [CrossRef]
- Ozgen, M.; Reese, R.N.; Tulio, A.Z.; Scheerens, J.C.; Miller, A.R. Modified 2, 2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method to measure anti- oxidant capacity of selected small fruits and comparison to ferric reducing anti- oxidant power (FRAP) and 2,2′-diphenyl-1-picrylhydrazyl (DPPH) methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Ranganna, S. Manual of Analysis of Fruit and Vegetable Products; McGraw-Hill Publishing Co. Ltd.: New Delhi, India, 1977. [Google Scholar]
- Amodio, M.L.; Cabezas-Serrano, A.B.; Rinaldi, R.; Colelli, G. Implementation of rating scales for visual quality evaluation of various vegetable crops. In Produce Quality Rating Scales and Color Charts Postharvest Horticulture; Series No. 23; Kader, A.A., Cantwell, M., Eds.; University of California: Davis, CA, USA, 2007. [Google Scholar]
- Andreu-Coll, L.; García-Pastor, M.; Valero, D.; Amorós, A.; Almansa, M.S.; Legua, P.; Hernández, F. Influence of Storage on Physiological Properties, Chemical Composition, and Bioactive Compounds on Cactus Pear Fruit (Opuntia ficus-indica (L.) Mill.). Agriculture 2021, 11, 62. [Google Scholar] [CrossRef]
- Jain, V.; Chawla, S.; Choudhary, P.; Jain, S. Post-harvest calcium chloride treatments influence fruit firmness, cell wall components and cell wall hydrolyzing enzymes of Ber (Ziziphus mauritiana Lamk.) fruits during storage. J. Food Sci. Technol. 2019, 56, 4535–4542. [Google Scholar] [CrossRef]
- García, J.; Herrera, S.; Morilla, A. Effects of Postharvest Dips in Calcium Chloride on Strawberry. J. Agric. Food Chem. 1996, 44, 30–33. [Google Scholar] [CrossRef]
- Irfan, P.; Vanjakshi, V.; Prakash, M.K.; Ravi, R.; Kudachikar, V. Calcium chloride extends the keeping quality of fig fruit (Ficus carica L.) during storage and shelf-life. Postharvest Biol. Technol. 2013, 82, 70–75. [Google Scholar] [CrossRef]
- Singh Bhooriya, M.; Bisen, B.P.; Pandey, S.K. Effect of post-harvest treatments on shelf life and quality of Guava (Psidiun guavajava) fruits. Int. J. Chem. Stud. 2018, 6, 2559–2564. [Google Scholar]
- Manganaris, G.; Vasilakakis, M.; Diamantidis, G.; Mignani, I. The effect of postharvest calcium application on tissue calcium concentration, quality attributes, incidence of flesh browning and cell wall physicochemical aspects of peach fruits. Food Chem. 2007, 100, 1385–1392. [Google Scholar] [CrossRef]
- Gheribi, R.; Khwaldia, K. Cactus Mucilage for Food Packaging Applications. Coatings 2019, 9, 655. [Google Scholar] [CrossRef] [Green Version]
- Ochoa-Velasco, C.E.; Guerrero-Beltrán, J. Ángel Postharvest quality of peeled prickly pear fruit treated with acetic acid and chitosan. Postharvest Biol. Technol. 2014, 92, 139–145. [Google Scholar] [CrossRef]
- Allegra, A.; Sortino, G.; Miciletta, G.; Riotto, M.; Fasciana, T.; Inglese, P. The influence of harvest period and fruit ripeness at harvest on minimally processed cactus pears (Opuntia ficus-indica L. Mill.) stored under passive atmosphere. Postharvest Biol. Technol. 2015, 104, 57–62. [Google Scholar] [CrossRef]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Betalain Stability and Degradation?Structural and Chromatic Aspects. J. Food Sci. 2006, 71, R41–R50. [Google Scholar] [CrossRef]
- Howard, L.; Hernandez-Brenes, C. Antioxidant content and market quality of jalapeno pepper rings as affected by minimal processing and modified atmosphere packaging. J. Food Qual. 1998, 21, 317–327. [Google Scholar] [CrossRef]
- El-Samahy, S.; El-Hady, A.; Habiba, R.; Moussa, T. Chemical and rheological characteristics of orange-yellow cactus-pear pulp from Egypt. J. Prof. Assoc. Cactus. 2006, 8, 39–51. [Google Scholar]
- Felker, P.; Rodriguez, S.D.C.; Casoliba, R.; Filippini, R.; Medina, D.; Zapata, R. Comparison of Opuntia ficus indica varieties of Mexican and Argentine origin for fruit yield and quality in Argentina. J. Arid. Environ. 2005, 60, 405–422. [Google Scholar] [CrossRef]
- Saenz, C. Processing technologies: An alternative for cactus pear (Opuntia spp.) fruits and cladodes. J. Arid. Environ. 2000, 46, 209–225. [Google Scholar] [CrossRef] [Green Version]
- Brackett, R. Microbiological consequences of minimally processed fruits and vegetables. J. Food Qual. 1987, 10, 195–206. [Google Scholar] [CrossRef]
- Potter, A.; Murray, J.; Lawson, B.; Graham, S. Trends in product recalls within the agri-food industry: Empirical evidence from the USA, UK and the Republic of Ireland. Trends Food Sci. Technol. 2012, 28, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Miceli, A.; Settanni, L. Influence of agronomic practices and pre-harvest conditions on the attachment and development of Listeria monocytogenes in vegetables. Ann. Microbiol. 2019, 69, 185–199. [Google Scholar] [CrossRef]
- Choi, M.H.; Park, Y.J.; Kim, M.; Seo, Y.H.; Kim, Y.A.; Choi, J.Y.; Yong, D.; Jeong, S.H.; Lee, K. Increasing Incidence of Listeriosis and Infection-associated Clinical Outcomes. Ann. Lab. Med. 2018, 38, 102–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacxsens, L.; Devlieghere, F.; Debevere, J. Behaviour of Listeria monocytogenes and Aeromonas spp. on fresh-cut produce packaged under equilibrium modified atmosphere. J. Food Prot. 1999, 62, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Jay, J.M.; Loessner, M.J.; Golden, D.A. Microbiologia Degli Alimenti; Springer Science and Business Media LLC: Mailand, Italy, 2009. [Google Scholar]
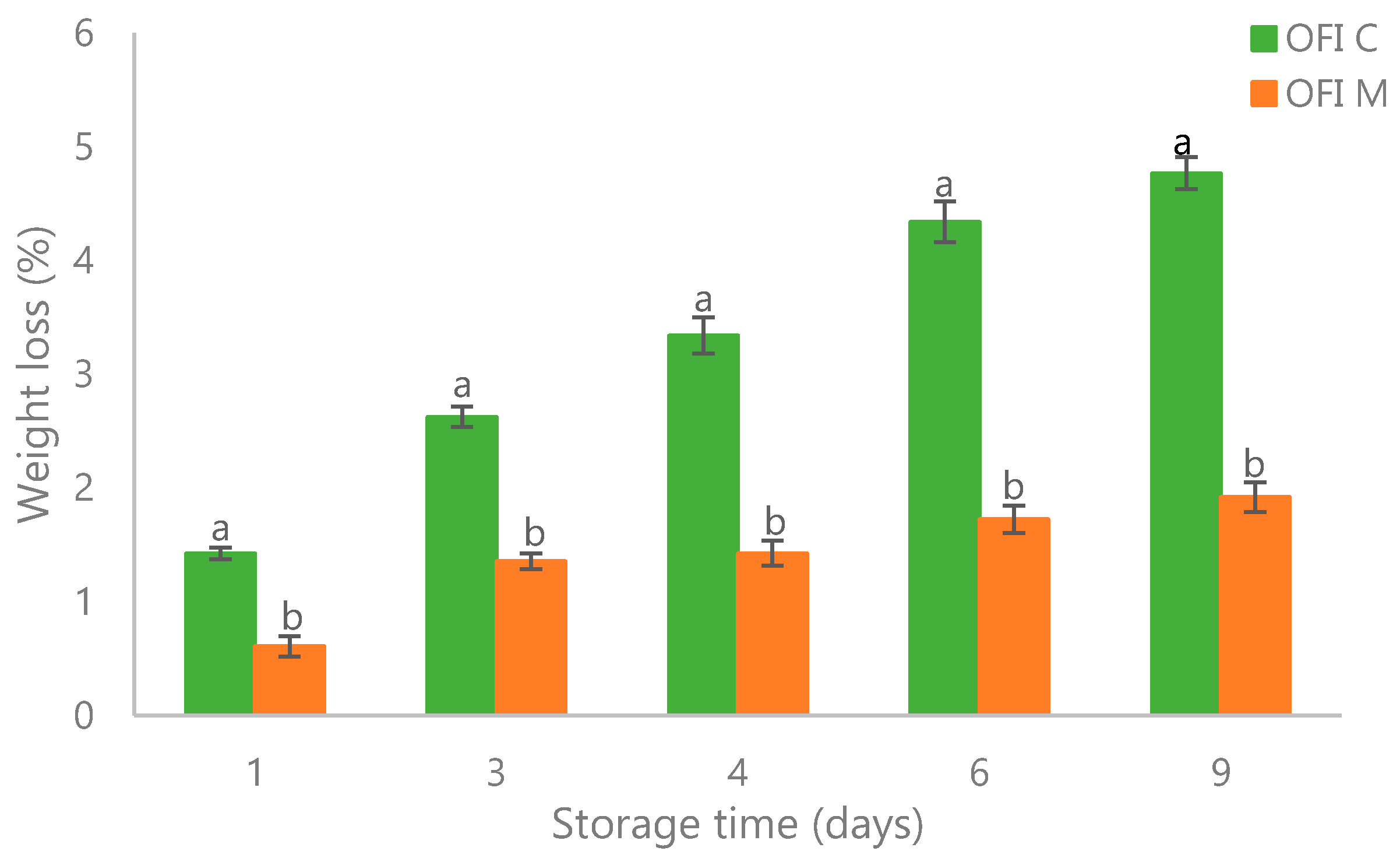


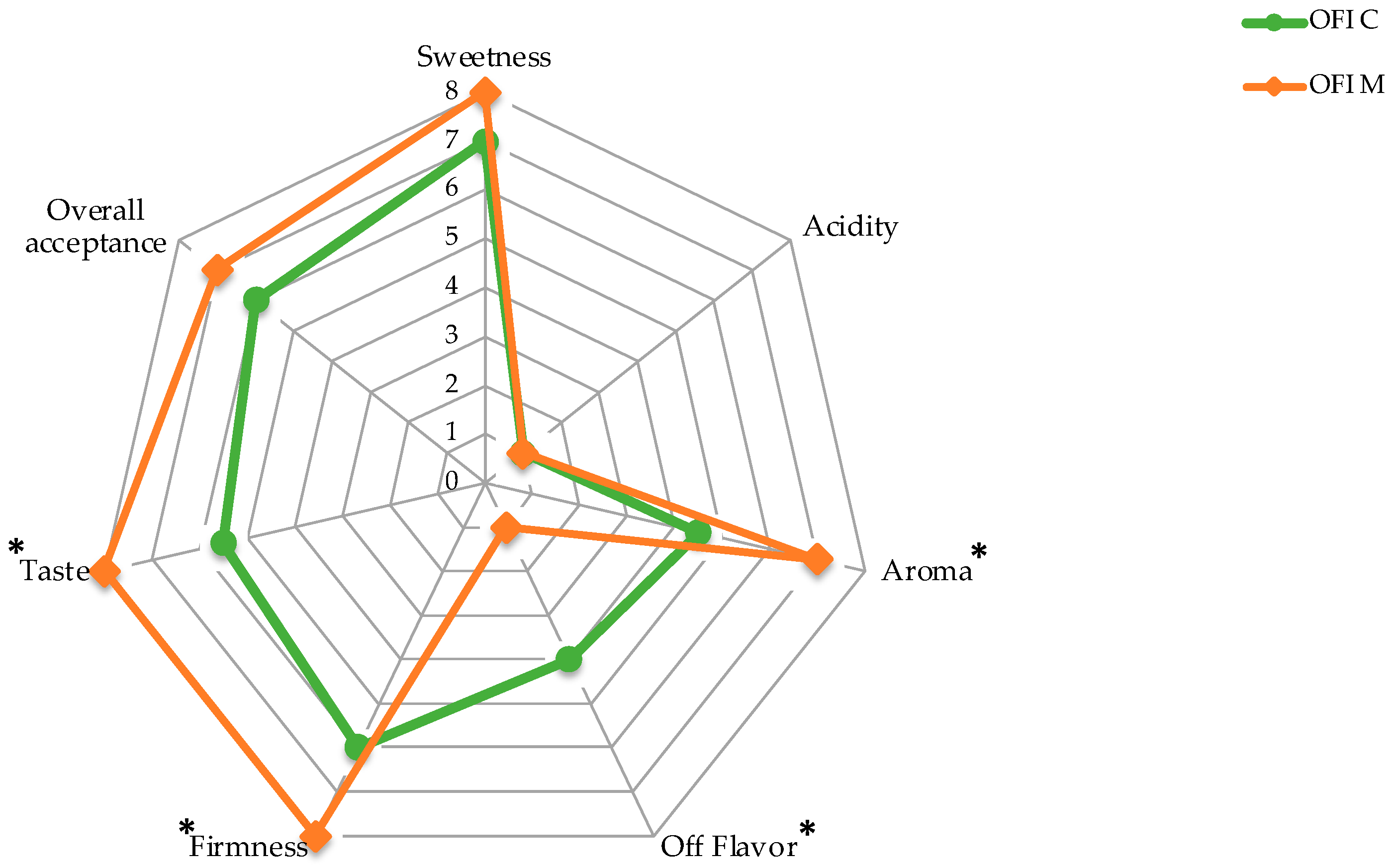
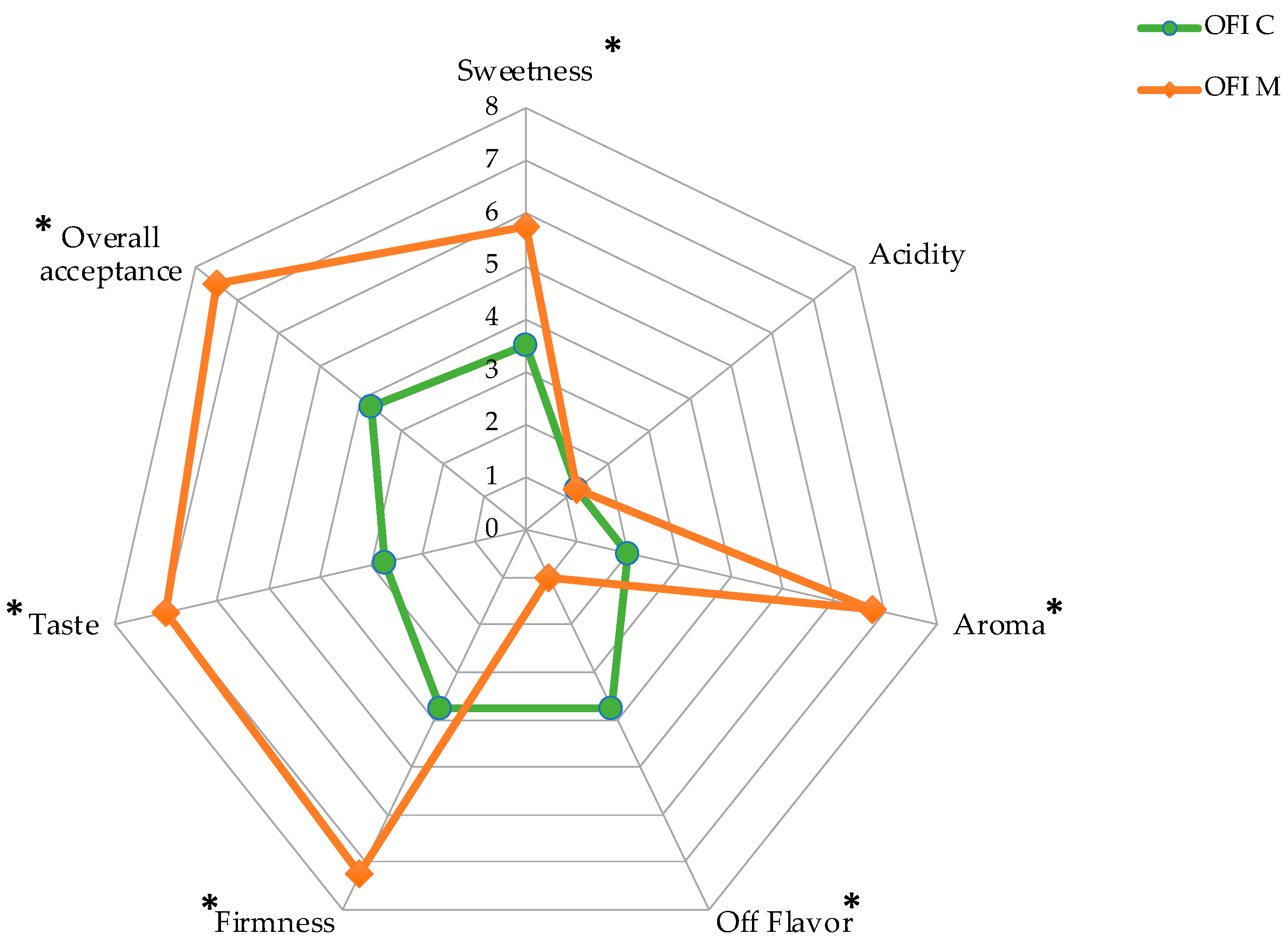

| Storage Time | Firmness | Total Soluble Solids (TSS) | Titratable Acidity (TA) | |||
|---|---|---|---|---|---|---|
| (days) | (N) | (°Brix) | (g citric acid 100 g−1 FW) | |||
| OFI C | OFI M | OFI C | OFI M | OFI C | OFI M | |
| T0 | 18.50 ± 0.71 a | 18.50 ± 0.71 a | 13.95 ± 0.42 | 13.95 ± 0.42 | 0.058 ± 0.002 | 0.058 ± 0.002 |
| T3 | 15.41 ± 0.89 b | 17.97 ± 0.92 a | 14.75 ± 0.35 | 14.11 ± 0.41 | 0.053 ± 0.003 | 0.054 ± 0.001 |
| T6 | 13.62 ± 0.84 b | 16.32 ± 0.91 a | 14.91 ± 0.59 | 14.32 ± 0.51 | 0.052 ± 0.002 | 0.053 ± 0.003 |
| T9 | 9.11 ± 0.97 b | 15.93 ± 0.88 a | 14.95 ± 0.47 | 14.42 ± 0.41 | 0.051 ± 0.002 | 0.053 ± 0.001 |
| Storage Time | Indicaxantin | Betanin | Ascorbic Acid | DPPH | ||||
|---|---|---|---|---|---|---|---|---|
| (days) | (mg 100 g−1 FW) | (mg 100 g−1 FW) | (mg 100 g−1 FW) | (mmol TE 100 g−1 FW) | ||||
| OFI C | OFI M | OFI C | OFI M | OFI C | OFI M | OFI C | OFI M | |
| T0 | 8.12 ± 0.32 a | 8.93 ± 0.33 a | 0.45 ± 0.02 a | 0.49 ± 0.02 a | 30.5 ± 0.21 a | 30.5 ± 0.21 a | 4.61 ± 0.32 a | 4.67 ± 0.13 a |
| T3 | 6.86 ± 0.28 b | 8.29 ± 0.32 a | 0.38 ± 0.04 a | 0.46 ± 0.03 a | 27.3 ± 0.23 b | 29.4 ± 0.34 a | 4.54 ± 0.18 a | 4.80 ± 0.28 a |
| T6 | 6.91 ± 0.17 b | 8.27 ± 0.23 a | 0.38 ± 0.05 a | 0.45 ± 0.03 a | 26.8 ± 0.15 b | 29.1 ± 0.30 a | 3.40 ± 0.20 b | 4.71 ± 0.47 a |
| T9 | 6.89 ± 0.21 b | 8.89 ± 0.51 a | 0.37 ± 0.02 a | 0.49 ± 0.01 a | 25.1 ± 0.21 b | 28.8 ± 0.15 a | 2.24 ± 0.23 b | 4.82 ± 0.33 a |
| Microorganisms | OFI C | OFI M | Statistical Significance | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 d | 3 d | 6 d | 9 d | 0 d | 3 d | 6 d | 9 d | OFI C * OFI M | |
| TMM | <2 a | 3.9 ± 0.2 a | 4.9 ± 0.2 a | 5.7 ± 0.2 a | <2 a | 2.7 ± 0.2 b | 3.5 ± 0.3 b | 4.8 ± 0.2 b | *** |
| TPM | <2 a | 2.8 ± 0.2 a | 3.7 ± 0.2 a | 4.4 ± 0.3 a | <2 a | 2.0 ± 0.0 b | 2.9 ± 0.1 b | 3.5 ± 0.2 b | *** |
| Pseudomonads | <2 a | 3.7 ± 0.3 a | 4.5 ± 0.2 a | 4.9 ± 0.3 a | <2 a | 2.1 ± 0.1 b | 2.9 ± 0.3 b | 3.7 ± 0.2 b | *** |
| Enterobacteriaceae | <2 a | <2 a | 2.7 ± 0.1 a | 3.5 ± 0.3 b | <2 a | <2 a | <2 b | 2.3 ± 0.1 b | * |
| Yeasts | <2 a | 3.6 ± 0.4 a | 4.6 ± 0.2 b | 5.8 ± 0.1 b | <2 a | 2.5 ± 0.3 b | 3.2 ± 0.1 b | 4.3 ± 0.2 a | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liguori, G.; Gaglio, R.; Greco, G.; Gentile, C.; Settanni, L.; Inglese, P. Effect of Opuntia ficus-indica Mucilage Edible Coating on Quality, Nutraceutical, and Sensorial Parameters of Minimally Processed Cactus Pear Fruits. Agronomy 2021, 11, 1963. https://doi.org/10.3390/agronomy11101963
Liguori G, Gaglio R, Greco G, Gentile C, Settanni L, Inglese P. Effect of Opuntia ficus-indica Mucilage Edible Coating on Quality, Nutraceutical, and Sensorial Parameters of Minimally Processed Cactus Pear Fruits. Agronomy. 2021; 11(10):1963. https://doi.org/10.3390/agronomy11101963
Chicago/Turabian StyleLiguori, Giorgia, Raimondo Gaglio, Giuseppe Greco, Carla Gentile, Luca Settanni, and Paolo Inglese. 2021. "Effect of Opuntia ficus-indica Mucilage Edible Coating on Quality, Nutraceutical, and Sensorial Parameters of Minimally Processed Cactus Pear Fruits" Agronomy 11, no. 10: 1963. https://doi.org/10.3390/agronomy11101963






