Spatial Distribution of Available Trace Metals in Four Typical Mediterranean Soils: The Caia Irrigation Perimeter Case Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling
2.2. Analytical Methods
2.3. Statistical Analysis
3. Results
3.1. Heavy Metals
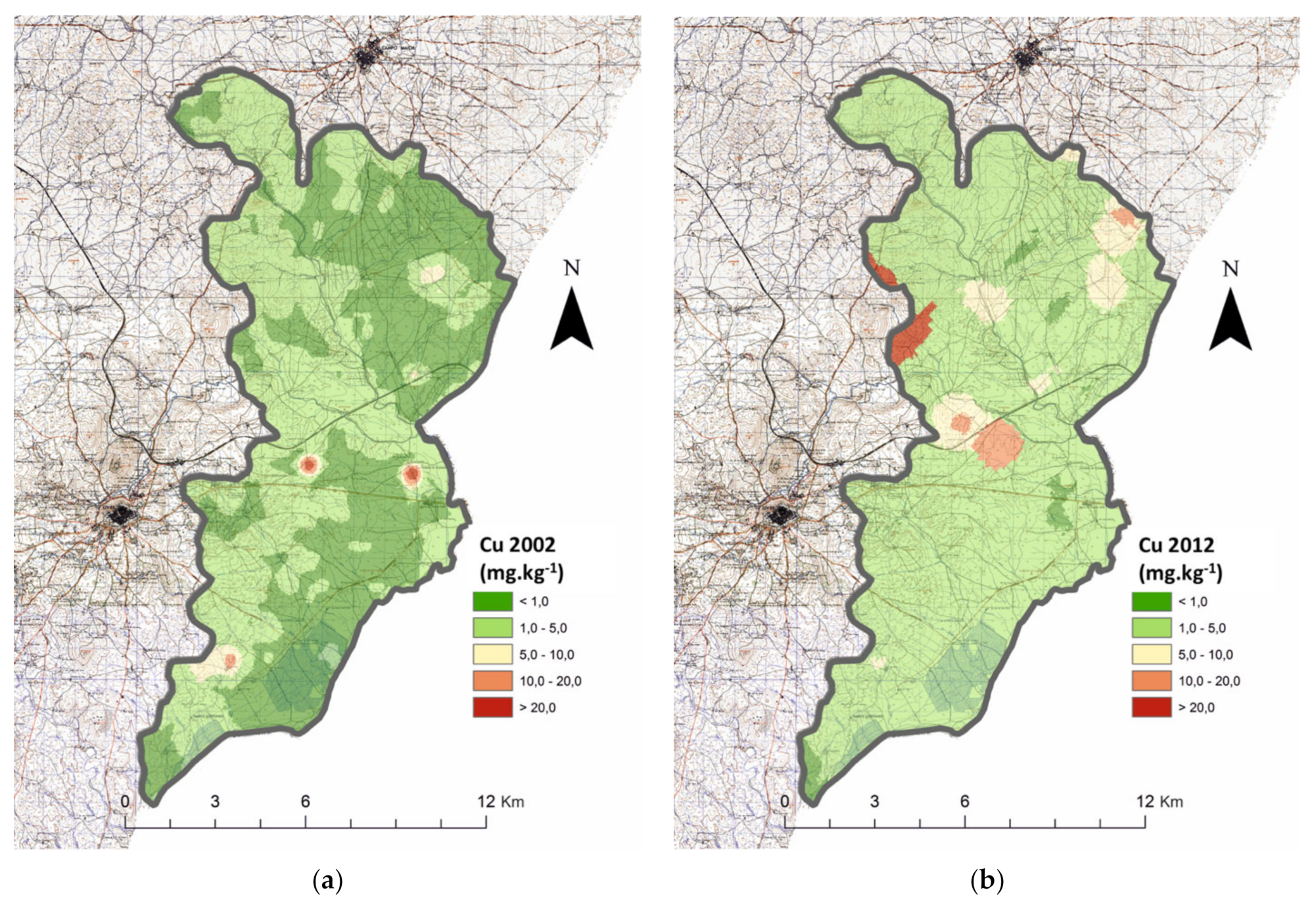
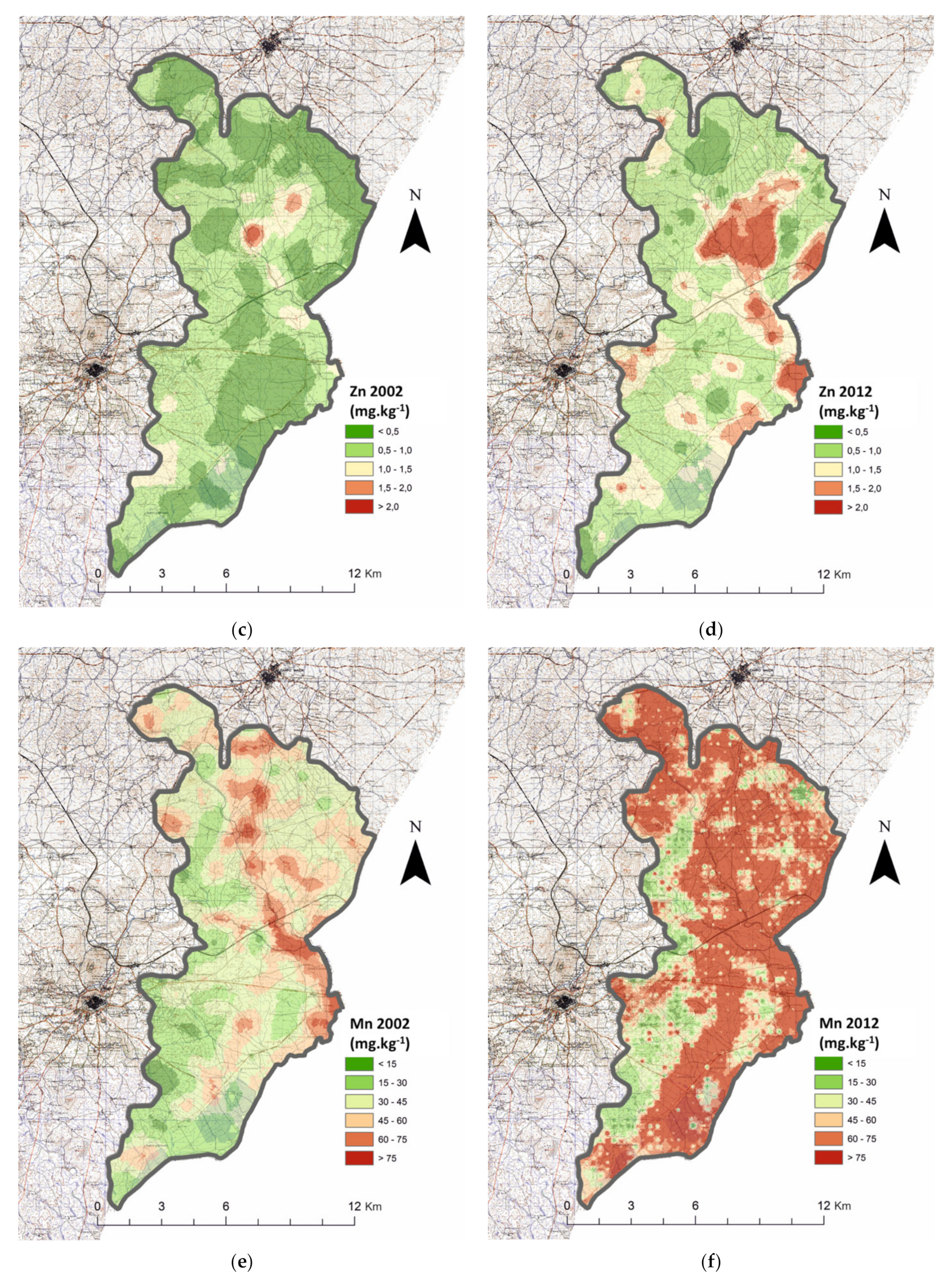
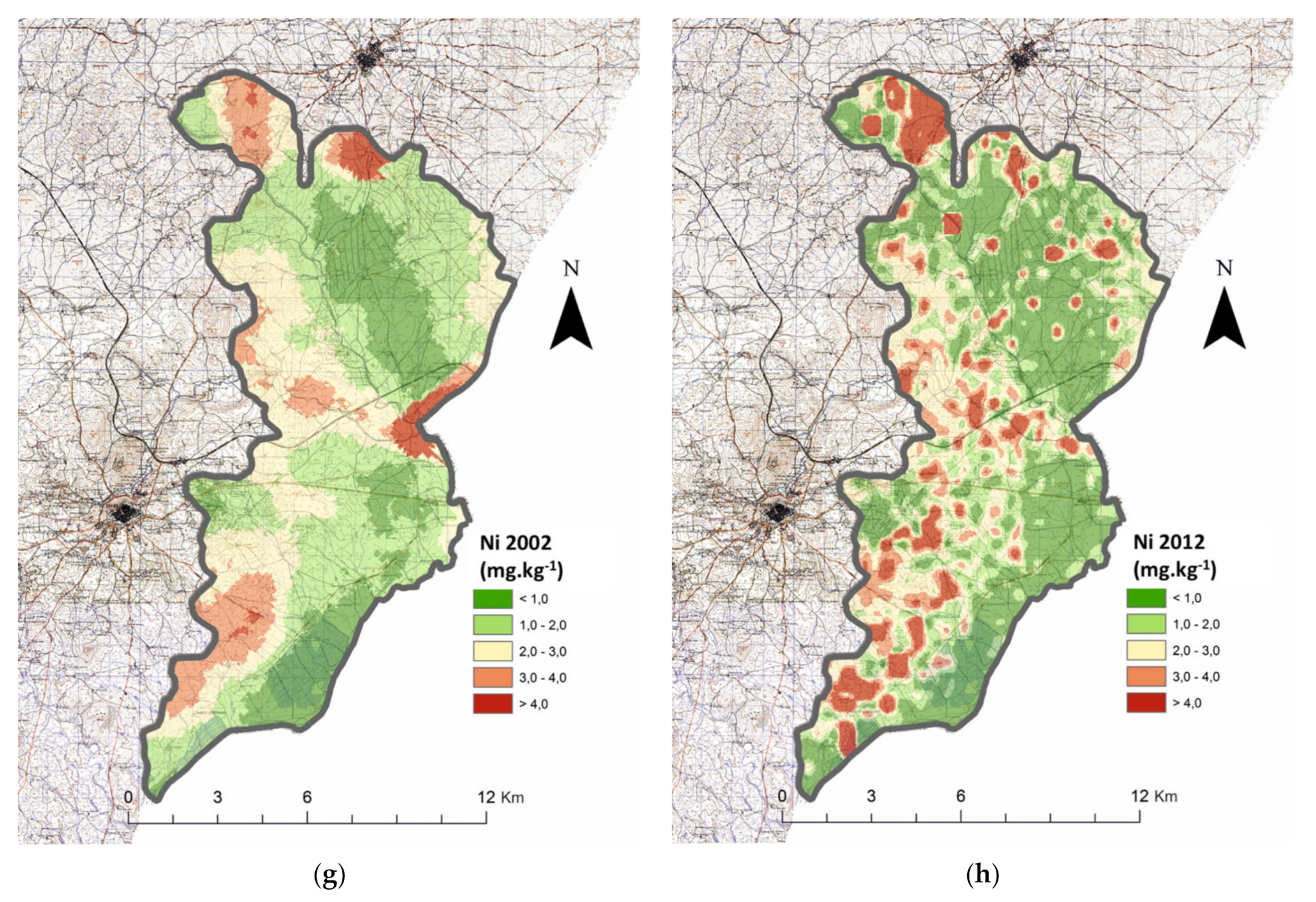
3.2. Cation Micronutrients
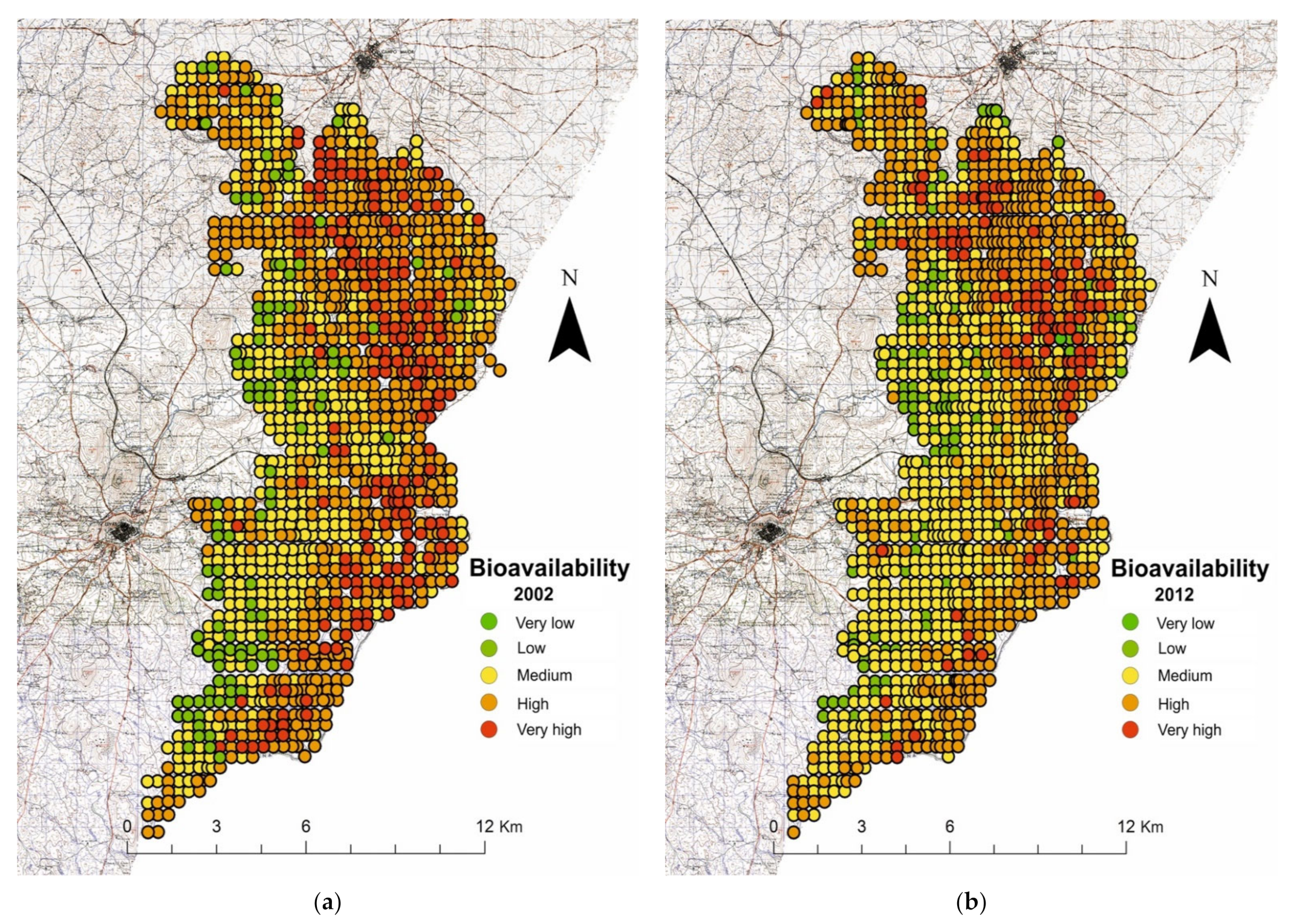
3.3. Bioavailability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jameel, M.; Shoeb, M.; Khan, M.T.; Ullah, R.; Mobin, M.; Farooqi, M.K.; Adnan, S.M. Enhanced insecticidal activity of thiamethoxam by zinc oxide nanoparticles: A Novel nanotechnology approach for pest control. ACS Omega 2020, 5, 1607–1615. [Google Scholar] [CrossRef]
- Kumpiene, J.; Giagnoni, L.; Marschner, B.; Denys, S.; Mench, M.; Adriaensen, K.; Vangronsveld, J.; Puschenreiter, M.; Renellal, G. Assessment of methods for determining bioavailability of trace elements in soils: A review. Pedosphere 2017, 27, 389–406. [Google Scholar] [CrossRef]
- Tóth, G.; Hermann, T.; Da Silva, M.R.; Montanarella, L. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Jebreen, H.; Wohnlich, S.; Banning, A.; Wisotzky, F.; Niedermayr, A.; Ghanem, M. Recharge, geochemical processes and water quality in karst aquifers: Central west bank, palestine. Env. Earth Sci. 2018, 77, 261. [Google Scholar] [CrossRef]
- Rambeau, C.M.C.; Baize, D.; Saby, N.; Matera, V.; Adatte, T.; Föllmi, K.B. High cadmium concentrations in Jurassic limestone as the cause for elevated cadmium levels in deriving soils: A case study in Lower Burgundy, France. Env. Earth Sci. 2010, 61, 1573–1585. [Google Scholar] [CrossRef] [Green Version]
- Alloway, B.J. Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability. In Environmental Pollution, 2nd ed.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 22, pp. 11–50. [Google Scholar]
- Adriano, D.C. Trace Elements in Terrestrial Environments: Biogeochemistry, Bioavailability and Risks of Metals, 2nd ed.; Springer: New York, NY, USA, 2001; p. 867. [Google Scholar]
- Abollino, O.; Aceto, M.; Malandrino, M.; Mentasti, E.; Sarzanini, C.; Petrella, F. Heavy metals in agricultural soils from Piedmont, Italy. Distribution, speciation and chemometric data treatment. Chemosphere 2002, 49, 545–557. [Google Scholar] [CrossRef]
- Vig, K.; Megharaj, M.; Sethunathan, N.; Naidu, R. Bioavailability and toxicity of cadmium to microorganisms and their activities in soil: A review. Adv. Environ. Res. 2003, 8, 121–135. [Google Scholar] [CrossRef]
- Nunes, J.; Ramos-Miras, J.; Lopez-Piñeiro, A.; Loures, L.; Gil, C.; Coelho, J.; Loures, A. Concentrations of available heavy metals in mediterranean agricultural soils and their relation with some soil selected properties: A case study in typical Mediterranean soils. Sustainability 2014, 6, 9124–9138. [Google Scholar] [CrossRef] [Green Version]
- Vega, F.A.; Covelo, E.F.; Andrade, M.L.; Marcet, P. Relationships between heavy metals content and soil properties in minesoils. Anal. Chim. Acta 2004, 524, 141–150. [Google Scholar] [CrossRef]
- Businelli, D.; Massaccesi, L.; Said-Pullicino, D.; Gigliotti, G. Long-term distribution, mobility and plant availability of compost-derived heavy metals in a landfill covering soil. Sci. Total. Environ. 2009, 407, 1426–1435. [Google Scholar] [CrossRef]
- Römkens, P.F.; Guo, H.-Y.; Chu, C.-L.; Liu, T.-S.; Chiang, C.-F.; Koopmans, G.F. Characterization of soil heavy metal pools in paddy fields in Taiwan: Chemical extraction and solid-solution partitioning. J. Soils Sediments 2009, 9, 216–228. [Google Scholar] [CrossRef]
- Tarvainen, T.; Jarva, J.; Kahelin, H. Geochemical baselines in relation to analytical methods in the Itä-Uusimaa and Pirkanmaa regions, Finland. Geochem. Explor. Environ. Anal. 2009, 9, 81–92. [Google Scholar] [CrossRef]
- Acosta, J.A.; Faz, A.; Martinez-Martinez, S. Identification of heavy metal sources by multivariable analysis in a typical Mediterranean city (SE Spain). Environ. Monit. Assess. 2010, 169, 519–530. [Google Scholar] [CrossRef]
- Afifi, A.A.; El-Rheem, K.M.A.; Youssef, R.A. Influence of sewage water reuse application on soil and the distribution of heavy metals. Nat. Sci. 2011, 4, 9. [Google Scholar]
- Micó, C.; Recatalá, L.; Peris, M.; Sánchez, J. Assessing heavy metal sources in agricultural soils of an European Mediterranean area by multivariate analysis. Chemosphere 2006, 5, 863–872. [Google Scholar] [CrossRef]
- Dias, R.M.S.; Simões, A.M.O.; Soveral-Dias, J.C.; Oliveira, R.; Rodrigues, P.C. Cádmio, Cobre, Níquel e Zinco em solos com ocupação agrí- cola em Portugal. Rev. De Ciências Agrárias 2007, 30, 358–368. [Google Scholar]
- Inácio, M.; Pereira, V.; Pinto, M. The soil geochemical atlas of Portugal: Overview and applications. J. Geochem. Explor. 2008, 98, 22–33. [Google Scholar] [CrossRef]
- Kelepertzis, E. Accumulation of heavy metals in agricultural soils of Mediterranean: Insights from Argolida basin, Peloponnese, Greece. Geoderma 2014, 221–222, 82–90. [Google Scholar] [CrossRef]
- Bakó, G.; Molnár, Z.; Bakk, L.; Horváth, F.; Fehér, L.; Ábrám, Ö.; Morvai, E.; Biro, C.; Pápay, G.; Fűrész, A.; et al. Toward a high spatial resolution aerial monitoring network for nature conservation—How can remote sensing help protect natural areas? Sustainability 2021, 13, 8807. [Google Scholar] [CrossRef]
- Telo da Gama, J.; Rato Nunes, J.; Loures, L.; Lopez Piñeiro, A.; Vivas, P. Assessing spatial and temporal variability for some edaphic characteristics of Mediterranean rainfed and irrigated soils. Agronomy 2019, 9, 132. [Google Scholar] [CrossRef] [Green Version]
- Food and Agriculture Organization (FAO). World Reference Base for Soil Resources 2014: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; FAO: Rome, Italy, 2015. [Google Scholar]
- Loures, L.; Gama, J.; Nunes, J.; Lopez-Piñeiro, A. Assessing the sodium exchange capacity in rainfed and irrigated soils in the Mediterranean basin using GIS. Sustainability 2017, 9, 405. [Google Scholar] [CrossRef] [Green Version]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Hooda, P.S.; Alloway, B.J. The plant availability and DTPA extractability of trace metals in sludge-amended soils. Sci. Total. Environ. 1994, 149, 39–51. [Google Scholar] [CrossRef]
- Soriano-Disla, J.M.; Gómez, I.; Guerrero, C.; Jordan, M.M.; Navarro-Pedreño, J. Soil factors related to heavy metal bioavailability after sewage sludge application. Fresenius Environ. Bull. 2008, 17, 8. [Google Scholar]
- Hao, X.-Z.; Zhou, D.-M.; Huang, D.-Q.; Cang, L.; Zhang, H.L.; Wang, H. Heavy metal transfer from soil to vegetable in southern Jiangsu Province, China. Pedosphere 2009, 19, 305–311. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591. [Google Scholar] [CrossRef]
- Razali, N.M.; Wah, Y.B. Power comparisons of shapiro-wilk, kolmogorov-smirnov, lilliefors and anderson-darling tests. J. Stat. Modeling Anal. 2011, 2, 21–33. [Google Scholar]
- Cramer, D. Fundamental Statistics for Social Research: Step-by-Step Calculations and Computer Techniques Using SPSS for Windows; Routledge: Abingdon, UK, 1988. [Google Scholar]
- Cramer, D.; Howitt, D.L. The Sage Dictionary of Statistics: A Practical Resource for Students in the Social Sciences; Sage: London, UK, 2004. [Google Scholar]
- Doane, D.P.; Seward, L.E. Measuring skewness: A forgotten statistic? J. Stat. Educ. 2011, 19, 1–18. [Google Scholar] [CrossRef]
- Nordstokke, D.W.; Zumbo, B.D. A new nonparametric Levene test for equal variances. Psicol. Int. J. Methodol. Exp. Psychol. 2010, 31, 401–430. [Google Scholar]
- Nordstokke, D.W.; Zumbo, B.D.; Cairns, S.L.; Saklofske, D.H. The operating characteristics of the nonparametric Levene test for equal variances with assessment and evaluation data. Pract. Assess. Res. Eval. 2011, 16, 5. [Google Scholar]
- Ferreira, M. Dados Geoquímicos de Base de Solos de Portugal Continental, Utilizando Amostragem de Baixa Densidade. Ph.D. Thesis, University of Aveiro, Aveiro, Portugal, 19 December 2004. [Google Scholar]
- Harmens, H.; Norris, D.A.; Koerber, G.R.; Buse, A.; Steinnes, E.; Rühling, Å. Temporal trends (1990–2000) in the concentration of cadmium, lead and mercury in mosses across Europe. Environ. Pollut. 2008, 151, 368–376. [Google Scholar] [CrossRef] [Green Version]
- Hengl, T.; Heuvelink, G.B.M.; Stein, A. A generic framework for spatial prediction of soil variables based on regression-kriging. Geoderma 2004, 120, 75–93. [Google Scholar] [CrossRef] [Green Version]
- Baxter, S.J.; Oliver, M.A. The spatial prediction of soil mineral N and potentially available N using elevation. Geoderma 2005, 128, 325–339. [Google Scholar] [CrossRef]
- Li, Y. Can the spatial prediction of soil organic matter contents at various sampling scales be improved by using regression kriging with auxiliary information? Geoderma 2010, 159, 63–75. [Google Scholar] [CrossRef]
- Sun, W.; Minasny, B.; McBratney, A. Analysis and prediction of soil properties using local regression-kriging. Geoderma 2012, 171–172, 16–23. [Google Scholar] [CrossRef]
- Behera, S.K.; Shukla, A.K. Spatial distribution of surface soil acidity, electrical conductivity, soil organic carbon content and exchangeable potassium, calcium and magnesium in some cropped acid soils of India. Land Degrad. Dev. 2015, 26, 71–79. [Google Scholar] [CrossRef]
- Chen, T.; Chang, Q.; Liu, J.; Clevers, J.G.P.W.; Kooistra, L. Identification of soil heavy metal sources and improvement in spatial mapping based on soil spectral information: A case study in northwest China. Sci. Total Environ. 2016, 565, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; O’Connor, D.; Nathanail, P.; Tian, L.; Ma, Y. Integrated GIS and multivariate statistical analysis for regional scale assessment of heavy metal soil contamination: A critical review. Environ. Pollut. 2017, 231, 1188–1200. [Google Scholar] [CrossRef]
- Tziachris, P.; Metaxa, E.; Papadopoulos, F.; Papadopoulou, M. Spatial modelling and prediction assessment of soil iron using Kriging interpolation with pH as auxiliary information. ISPRS Int. J. Geo-Inf. 2017, 6, 283. [Google Scholar] [CrossRef] [Green Version]
- Team QD. QGIS Geographic Information System. Open Source Geospatial Foundation Project, Versão. 2015, 2. Available online: https://www.qgis.org/en/site/ (accessed on 1 March 2021).
- Fairbrother, A.; Wenstel, R.; Sappington, K.; Wood, W. Framework for metals risk assessment. Ecotoxicol. Environ. Safety 2007, 68, 145–227. [Google Scholar] [CrossRef]
- Robson, T.C.; Braungardt, C.B.; Rieuwerts, J.; Worsfold, P. Cadmium contamination of agricultural soils and crops resulting from sphalerite weathering. Environ. Pollut. 2014, 184, 283–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khodaverdiloo, H.; Samadi, A. Batch equilibrium study on sorption, desorption, and immobilisation of cadmium in some semi-arid zone soils as affected by soil properties. Soil Res. 2011, 49, 444. [Google Scholar] [CrossRef]
- Alcântara, M.A.K.; De Camargo, O.A. Transporte de crômio trivalente influenciado pelo pH, horizonte do solo e fontes do crômio. Rev. Bras. Eng. Agríc. Ambient. 2001, 5, 497–501. [Google Scholar] [CrossRef]
- Salunkhe, P.B.; Dhakephalkar, P.K.; Paknikar, K.M. Bioremediation of hexavalent chromium in soil microcosms. Biotechnol. Lett. 1998, 20, 749–751. [Google Scholar] [CrossRef]
- Gonçalves Junior, A.C.; Luchese, E.B.; Lenzi, E. Avaliação da fitodisponibilidade de cádmio, chumbo e crômio, em soja cultivada em latossolo vermelho escuro tratado com fertilizantes comerciais. Quím Nova 2000, 23, 173–177. [Google Scholar] [CrossRef]
- Chirigiu, L.; Popescu, R.; Bubulica, M.-V.; Popescu, A. Determination of chromium, cooper, iron, zinc, cadmium and led by graphite furnace atomic absorption spectrometry in seven phytopharmaceutical products. Rev. Chim. 2012, 63, 874–876. [Google Scholar]
- Rodríguez Martín, J.A.; Ramos-Miras, J.J.; Boluda, R.; Gil, C. Spatial relations of heavy metals in arable and greenhouse soils of a Mediterranean environment region (Spain). Geoderma 2013, 200–201, 180–188. [Google Scholar] [CrossRef]
- Obrador, A.; Gonzalez, D.; Alvarez, J.M. Effect of inorganic and organic copper fertilizers on copper nutrition in spinacia oleracea and on labile copper in soil. J. Agric. Food Chem. 2013, 61, 4692–4701. [Google Scholar] [CrossRef]
- Romic, M.; Matijevic, L.; Bakic, H.; Romic, D. Copper accumulation in vineyard soils: Distribution, fractionation and bioavailability assessment. In Environmental Risk Assessment of Soil Contamination; Hernandez Soriano, M.C., Ed.; BoD: Norderstedt, Germany, 2014; Available online: http://www.intechopen.com/books/environmental-risk-assessment-of-soil-contamination/copper-accumulation-in-vineyard-soils-distribution-fractionation-and-bioavailability-assessment (accessed on 27 September 2021).
- Watts, C.; Aslam, M.; Gunaratna, N.; Shankar, A.; Groote, H.D.; Sharp, P. Agronomic Biofortification of maize with zinc fertilizers increases zinc uptake from maize flour by human intestinal caco-2 cells. Curr. Dev. Nutr. 2020, 4, 1853. [Google Scholar] [CrossRef]
- Safaya, N.M. Phosphorus-zinc interaction in relation to absorption rates of phosphorus, zinc, copper, manganese, and iron in corn. Soil Sci. Soc. Am. J. 1976, 40, 719–722. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R.; Weil, R.R. The Nature and Properties of Soils; Prentice Hall: Upper Saddle River, NJ, USA, 2010; Volume 13, pp. 662–710. [Google Scholar]
- Brooks, R.R. Serpentine and its Vegetation: A Multidisciplinary Approach; Dioscorides Press: Portland, OR, USA, 1987. [Google Scholar]

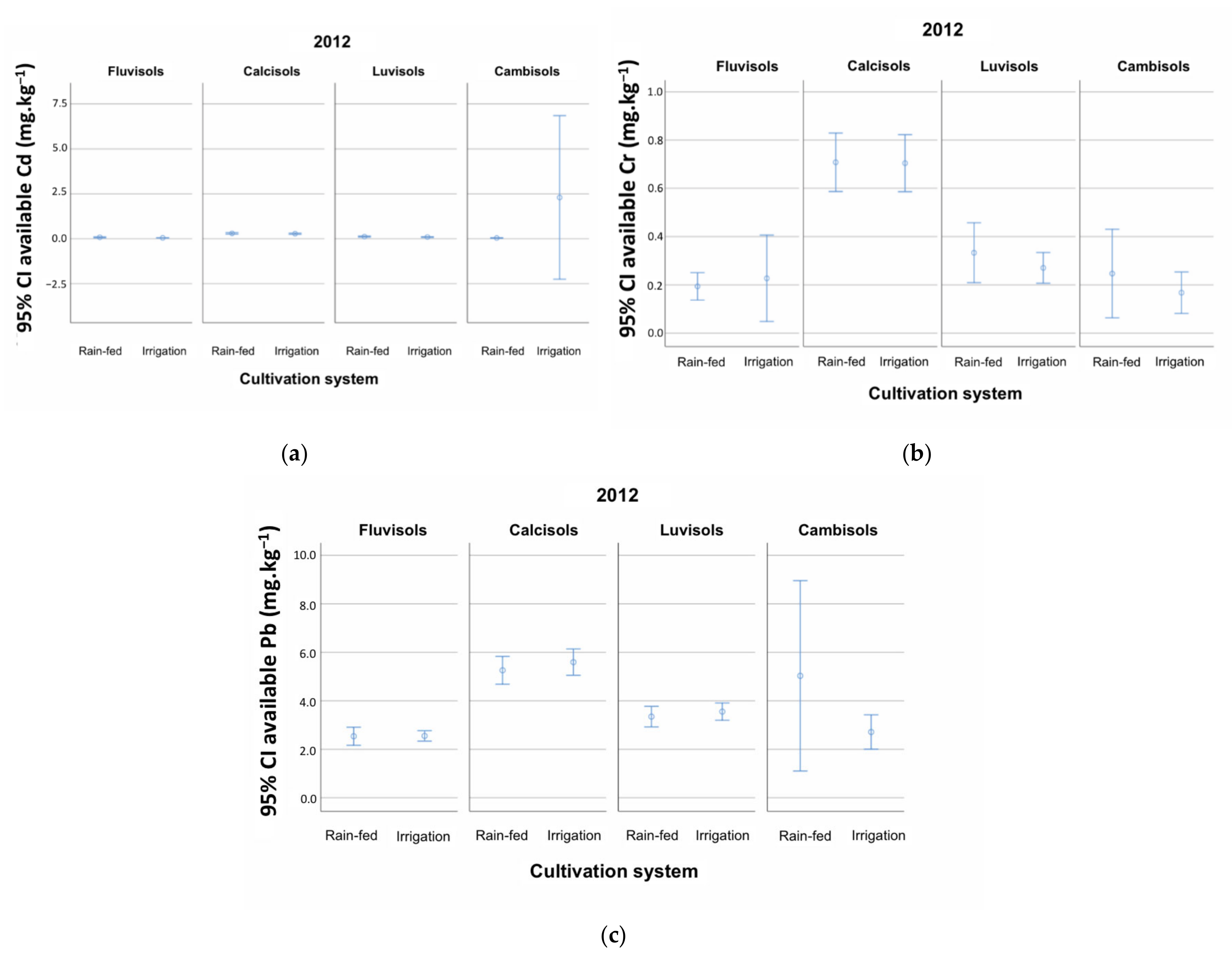
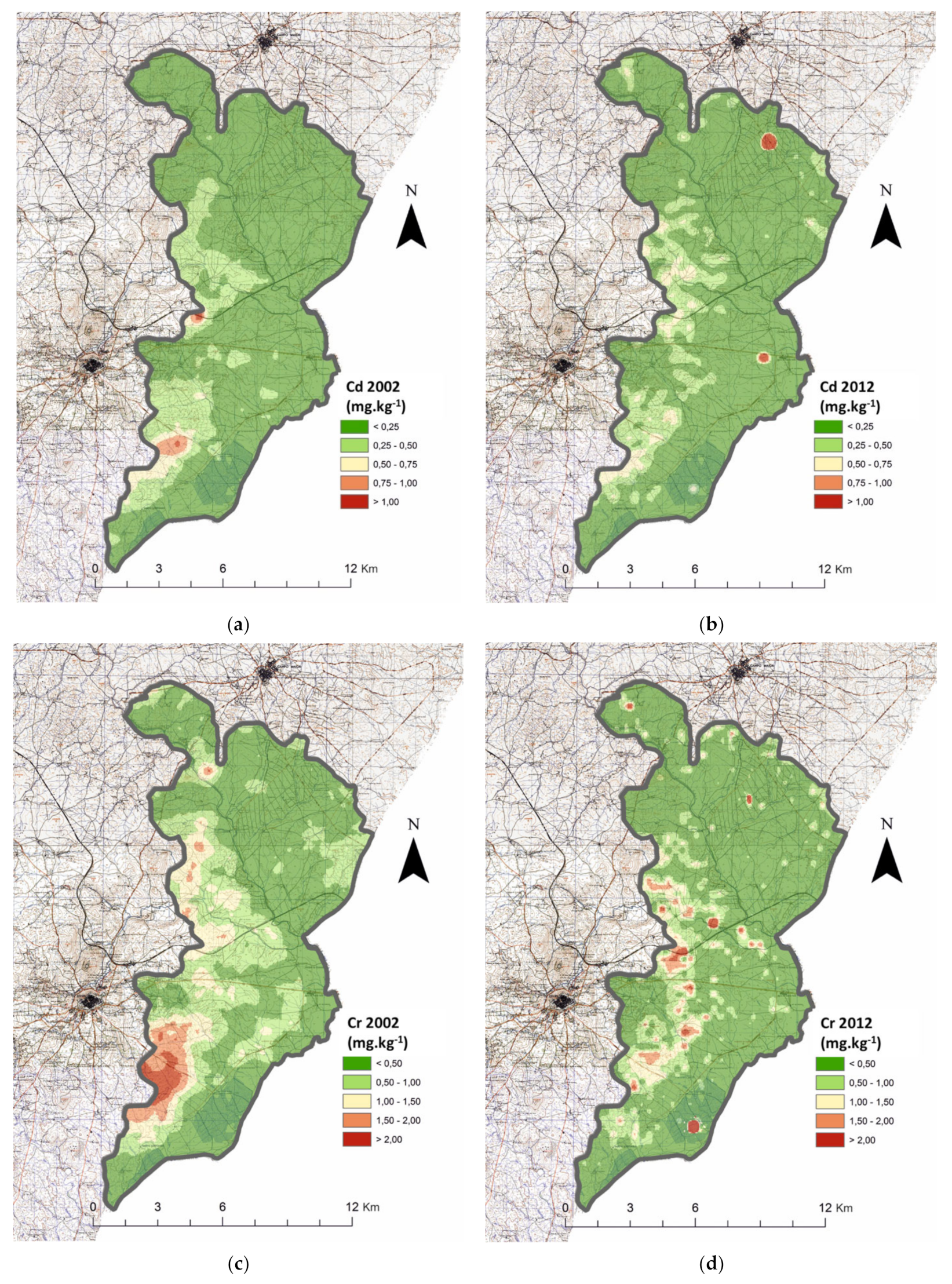
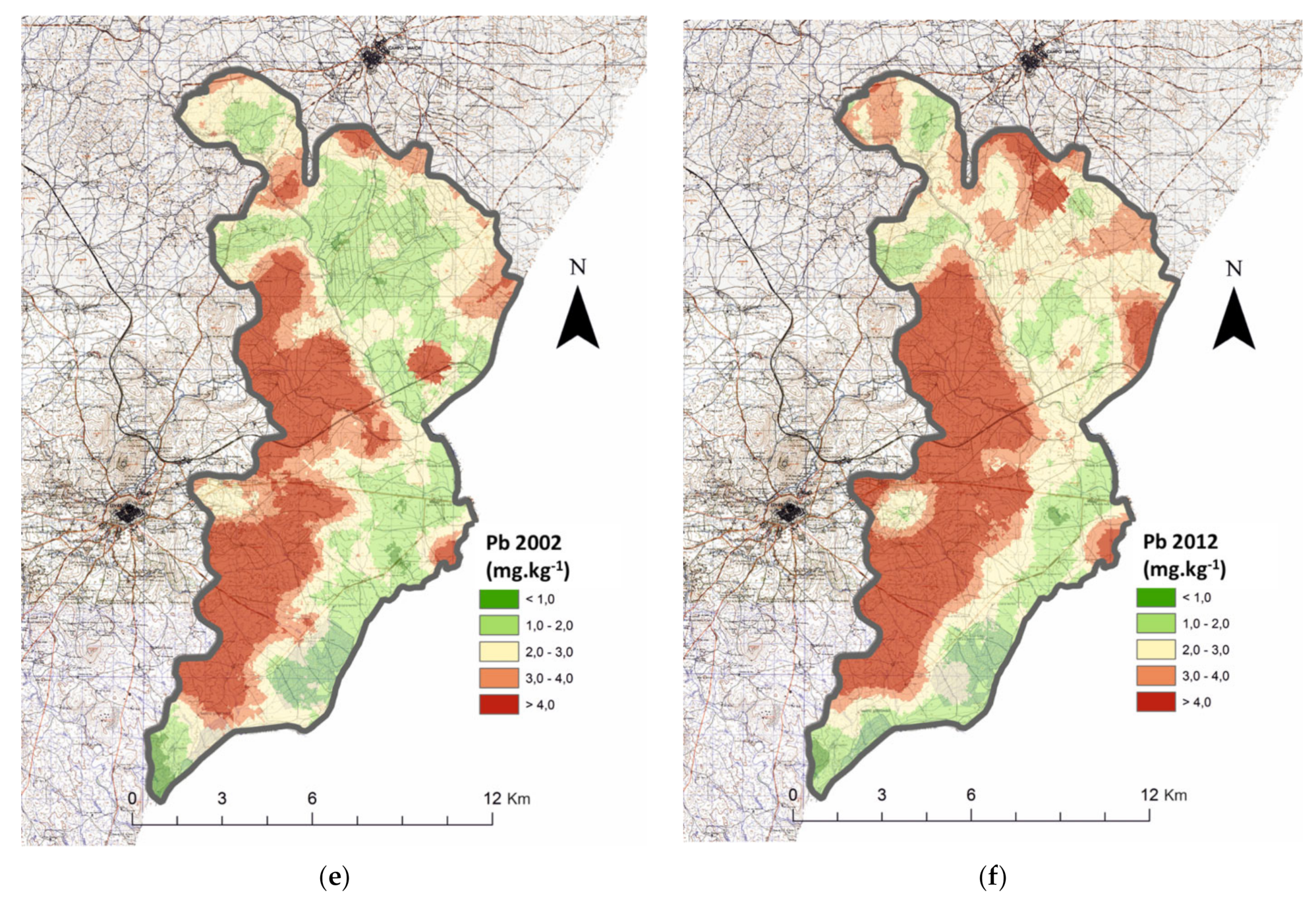
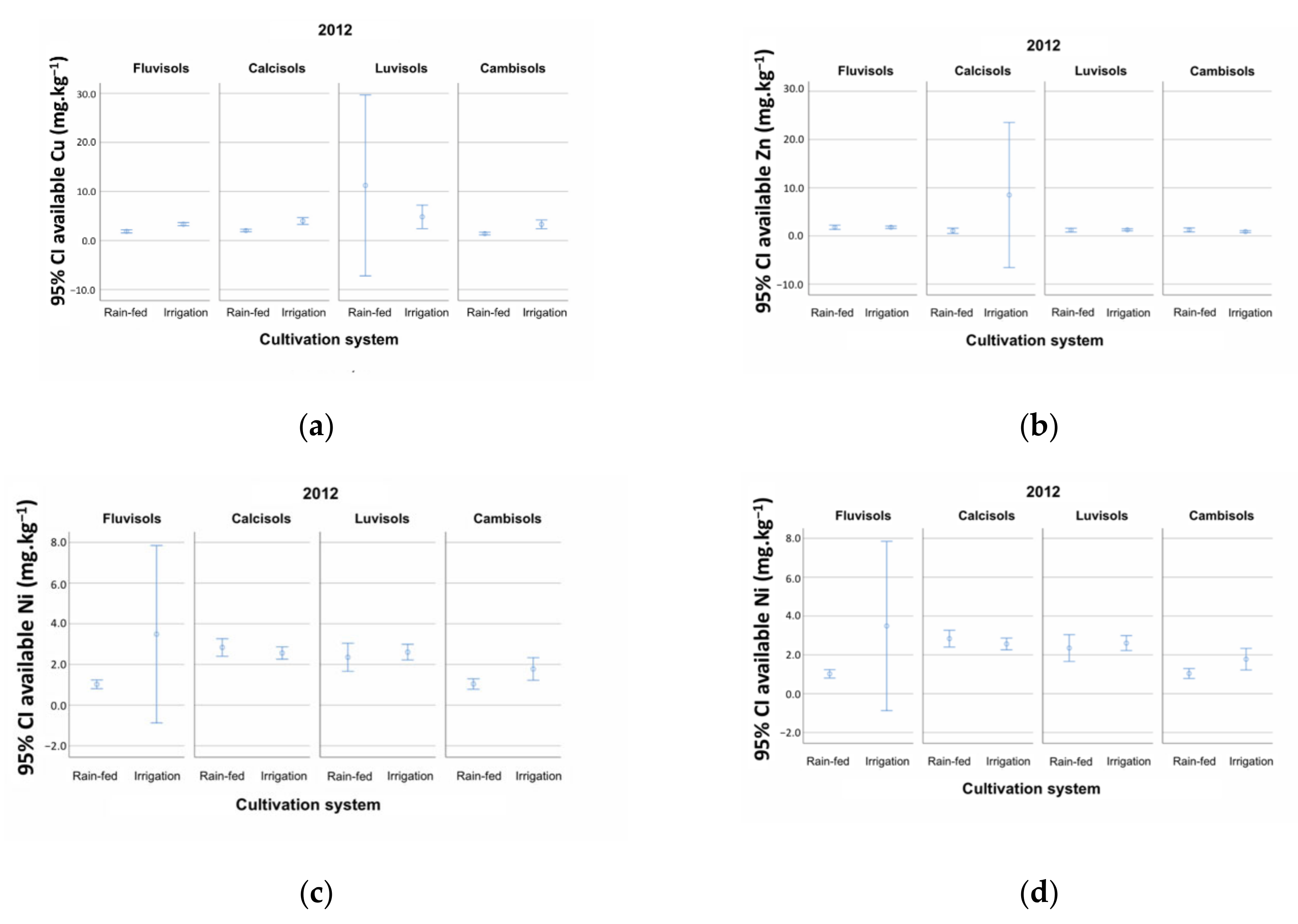
| Parameter | Year | RSG | CS | N | MAI | Test | p | N | HAI | N | Average | Test | p | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (a) | Cd (mg kg−1) | 2002 | n.a. | n.a. | 620 | 0.065 | X: 9857 | 0.002 | 675 | 0.510 | 1295 | 0.101 | T (2588): −0.649 | 0.517 |
| 2012 | 512 | 0.020 | 783 | 0.495 | 1295 | 0.089 | ||||||||
| Cr (mg kg−1) | 2002 | n.a. | n.a. | 620 | 0.380 | X: 59,136 | 0.000 | 675 | 1.60 | 1295 | 0.440 | U: 599,533,000 | 0.000 | |
| 2012 | 512 | 0.000 | 784 | 1.35 | 1296 | 0.223 | ||||||||
| Pb (mg kg−1) | 2002 | n.a. | n.a. | 620 | 2.55 | X: 1.201 | 0.026 | 675 | 6.70 | 1295 | 2.71 | U: 763,037,000 | 0.000 | |
| 2012 | 513 | 2.70 | 784 | 8.02 | 1297 | 3.08 | ||||||||
| (b) | Cd (mg kg−1) | 2012 | Fluvisols | n.a. | 198 | 0.000 b | X2(3): 59,760 | 0.000 | 430 | 0.245 | n.a. | |||
| Luvisols | 151 | 0.015 b | 188 | 0.475 | ||||||||||
| Calcisols | 98 | 0.315 a | 130 | 0.612 | ||||||||||
| Cambisols | 64 | 0.000 b | 36 | 12.5 | ||||||||||
| Cr (mg kg−1) | 2012 | Fluvisols | n.a. | 198 | 0.000 b | X2(3): 36,140 | 0.000 | 430 | 0.845 | n.a. | ||||
| Luvisols | 152 | 0.000 b | 188 | 1.21 | ||||||||||
| Calcisols | 98 | 0.675 a | 130 | 1.93 | ||||||||||
| Cambisols | 64 | 0.000 b | 36 | 0.687 | ||||||||||
| Pb (mg kg−1) | 2012 | Fluvisols | n.a. | 198 | 1.89 c | X2(3): 63,747 | 0.000 | 430 | 6.64 | n.a. | ||||
| Luvisols | 152 | 2.86 b | 188 | 7.86 | ||||||||||
| Calcisols | 99 | 5.51 a | 130 | 12.4 | ||||||||||
| Cambisols | 64 | 2.34 bc | 36 | 6.87 | ||||||||||
| (c) | Cd (mg kg−1) | 2012 | n.a. | Rainfed | n.a. | n.a. | 512 | 0.106 | T (1293): 0.566 | 0.572 | ||||
| Irrigation | 783 | 0.078 | ||||||||||||
| Cr (mg kg−1) | 2012 | n.a. | Rainfed | n.a. | n.a. | 512 | 0.259 | T (1294): −0.386 | 0.700 | |||||
| Irrigation | 784 | 0.199 | ||||||||||||
| Pb (mg kg−1) | 2012 | n.a. | Rainfed | n.a. | n.a. | 513 | 3.11 | U: 199,147,500 | 0.768 | |||||
| Irrigation | 784 | 3.06 | ||||||||||||
| (d) | Cd (mg kg−1) | 2012 | Fluvisols c | Rainfed | n.a. | n.a. | 198 | 0.049 | U: 39,772,000 | 0.147 | ||||
| Irrigation | 429 | 0.032 | ||||||||||||
| Luvisols b | Rainfed | n.a. | n.a. | 151 | 0.100 | U: 13,687,000 | 0.552 | |||||||
| Irrigation | 188 | 0.076 | ||||||||||||
| Calcisols a | Rainfed | n.a. | n.a. | 99 | 0.295 | T (227): −0.647 | 0.518 | |||||||
| Irrigation | 130 | 0.276 | ||||||||||||
| Cambisols bc | Rainfed | n.a. | n.a. | 64 | 0.032 | U: 1,055,500 | 0.461 | |||||||
| Irrigation | 36 | 0.049 | ||||||||||||
| Cr (mg kg−1) | 2012 | Fluvisols c | Rainfed | n.a. | n.a. | 198 | 0.129 | T (626): 0.246 | 0.805 | |||||
| Irrigation | 430 | 0.094 | ||||||||||||
| Luvisols b | Rainfed | n.a. | n.a. | 152 | 0.228 | T (338): −0.938 | 0.349 | |||||||
| Irrigation | 188 | 0.209 | ||||||||||||
| Calcisols a | Rainfed | n.a. | n.a. | 98 | 0.678 | T (226): −0.043 | 0.966 | |||||||
| Irrigation | 130 | 0.660 | ||||||||||||
| Cambisols c | Rainfed | n.a. | n.a. | 64 | 0.129 | T (98): −0.627 | 0.532 | |||||||
| Irrigation | 36 | 0.137 | ||||||||||||
| Pb (mg kg−1) | 2012 | Fluvisols c | Rainfed | n.a. | n.a. | 198 | 0.129 | T (626): 0.073 | 0.942 | |||||
| Irrigation | 430 | 0.094 | ||||||||||||
| Luvisols b | Rainfed | n.a. | n.a. | 152 | 0.228 | T (338): 0.739 | 0.460 | |||||||
| Irrigation | 188 | 0.209 | ||||||||||||
| Calcisols a | Rainfed | n.a. | n.a. | 99 | 0.678 | T (227): 0.836 | 0.404 | |||||||
| Irrigation | 130 | 0.660 | ||||||||||||
| Cambisols c | Rainfed | n.a. | n.a. | 64 | 0.129 | T (98): −0.878 | 0.382 | |||||||
| Irrigation | 36 | 0.137 | ||||||||||||
| Parameter | Year | RSG | CS | N | MAI | Test | p | N | HAI | N | Aver. | Test | p | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (a) | Cu (mg kg−1) | 2002 | n.a. | n.a. | 620 | 0.995 | X: 78,782 | 0.000 | 675 | 3.62 | 1295 | 1.07 | U: 411,473,500 | 0.000 |
| 2012 | 513 | 1.61 | 783 | 10.1 | 1295 | 2.39 | ||||||||
| Zn (mg kg−1) | 2002 | n.a. | n.a. | 620 | 0.505 | X: 64,306 | 0.000 | 675 | 2.92 | 1295 | 0.646 | U: 581,192,500 | 0.000 | |
| 2012 | 513 | 0.715 | 784 | 5.43 | 1297 | 1.06 | ||||||||
| Mn (mg kg−1) | 2002 | n.a. | n.a. | 620 | 41.5 | X: 17,090 | 0.000 | 675 | 150 | 1295 | 48.3 | U: 639,113,500 | 0.000 | |
| 2012 | 513 | 55.1 | 783 | 164 | 1296 | 67.5 | ||||||||
| Ni (mg kg−1) | 2002 | n.a. | n.a. | 620 | 1.80 | X: 7350 | 0.007 | 675 | 4.10 | 1295 | 1.62 | T (2581): −0.942 | 0.346 | |
| 2012 | 507 | 1.35 | 781 | 5.42 | 1288 | 1.57 | ||||||||
| (b) | Cu (mg kg−1) | 2012 | Fluvisols | n.a. | 198 | 1.398 b | X2(3): 20,762 | 0.000 | 430 | 9.22 | n.a. | |||
| Luvisols | 152 | 1.645 ab | 188 | 12.5 | ||||||||||
| Calcisols | 99 | 1.870 a | 130 | 13.3 | ||||||||||
| Cambisols | 64 | 1.070 b | 36 | 9.00 | ||||||||||
| Zn (mg kg−1) | 2012 | Fluvisols | n.a. | 198 | 0.743 a | X2(3): 6365 | 0.095 | 430 | 6.77 | n.a. | ||||
| Luvisols | 152 | 0.728 a | 188 | 4.73 | ||||||||||
| Calcisols | 99 | 0.650 a | 130 | 2.00 | ||||||||||
| Cambisols | 64 | 0.778 a | 36 | 2.27 | ||||||||||
| Mn (mg kg−1) | 2012 | Fluvisols | n.a. | 198 | 60.5 a | X2(3): 9326 | 0.025 | 430 | 157 | n.a. | ||||
| Luvisols | 152 | 56.9 a | 188 | 182 | ||||||||||
| Calcisols | 99 | 31.4 b | 130 | 166 | ||||||||||
| Cambisols | 64 | 63.3 a | 36 | 166 | ||||||||||
| Ni (mg kg−1) | 2012 | Fluvisols | n.a. | 198 | 1.89 c | X2(3): 104,579 | 0.000 | 430 | 4.16 | n.a. | ||||
| Luvisols | 152 | 2.86 b | 188 | 6.11 | ||||||||||
| Calcisols | 99 | 5.51 a | 130 | 6.63 | ||||||||||
| Cambisols | 64 | 2.34 bc | 36 | 5.72 | ||||||||||
| (c) | Cu (mg kg−1) | 2012 | n.a. | Rainfed | n.a. | n.a. | 513 | 1.68 | T (1294): −0.362 | 0.717 | ||||
| Irrigation | 783 | 2.95 | ||||||||||||
| Zn (mg kg−1) | 2012 | n.a. | Rainfed | n.a. | n.a. | 513 | 1.40 | T (1295): 0.852 | 0.394 | |||||
| Irrigation | 784 | 2.74 | ||||||||||||
| Mn (mg kg−1) | 2012 | n.a. | Rainfed | n.a. | n.a. | 513 | 62.0 | T (1294): 3.218 | 0.007 | |||||
| Irrigation | 783 | 71.1 | ||||||||||||
| Ni (mg kg−1) | 2012 | n.a. | Rainfed | n.a. | n.a. | 507 | 1.51 | T (1286): 0.833 | 0.405 | |||||
| Irrigation | 781 | 1.61 | ||||||||||||
| (d) | Cu (mg kg−1) | 2012 | Fluvisols c | Rainfed | n.a. | n.a. | 198 | 1.56 | U: 26,567,000 | 0.000 | ||||
| Irrigation | 429 | 2.86 | ||||||||||||
| Luvisols ac | Rainfed | 152 | 1.82 | T (338): −0.754 | 0.451 | |||||||||
| Irrigation | 188 | 2.86 | ||||||||||||
| Calcisols a | Rainfed | 99 | 1.91 | U: 3,438,000 | 0.000 | |||||||||
| Irrigation | 130 | 3.38 | ||||||||||||
| Cambisols b | Rainfed | 64 | 1.32 | U: 1,055,500 | 0.000 | |||||||||
| Irrigation | 36 | 3.08 | ||||||||||||
| Zn (mg kg−1) | 2012 | Fluvisols b | Rainfed | n.a. | n.a. | 198 | 1.79 | T (626): 0.028 | 0.978 | |||||
| Irrigation | 430 | 1.80 | ||||||||||||
| Luvisols a | Rainfed | 152 | 1.19 | T (338): 0.324 | 0.746 | |||||||||
| Irrigation | 188 | 1.26 | ||||||||||||
| Calcisols a | Rainfed | 99 | 1.06 | T (227): 0.852 | 0.395 | |||||||||
| Irrigation | 130 | 8.50 | ||||||||||||
| Cambisols ab | Rainfed | 64 | 0.964 | U: 1,106,000 | 0.741 | |||||||||
| Irrigation | 36 | 0.823 | ||||||||||||
| Mn (mg kg−1) | 2012 | Fluvisols a | Rainfed | n.a. | n.a. | 198 | 66.8 | T (626): 2.691 | 0.007 | |||||
| Irrigation | 430 | 78.0 | ||||||||||||
| Luvisols a | Rainfed | 152 | 61.2 | U: 11,837,000 | 0.008 | |||||||||
| Irrigation | 187 | 76.6 | ||||||||||||
| Calcisols b | Rainfed | 99 | 48.3 | T (227): −1.286 | 0.200 | |||||||||
| Irrigation | 130 | 38.6 | ||||||||||||
| Cambisols a | Rainfed | 64 | 70.1 | T (98): 0.468 | 0.641 | |||||||||
| Irrigation | 36 | 76.3 | ||||||||||||
| Ni (mg kg−1) | 2012 | Fluvisols c | Rainfed | n.a. | n.a. | 196 | 0.840 | T (626): 0.073 | 0.942 | |||||
| Irrigation | 429 | 1.079 | ||||||||||||
| Luvisols b | Rainfed | 150 | 1.944 | T (338): 0.739 | 0.460 | |||||||||
| Irrigation | 187 | 2.337 | ||||||||||||
| Calcisols a | Rainfed | 98 | 2.650 | T (227): 0.836 | 0.404 | |||||||||
| Irrigation | 129 | 2.421 | ||||||||||||
| Cambisols c | Rainfed | 63 | 0.958 | U: 784,500 | 0.011 | |||||||||
| Irrigation | 36 | 1.608 | ||||||||||||
| RSG | CS | N | pH | SOM (%) | Bioavailability * | Study Area | |
|---|---|---|---|---|---|---|---|
| (ha) | (%) | ||||||
| Fluvisols | Rainfed | 196 | 6.50 | 1.48 | High | 6761 | 45.5 |
| Irrigation | 430 | 6.63 | 1.11 | High | |||
| Luvisols | Rainfed | 145 | 7.10 | 1.52 | Medium | 4465 | 30.1 |
| Irrigation | 189 | 7.29 | 1.22 | Medium | |||
| Calcisols | Rainfed | 99 | 7.97 | 1.65 | Medium | 2808 | 18.9 |
| Irrigation | 132 | 8.06 | 1.46 | Medium | |||
| Cambisols | Rainfed | 59 | 6.36 | 1.42 | High | 818 | 5.50 |
| Irrigation | 36 | 6.39 | 1.35 | High | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Telo da Gama, J.; Loures, L.; López-Piñeiro, A.; Nunes, J.R. Spatial Distribution of Available Trace Metals in Four Typical Mediterranean Soils: The Caia Irrigation Perimeter Case Study. Agronomy 2021, 11, 2024. https://doi.org/10.3390/agronomy11102024
Telo da Gama J, Loures L, López-Piñeiro A, Nunes JR. Spatial Distribution of Available Trace Metals in Four Typical Mediterranean Soils: The Caia Irrigation Perimeter Case Study. Agronomy. 2021; 11(10):2024. https://doi.org/10.3390/agronomy11102024
Chicago/Turabian StyleTelo da Gama, José, Luis Loures, António López-Piñeiro, and José Rato Nunes. 2021. "Spatial Distribution of Available Trace Metals in Four Typical Mediterranean Soils: The Caia Irrigation Perimeter Case Study" Agronomy 11, no. 10: 2024. https://doi.org/10.3390/agronomy11102024
APA StyleTelo da Gama, J., Loures, L., López-Piñeiro, A., & Nunes, J. R. (2021). Spatial Distribution of Available Trace Metals in Four Typical Mediterranean Soils: The Caia Irrigation Perimeter Case Study. Agronomy, 11(10), 2024. https://doi.org/10.3390/agronomy11102024







