Parthenocarpy and Self-Incompatibility in Mandarins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Procedure
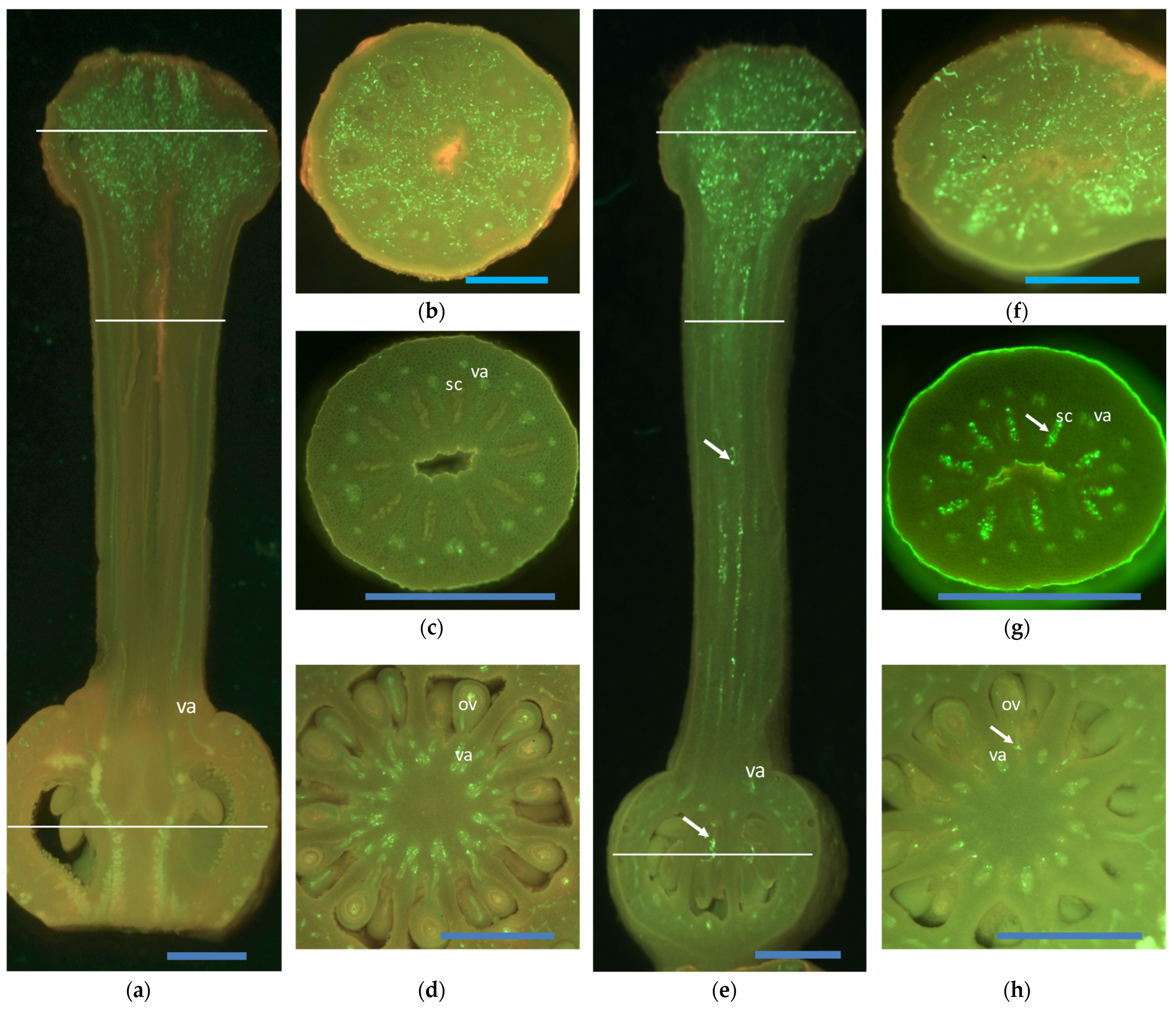
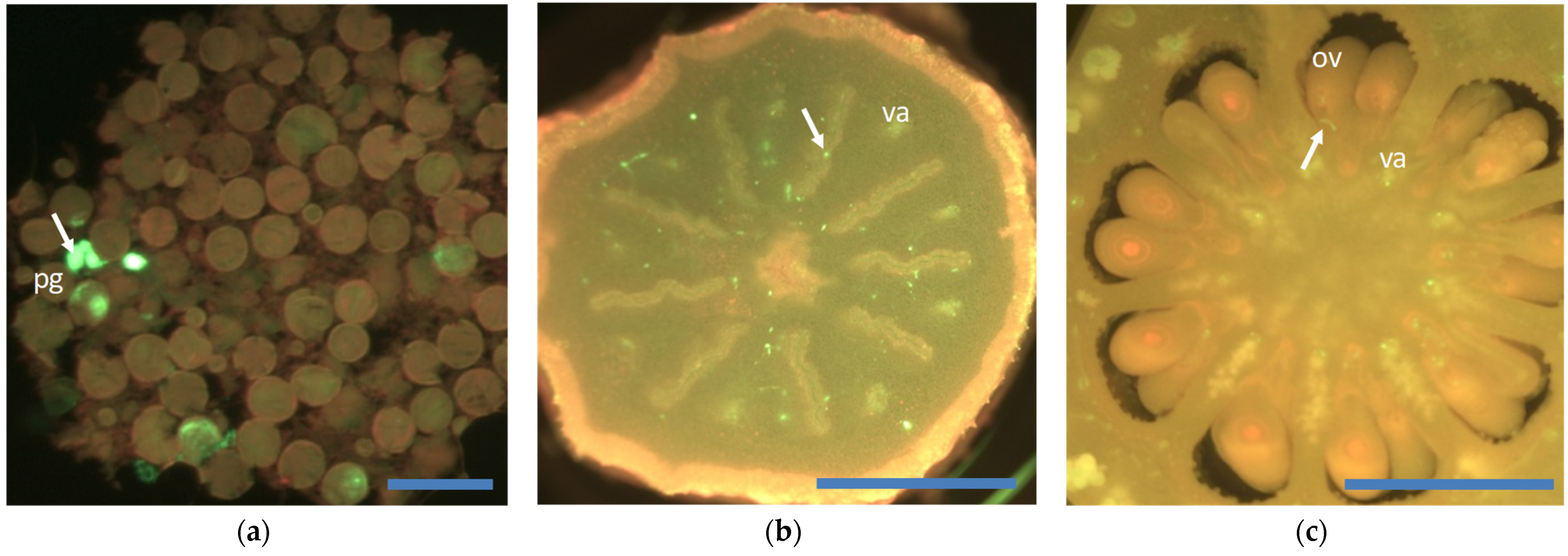
2.3. Histological Observations
2.4. Statistical Analysis
2.5. Genetic Analysis with Simple Sequence Repeat (SSR) Markers
3. Results and Discussion
3.1. Self-Incompatibility
3.2. Assessing Parthenocarpic Ability (PA) and Testing the Pollination Stimulus Requirement for Fruit to Set
3.3. Parthenocarpic Classification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garcia-Lor, A.; Luro, F.; Ollitrault, P.; Navarro, L. Genetic diversity and population structure analysis of mandarin germplasm by nuclear, chloroplastic and mitochondrial markers. Tree Genet. Genomes 2015, 11. [Google Scholar] [CrossRef]
- Ollitrault, P.; Froelicher, Y.; Dambier, D.; Luro, F.; Yamamoto, M. Seedlessness and ploidy manipulation. In Citrus Genetics, Breeding and Biotechnology; Khan, I., Ed.; CABI: Wallingfor, UK, 2007; pp. 197–218. [Google Scholar]
- Picarella, M.E.; Mazzucato, A. The Occurrence of Seedlessness in Higher Plants; Insights on Roles and Mechanisms of Par-thenocarpy. Front. Plant Sci. 2019, 9, 1997. [Google Scholar] [CrossRef] [Green Version]
- Mesejo, C.; Muñoz-Fambuena, N.; Reig, C.; Martínez-Fuentes, A.; Agustí, M. Cell division interference in newly fertilized ovules induces stenospermocarpy in cross-pollinated citrus fruit. Plant Sci. 2014, 225, 86–94. [Google Scholar] [CrossRef]
- Yamamoto, M. Progress on studies for seedless breeding of citrus in Japan. Adv. Hortic. Sci. 2014, 28, 64–72. [Google Scholar]
- Wilms, H.J.; Van Went, J.L.; Cresti, M.; Ciampolini, F. Structural aspects of female sterility in Citrus limon. Acta Bot. Neerl. 1983, 32, 87–96. [Google Scholar] [CrossRef]
- Osawa, I. Cytological and experimental studies in Citrus. J. Coll. Agric. Tokyo Univ. 1912, 4, 83–116. [Google Scholar]
- Wong, C.Y. The influence of pollination on seed development in certain varieties of citrus. Soc. Hortic. Sci. 1939, 37, 161–164. [Google Scholar]
- Yamasaki, A.; Kitajima, A.; Ohara, N.; Tanaka, M.; Hasegawa, K. Characteristics of Arrested Seeds in Mukaku Kishu-type Seedless Citrus. J. Jpn. Soc. Hortic. Sci. 2009, 78, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Yamasaki, A.; Kitajima, A.; Ohara, N.; Tanaka, M.; Hasegawa, K. Histological Study of Expression of Seedlessness in Citrus kinokuni ‘Mukaku Kishu’ and Its Progenies. J. Am. Soc. Hortic. Sci. 2007, 132, 869–875. [Google Scholar] [CrossRef] [Green Version]
- Goto, S.; Yoshioka, T.; Ohta, S.; Kita, M.; Hamada, H.; Shimizu, T. Segregation and Heritability of Male Sterility in Populations Derived from Progeny of Satsuma Mandarin. PLoS ONE 2016, 11, e0162408. [Google Scholar] [CrossRef]
- Goto, S.; Yoshioka, T.; Ohta, S.; Kita, M.; Hamada, H.; Shimizu, T. QTL mapping of male sterility and transmission pattern in progeny of Satsuma Mandarin. PLoS ONE 2018, 13. [Google Scholar] [CrossRef]
- Goldenberg, L.; Yaniv, Y.; Porat, R.; Carmi, N. Effects of Gamma-Irradiation Mutagenesis for Induction of Seedlessness, on the Quality of Mandarin Fruit. Food Nutr. Sci. 2014, 5, 943–952. [Google Scholar] [CrossRef] [Green Version]
- Bermejo, A.; Pardo, J.; Cano, A. Influence of gamma irradiation on seedless citrus production: Pollen germination and fruit quality. Food Nutr. Sci. 2011, 2, 169. [Google Scholar]
- Navarro, L.; Aleza, P.; Cuenca, J.; Juárez, J.; Pina, J.A.; Ortega, C.; Navarro, A.; Ortega, V. The Mandarin Triploid Breeding Program in Spain; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2015; Volume 1065, pp. 389–396. [Google Scholar]
- Ollitrault, P.; Dambier, D.; Francois, L.; Froelicher, Y. Ploidy Manipulation for Breeding Seedless Triploid Citrus. In Plant Breed Rev; John Wiley & Sons: New York, NY, USA, 2008; Volume 30, pp. 323–352. ISBN 9780470380130. [Google Scholar]
- Otto, S.P.; Whitton, J. Polyploid incidence and evolution. Annu. Rev. Genet. 2000, 34, 401–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aleza, P.; Juárez, J.; Cuenca, J.; Ollitrault, P.; Navarro, L. Recovery of citrus triploid hybrids by embryo rescue and flow cytometry from 2x × 2x sexual hybridisation and its application to extensive breeding programs. Plant Cell Rep. 2010, 29, 1023–1034. [Google Scholar] [CrossRef]
- Cuenca, J.; Froelicher, Y.; Aleza, P.; Juárez, J.; Navarro, L.; Ollitrault, P. Multilocus half-tetrad analysis and centromere mapping in citrus: Evidence of SDR mechanism for 2n megagametophyte production and partial chiasma interference in mandarin cv “Fortune”. Heredity (Edinburgh) 2011, 107, 462–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aleza, P.; Juárez, J.; Hernández, M.; Ollitrault, P.; Navarro, L. Implementation of extensive citrus triploid breeding programs based on 4x × 2x sexual hybridisations. Tree Genet. Genomes 2012, 8, 1293–1306. [Google Scholar] [CrossRef]
- Aleza, P.; Juárez, J.; Cuenca, J.; Ollitrault, P.; Navarro, L. Extensive citrus triploid hybrid production by 2x × 4x sexual hybridizations and parent-effect on the length of the juvenile phase. Plant Cell Rep. 2012, 31, 1723–1735. [Google Scholar] [CrossRef]
- Liang, M.; Cao, Z.; Zhu, A.; Liu, Y.; Tao, M.; Yang, H.; Xu, Q.; Wang, S.; Liu, J.; Li, Y.; et al. Evolution of self-compatibility by a mutant Sm -RNase in citrus. Nat. Plants 2020, 6, 131–142. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kubo, T.; Tominaga, S. Self- and cross-incompatibility of various citrus accessions. J. Jpn. Soc. Hortic. Sci. 2006, 75, 372–378. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Liang, M.; Wang, N.; Xu, Q.; Deng, X.; Chai, L. Reproduction in woody perennial Citrus: An update on nucellar embryony and self-incompatibility. Plant Reprod. 2018, 31, 43–57. [Google Scholar] [CrossRef]
- Vardi, A.; Neumann, H.; Frydman-Shani, A.; Yaniv, Y.; Spiegel-Roy, P. Tentative model on the inheritance of juvenility, self-incompatibility and parthenocarpy. Acta Hortic. 2000, 535, 199–206. [Google Scholar] [CrossRef]
- Esen, A.; Soost, R.K. Adventive Embroyogenesis in Citrus and its Relation to Pollination and Fertilization. Am. J. Bot. 1977, 64, 607–614. [Google Scholar] [CrossRef]
- Koltunow, A.M. Apomixis: Embryo Sacs and Embryos Formed without Meiosis or Fertilization in Ovules. Plant Cell 1993, 5, 1425–1437. [Google Scholar] [CrossRef] [Green Version]
- Vardi, A.; Levin, I.; Carmi, N.; Sciences, P.; Box, P.O. Induction of Seedlessness in Citrus: From Classical Techniques to Emerging Biotechnological Approaches. J. Amer. Soc. Hort. Sci. 2008, 133, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Ozga, J.A.; Reinecke, D.M. Hormonal Interactions in Fruit Development. J. Plant Growth Regul. 2003, 22, 73–81. [Google Scholar] [CrossRef]
- Talon, M.; Zacarias, L.; Primo-Millo, E. Hormonal changes associated with fruit set and development in mandarins differing in their parthenocarpic ability. Physiol. Plant. 1990, 79, 400–406. [Google Scholar] [CrossRef]
- Talon, M.; Zacarias, L.; Primo-Millo, E. Gibberellins and parthenocarpic ability in developing ovaries of seedless mandarins. Plant Physiol. 1992, 99, 1575–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesejo, C.; Yuste, R.; Reig, C.; Martínez-Fuentes, A.; Iglesias, D.J.; Muñoz-Fambuena, N.; Bermejo, A.; Germanà, M.A.; Primo-Millo, E.; Agustí, M. Gibberellin reactivates and maintains ovary-wall cell division causing fruit set in parthenocarpic Citrus species. Plant Sci. 2016, 247, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Mesejo, C.; Yuste, R.; Martínez-Fuentes, A.; Reig, C.; Iglesias, D.J.; Primo-Millo, E.; Agustí, M. Self-pollination and parthenocarpic ability in developing ovaries of self-incompatible Clementine mandarins (Citrus clementina). Physiol. Plant. 2013, 148, 87–96. [Google Scholar] [CrossRef]
- Sykes, S.R. Segregation in an “Imperial” mandarin X “Ellendale” tangor family for characteristics that contribute to the seedless phenotype. J. Hortic. Sci. Biotechnol. 2008, 83, 719–724. [Google Scholar] [CrossRef]
- Garcia-Papi, M.A.; Garcia-Martinez, J.L. Endogenous plant growth substances content in young fruits of seeded and seedless Clementine mandarin as related to fruit set and development. Sci. Hortic. (Amsterdam) 1984, 22, 265–274. [Google Scholar] [CrossRef] [Green Version]
- Hodgson, R.W. Horticultural Varieties of Citrus. In The Citrus Industry. Volume I. History, World Distribution, Botany, and Varieties; Reuther, W., Webber, H., Batchelor, L., Eds.; University of California: Berkeley, CA, USA, 1967; pp. 431–611. [Google Scholar]
- Sykes, S.R. The effect on Citrus fruit of excluding pollinating insects at flowering and implications for breeding new seedless cultivars. J. Hortic. Sci. Biotechnol. 2008, 83, 713–718. [Google Scholar] [CrossRef]
- Sykes, S.R.; Possingham, J. V The effect of excluding insect pollinators on seediness of Imperial mandarin fruits. Aust. J. Exp. Agric. 1992, 32, 409–411. [Google Scholar] [CrossRef]
- Vithanage, V. Incompatibility relationships among some mandarin cultivars. Plant Cell Incompat. Newsl. 1986, 18, 41–45. [Google Scholar]
- Vithanage, V. Effect of different pollen parents on seediness and quality of ‘Ellendale’ tangor. Sci. Hortic. (Amsterdam) 1991, 48, 253–260. [Google Scholar] [CrossRef]
- Wallace, H.M.; Lee, L.S. Pollen source, fruit set and xenia in mandarins. J. Hortic. Sci. Biotechnol. 1999, 74, 82–86. [Google Scholar] [CrossRef]
- Navarro, L.; Pina, J.A.; Juárez, J.; Ballester-Olmos, J.F.; Arregui, J.M.; Ortega, C.; Navarro, A.; Duran-Vila, N.; Guerri, J.; Moreno, P.; et al. The Citrus Variety Improvement Program in Spain in the Period 1975–2001; Duran-Vila, N., Milne, R., da Graça, J., Eds.; International Organization Citrus Virologists (IOCV): Riverside, CA, USA, 2002; pp. 306–316. [Google Scholar]
- Johansen, D. Plant Microtechniques; McGraw-Hill: New York, NY, USA, 1940. [Google Scholar]
- Montalt, R.; Cuenca, J.; Vives, M.C.; Navarro, L.; Ollitrault, P.; Aleza, P. Influence of temperature on the progamic phase in Citrus. Environ. Exp. Bot. 2019, 166, 103806. [Google Scholar] [CrossRef]
- Linskens, F.H.; Esser, K. Über eine spezifische anfarbung der pollenschlauche im griffel und die zahl der kallospefropfen nach slbstdung und femddung. Naturwissenschaften 1957, 44, 16. [Google Scholar] [CrossRef]
- Adhikari, P.B.; Liu, X.; Kasahara, R.D. Mechanics of Pollen Tube Elongation: A Perspective. Front. Plant Sci. 2020, 11, 1612. [Google Scholar] [CrossRef]
- Ollitrault, P.; Terol, J.; Chen, C.; Federici, C.T.; Lotfy, S.; Hippolyte, I.; Ollitrault, F.; Bérard, A.; Chauveau, A.; Cuenca, J.; et al. A reference genetic map of C. clementina hort. ex Tan.; citrus evolution inferences from comparative mapping. BMC Genomics 2012, 13, 593. [Google Scholar] [CrossRef] [PubMed]
- Eti, S.; Stosser, R. Pollen Tube Growth and Development of Ovules in Relation to Fruit Set in Mandarines, cv.’Clementine’ (Citrus reticulata Blanco); International Society for Horticultural Science (ISHS): Leuven, Belgium, 1992; pp. 621–625. [Google Scholar]
- Distefano, G.; Caruso, M.; la Malfa, S.; Gentile, A.; Tribulato, E. Histological and molecular analysis of pollen-pistil interaction in clementine. Plant Cell Rep. 2009, 28, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Distefano, G.; Las Casas, G.; La Malfa, S.; Gentile, A.; Tribulato, E.; Herrero, M. Pollen tube behavior in different Mandarin hybrids. J. Am. Soc. Hortic. Sci. 2009, 134, 583–588. [Google Scholar] [CrossRef] [Green Version]
- Froelicher, Y.; Dambier, D.; Bassene, J.B.; Constantino, G.; Lotfy, S.; Didout, C.; Beaumont, V.; Brottier, P.; Risterucci, A.M.; Luro, F.; et al. Characterization of microsatellite markers in mandarin orange (Citrus reticulata Blanco). Mol. Ecol. Resour. 2008, 8, 119–122. [Google Scholar] [CrossRef]
- García-Lor, A.; Luro, F.; Navarro, L.; Ollitrault, P. Comparative use of InDel and SSR markers in deciphering the interspecific structure of cultivated citrus genetic diversity: A perspective for genetic association studies. Mol. Genet. Genom. 2012, 287, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Kamiri, M.; Stift, M.; Srairi, I.; Costantino, G.; El Moussadik, A.; Hmyene, A.; Bakry, F.; Ollitrault, P.; Froelicher, Y. Evidence for non-disomic inheritance in a Citrus interspecific tetraploid somatic hybrid between C. reticulata and C. limon using SSR markers and cytogenetic analysis. Plant Cell Rep. 2011, 30, 1415–1425. [Google Scholar] [CrossRef]
- Kijas, J.M.H.; Thomas, M.R.; Fowler, J.C.S.; Roose, M.L. Integration of trinucleotide microsatellites into a linkage map of Citrus. Theor. Appl. Genet. 1997, 94, 701–706. [Google Scholar] [CrossRef]
- Aleza, P.; Froelicher, Y.; Schwarz, S.; Agustí, M.; Hernández, M.; Juárez, J.; Luro, F.; Morillon, R.; Navarro, L.; Ollitrault, P. Tetraploidization events by chromosome doubling of nucellar cells are frequent in apomictic citrus and are dependent on genotype and environment. Ann. Bot. 2011, 108, 37–50. [Google Scholar] [CrossRef] [Green Version]
- Ollitrault, P.; Ahmed, D.; Costantino, G.; Evrard, J.C.; Cardi, C.; Mournet, P.; Perdereau, A.; Froelicher, Y. Segregation distortion for male parents in high density genetic maps from reciprocal crosses between two self-incompatible cultivars confirms a gametophytic system for self-incompatibility in citrus. Agriculture 2021, 11, 379. [Google Scholar] [CrossRef]
- Kim, J.-H.; Handayani, E.; Wakana, A.; Sato, M.; Miyamoto, M.; Miyazaki, R.; Zhou, X.; Sakai, K.; Mizunoe, Y.; Shigyo, M.; et al. Distribution and evolution of Citrus accessions with S3 and/or S11 alleles for self-incompatibility with an emphasis on sweet orange [Citrus sinensis (L.) Osbeck; SfS3 or SfS3sm]. Genet. Resour. Crop Evol. 2020, 67, 2101–2117. [Google Scholar] [CrossRef]
- Ngo, B.X.; Wakana, A.; Park, S.M.; Nada, Y.; Fukudome, I. Pollen tube behaviors in self-incompatible and self-compatible Citrus cultivars. J. Fac. Agric. Kyushu Univ. 2001, 45, 443–457. [Google Scholar] [CrossRef]
- Distefano, G.; Hedhly, A.; Las Casas, G.; La Malfa, S.; Herrero, M.; Gentile, A. Male-female interaction and temperature variation affect pollen performance in Citrus. Sci. Hortic. (Amsterdam) 2012, 140, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Aloisi, I.; Distefano, G.; Antognoni, F.; Potente, G.; Parrotta, L.; Faleri, C.; Gentile, A.; Bennici, S.; Mareri, L.; Cai, G.; et al. Temperature-Dependent Compatible and Incompatible Pollen-Style Interactions in Citrus clementina Hort. ex Tan. Show Different Transglutaminase Features and Polyamine Pattern. Front. Plant Sci. 2020, 11, 1018. [Google Scholar] [CrossRef] [PubMed]
- Distefano, G.; Gentile, A.; Herrero, M. Pollen-pistil interactions and early fruiting in parthenocarpic citrus. Ann. Bot. 2011, 108, 499–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agustí, M.; García-Marí, F.; Guardiola, J.L. The influence of flowering intensity on the shedding of reproductive structures in sweet orange. Sci. Hortic. (Amsterdam) 1982, 17, 343–352. [Google Scholar] [CrossRef]
- Garcia-Papi, M.A.; Garcia-Martinez, J.L. Fruit set and development in seeded and seedless Clementine mandarin. Sci. Hortic. (Amsterdam) 1984, 22, 113–119. [Google Scholar] [CrossRef]
- Omura, M.; Shimada, T. Citrus breeding, genetics and genomics in Japan. Breed. Sci. 2016, 66, 3–17. [Google Scholar] [CrossRef] [Green Version]
- Nishiura, M.; Shichijo, T.; Ueno, I.; Iwamasa, M.; Kihara, T.; Yamada, Y. Kiyomi tangor: A new variety of Citrus. Bull. Fruit Tree Res. Stn. B 1983, 10, 1–9. [Google Scholar]
- de Teresa, E. Mandarin treee named “Mandarin Queen”. US PP22,062 P3, pp. 1–22. Available online: https://patents.google.com/patent/USPP22062P3/en (accessed on 9 August 2011).
- Tribulato, E.; La Rosa, G. Primosole e Simeto: Due nuovi ibridi di mandarino. Italus Hortus 1993, 1, 21–25. [Google Scholar]
- Chasan, R.; Walbot, V. Mechanisms of Plant Reproduction: Questions and Approaches. Plant Cell 1993, 5, 1139–1146. [Google Scholar] [CrossRef]
- Hedhly, A.; Hormaza, J.I.; Herrero, M. Global warming and sexual plant reproduction. Trends Plant Sci. 2009, 14, 30–36. [Google Scholar] [CrossRef]
- Iizumi, T.; Furuya, J.; Shen, Z.; Kim, W.; Okada, M.; Fujimori, S.; Hasegawa, T.; Nishimori, M. Responses of crop yield growth to global temperature and socioeconomic changes. Sci. Rep. 2017, 7, 7800. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.; Yao, Y.; Bassu, S.; Ciais, P.; et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [CrossRef] [Green Version]
- Koti, S.; Reddy, K.R.; Reddy, V.R.; Kakani, V.G.; Zhao, D. Interactive effects of carbon dioxide, temperature, and ultraviolet-B radiation on soybean (Glycine max L.) flower and pollen morphology, pollen production, germination, and tube lengths. J. Exp. Bot. 2005, 56, 725–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Distefano, G.; Gentile, A.; Hedhly, A.; La Malfa, S. Temperatures during flower bud development affect pollen germination, self-incompatibility reaction and early fruit development of clementine (Citrus clementina Hort. ex Tan.). Plant Biol. 2018. [Google Scholar] [CrossRef]
- Lora, J.; Herrero, M.; Hormaza, J.I. The coexistence of bicellular and tricellular pollen in Annona cherimola (Annonaceae): Implications for pollen evolution. Am. J. Bot. 2009, 96, 802–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lora, J.; Herrero, M.; Hormaza, J.I. Stigmatic receptivity in a dichogamous early-divergent angiosperm species, Annona cherimola (Annonaceae): Influence of temperature and humidity. Am. J. Bot. 2011, 98, 265–274. [Google Scholar] [CrossRef] [Green Version]
- Montalt, R.; Ollitrault, P.; Navarro, L.; Aleza, P. Evaluation of parthenocarpy ability in different citrus genotypes. In Proceedings of the 13 th International Citrus Congress, IAPAR, Foz do Iguaçu, Brazil; p. 100. Available online: https://www.semanticscholar.org/paper/Evaluation-of-parthenocarpy-ability-in-different-Montalt-Ollitrault/b706e659355dd0e57951258326d65abd6233c5d1 (accessed on 6 October 2021).
- Roose, M.L.; Williams, T.E. Mutation Breeding. In Citrus Genetics, Breeding and Biotechnology; Khan, I., Ed.; CABI: Wallingfor, UK, 2007; pp. 345–352. [Google Scholar]
- Caruso, M.; Smith, M.; Froelicher, Y.; Russo, G.; Gmitter, F. Traditional breeding. In The Genus Citrus; Talon, M., Caruso, M., Gmitter, F.G., Eds.; Woodhead Publishing: Duxford, UK, 2020; pp. 129–148. ISBN 9780128121634. [Google Scholar]
- Garmendia, A.; Beltrán, R.; Zornoza, C.; García-Breijo, F.J.; Reig, J.; Merle, H. Gibberellic acid in Citrus spp. flowering and fruiting: A systematic review. PLoS ONE 2019, 14, e0223147. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Pollen Tubes Growth | Number of Seeds per Fruit | ||||
|---|---|---|---|---|---|
| SC/SI | SP | CP | SP | CP | |
| ‘Clemenules’ | SI | 0 | 100 | 0 | 24.3 ± 2.6 |
| ‘Monreal’ | SC | 100 | 100 | 22.5 ± 3.6 (a) | 21.0 ± 4.4 (a) |
| ‘Campeona’ | SC | 100 | 100 | 4.9 ± 1.8 (a) | 9.9 ± 2.9 (b) |
| ‘Imperial’ | SI | 15 | 100 | 0.8 ± 1.4 (a) | 9.3 ± 2.5 (b) |
| ‘Salteñita’ | SC | 100 | 100 | 11.0 ± 4.3 (a) | 16.5 ± 4.1 (b) |
| ‘Fortune’ | SI | 0 | 100 | 0 | 20.4 ± 5.6 |
| ‘Moncada’ | SI | 0 | 100 | 0 | 11.6 ± 5.2 |
| ‘Ellendale’ | SI | 10 | 100 | 0.7 ± 1.0 (a) | 33.1 ± 5.8 (b) |
| ‘Serafines’ | NA | 0 | 100 | 0 | 5.3 ± 2.1 |
| E | SP | CP | |
|---|---|---|---|
| ‘Clemenules’ | 15 (a) | 16 (a) | 84 (b) |
| ‘Monreal’ | 0 | 68 (a) | 74 (a) |
| ‘Campeona’ | 9 (a) | 42 (b) | 56 (c) |
| ‘Imperial’ | 19 (a) | 33 (b) | 67 (c) |
| ‘Salteñita’ | 5 (a) | 58 (b) | 70 (c) |
| ‘Fortune’ | 0 | 9 (a) | 63 (b) |
| ‘Moncada’ | 34 (a) | 33 (a) | 72 (b) |
| ‘Ellendale’ | 5 (a) | 5 (a) | 60 (b) |
| ‘Serafines’ | 64 (a) | 66 (a) | 65 (a) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montalt, R.; Vives, M.C.; Navarro, L.; Ollitrault, P.; Aleza, P. Parthenocarpy and Self-Incompatibility in Mandarins. Agronomy 2021, 11, 2023. https://doi.org/10.3390/agronomy11102023
Montalt R, Vives MC, Navarro L, Ollitrault P, Aleza P. Parthenocarpy and Self-Incompatibility in Mandarins. Agronomy. 2021; 11(10):2023. https://doi.org/10.3390/agronomy11102023
Chicago/Turabian StyleMontalt, Rafael, María Carmen Vives, Luis Navarro, Patrick Ollitrault, and Pablo Aleza. 2021. "Parthenocarpy and Self-Incompatibility in Mandarins" Agronomy 11, no. 10: 2023. https://doi.org/10.3390/agronomy11102023
APA StyleMontalt, R., Vives, M. C., Navarro, L., Ollitrault, P., & Aleza, P. (2021). Parthenocarpy and Self-Incompatibility in Mandarins. Agronomy, 11(10), 2023. https://doi.org/10.3390/agronomy11102023






