In Vitro Propagation of Easter Island Curcuma longa from Rhizome Explants Using Temporary Immersion System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Explant Collection, Disinfection and Establishment of Rhizome Cultures
2.2. In Vitro Growth of Explants
2.3. In Vitro Proliferation of C. Longa by TIS
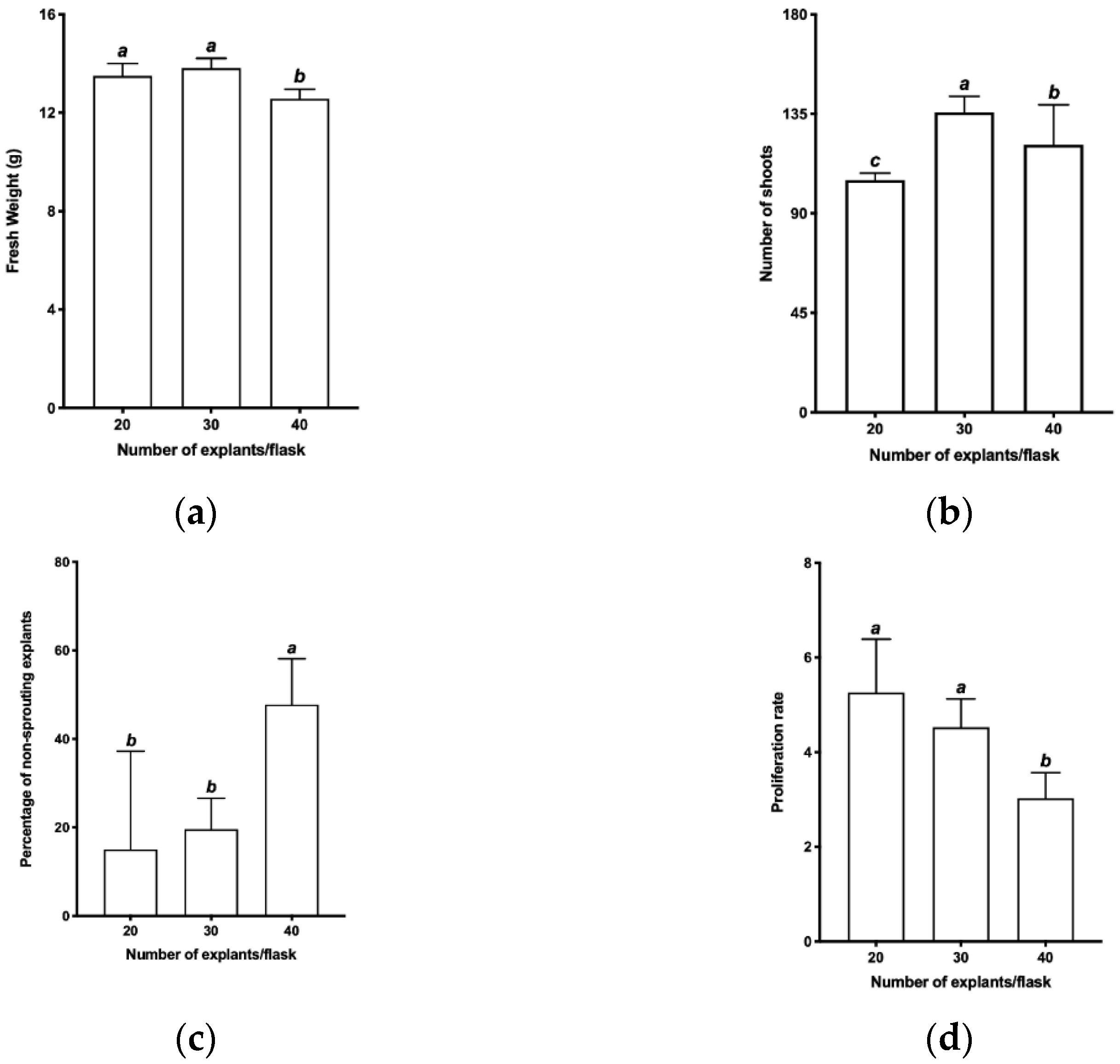
2.3.1. Evaluation of the Number of Explants per Flask
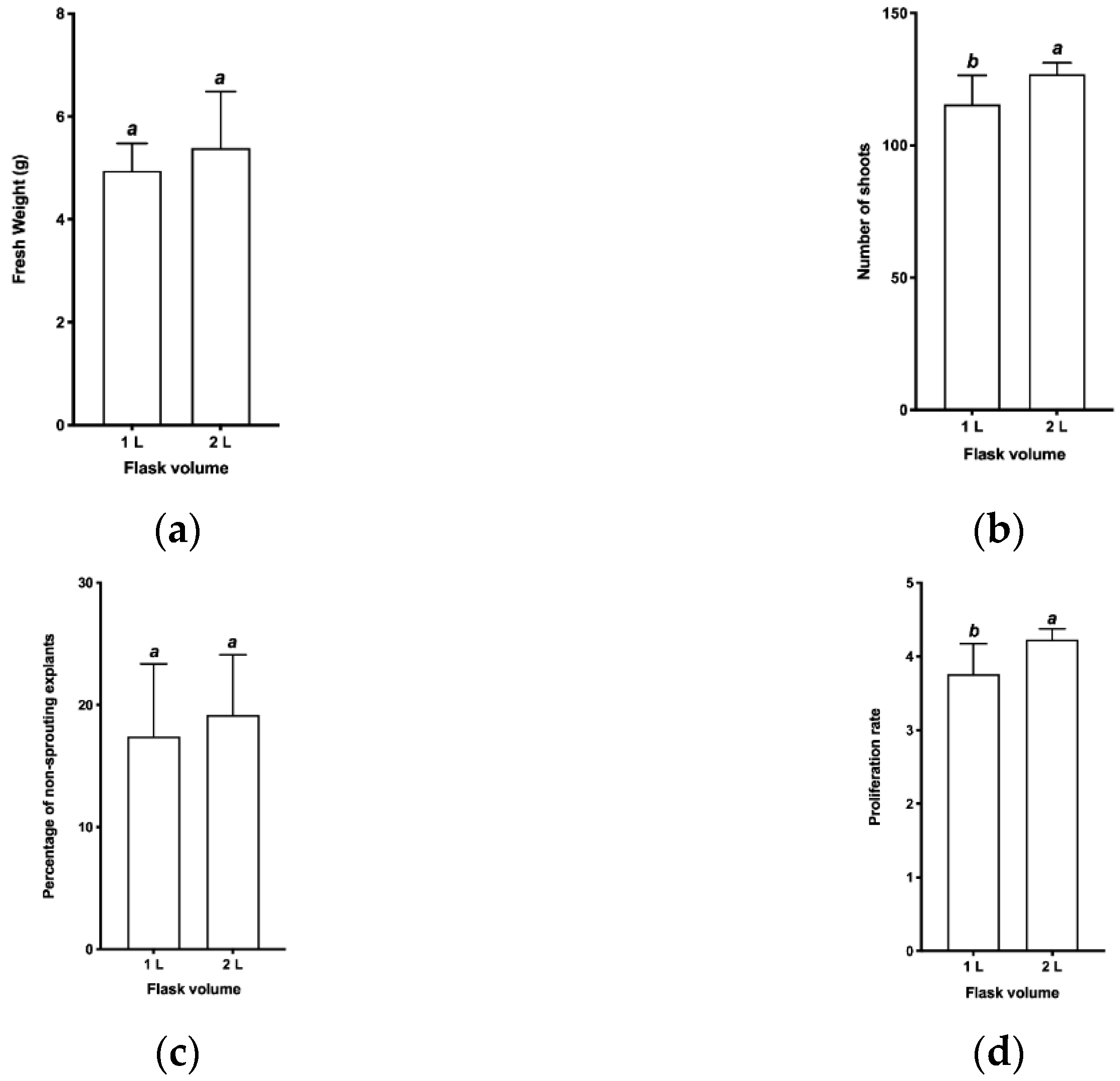
2.3.2. Evaluation of Flask Capacity
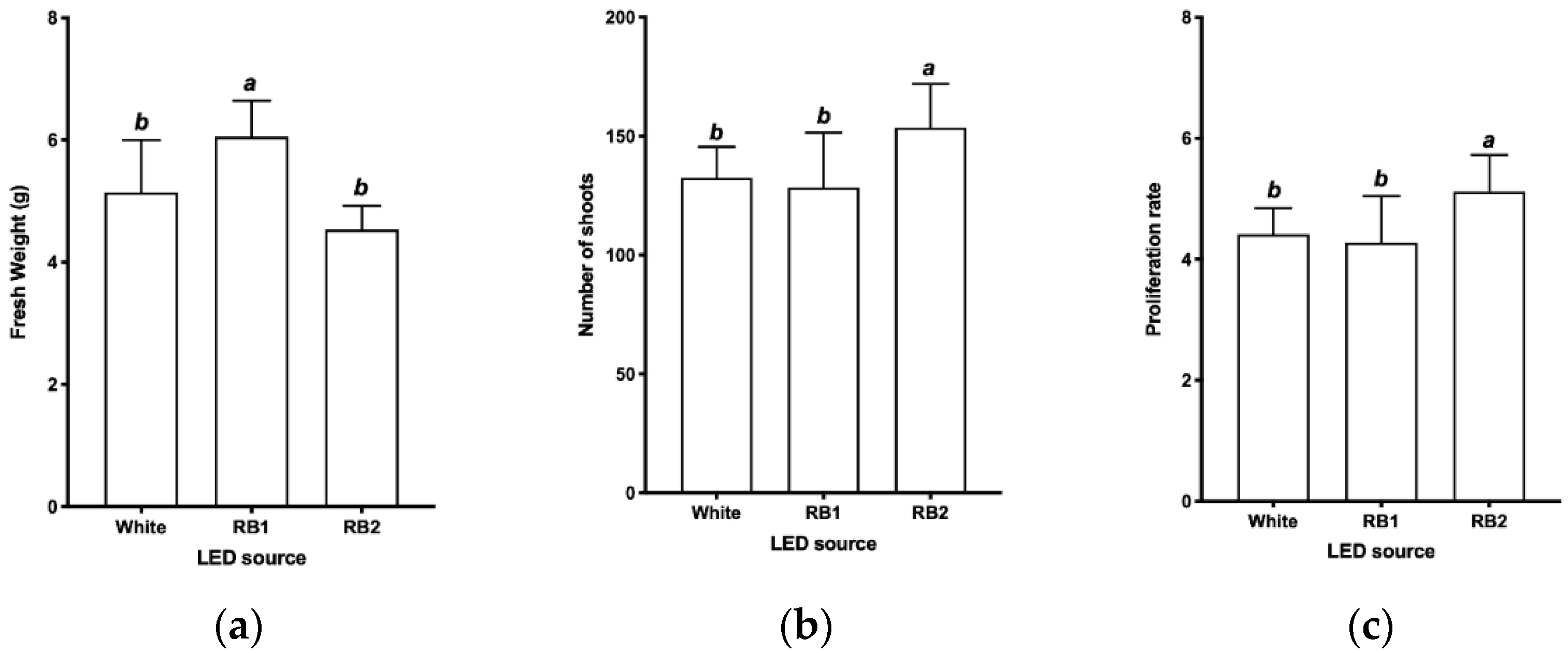
2.3.3. Evaluation of Light Spectra
2.4. Statistical Analysis
3. Results
3.1. Evaluation of the Number of Explants per Flask
3.2. Evaluation of the Volumetric Capacity of the Flasks
3.3. Evaluation of Light Spectra
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Holdsworth, D.K. A Preliminary Study of Medicinal Plants of Easter Island, South Pacific. Int. J. Pharmacogn. 1992, 30, 27–32. [Google Scholar] [CrossRef]
- Kocaadam, B.; Şanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Balada, C.; Castro, M.; Fassio, C.; Zamora, A.; Marchant, M.J.; Acevedo, W.; Guzmán, L. Genetic diversity and biological activity of Curcuma longa ecotypes from Rapa Nui using molecular markers. Saudi J. Biol. Sci. 2021, 28, 707–716. [Google Scholar] [CrossRef]
- Nasirujjaman, K.; Uddin, M.S.; Zaman, S.; Reza, M. Micropropagation of Turmeric (Curcuma longa Linn.) through in vitro Rhizome Bud Culture. J. Biol. Sci. 2005, 5, 490–492. [Google Scholar] [CrossRef] [Green Version]
- Etienne, H.; Berthouly, M. Temporary immersion systems in plant micropropagation. Plant Cell Tissue Organ 2002, 69, 215–231. [Google Scholar] [CrossRef]
- Berthouly, M.; Etienne, H. Temporary immersion system: A new concept for use liquid medium in mass propagation. In Liquid Culture Systems for In Vitro Plant Propagation; Springer: Dordrecht, The Netherlands, 2005; pp. 165–195. [Google Scholar]
- Roels, S.; Noceda, C.; Escalona, M.; Sandoval, J.; Canal-Villanueva, M.J.F.; Rodriguez, R.; DeBergh, P. The effect of headspace renewal in a Temporary Immersion Bioreactor on plantain (Musa AAB) shoot proliferation and quality. Plant Cell Tissue Organ 2005, 84, 155–163. [Google Scholar] [CrossRef]
- El-Hawaz, R.F.; Bridges, W.C.; Adelberg, J.W. In Vitro Growth of Curcuma longa L. in Response to Five Mineral Elements and Plant Density in Fed-Batch Culture Systems. PLoS ONE 2015, 10, e0118912. [Google Scholar] [CrossRef]
- Aragón, C.E.; Escalona, M.; Rodríguez, R.; Canal, M.J.; Capote, I.; Pina, D.; González-Olmedo, J.L. Effect of sucrose, light, and carbon dioxide on plantain micropropagation in temporary immersion bioreactors. Vitr. Cell. Dev. Biol. Anim. 2010, 46, 89. [Google Scholar] [CrossRef]
- Kevers, C.; Franck, T.; Strasser, R.J.; Dommes, J.; Gaspar, T. Hyperhydricity of Micropropagated Shoots: A Typically Stress-induced Change of Physiological State. Plant Cell Tissue Organ 2004, 77, 181–191. [Google Scholar] [CrossRef]
- José, M.C.S.; Blázquez, N.; Cernadas, M.J.; Janeiro, L.V.; Cuenca, B.; Sánchez, C.; Vidal, N. Temporary immersion systems to improve alder micropropagation. Plant Cell Tissue Organ 2020, 143, 265–275. [Google Scholar] [CrossRef]
- Hesami, M.; Jones, A.M.P. Application of artificial intelligence models and optimization algorithms in plant cell and tissue culture. Appl. Microbiol. Biotechnol. 2020, 104, 9449–9485. [Google Scholar] [CrossRef]
- Adelberg, J.; Driesse, T.; Halloran, S.; Bridges, W.C. Relationships between nutrients and plant density in liquid media during micropropagation and acclimatization of turmeric. Vitr. Cell. Dev. Biol. Anim. 2013, 49, 724–736. [Google Scholar] [CrossRef]
- Gupta, S.D.; Jatothu, B. Fundamentals and applications of light-emitting diodes (LEDs) in plant growth and morphogenesis. Plant Biotechnol. Rep. 2013, 7, 211–220. [Google Scholar] [CrossRef]
- Miler, N.; Kulus, D.; Woźny, A.; Rymarz, D.; Hajzer, M.; Wierzbowski, K.; Nelke, R.; Szeffs, L. Application of wide-spectrum light-emitting diodes in micropropagation of popular ornamental plant species: A study on plant quality and cost reduction. Vitr. Cell. Dev. Biol. Plant 2018, 55, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Wangchuk, K.; Manochai, B.; Chulaka, P.; Wongchaochant, S.; Chintakovid, W.; Pumprasert, J. Monitoring of active constituents of turmeric (Curcuma longa L.) rhizome stored under supplemented white LED-light with different light intensities. Acta Hortic. 2019, 131–138. [Google Scholar] [CrossRef]
- Mendonça, E.G.; Stein, V.C.; De Carvalho, H.H.; Santos, B.R.; Beijo, L.; Paiva, L.V. The Use of Continuous, Temporary Immersion Bioreactor System and Semisolid Culture Medium for the Production of Eucalyptus camaldulensis CLONES. Ciência Florest. 2016, 26, 1211–1224. [Google Scholar] [CrossRef] [Green Version]
- Escalona, M.; Lorenzo, J.C.; González, B.; Daquinta, M.; González, J.L.; Desjardins, Y.; Borroto, C.G. Pineapple (Ananas comosus L. Merr) micropropagation in temporary immersion systems. Plant Cell Rep. 1999, 18, 743–748. [Google Scholar] [CrossRef]
- Carvalho, L.S.O.; Ozudogru, E.A.; Lambardi, M.; Paiva, L.V. Temporary Immersion System for Micropropagation of Tree Species: A Bibliographic and Systematic Review. Not. Bot. Horti Agrobot. 2019, 47, 269–277. [Google Scholar] [CrossRef] [Green Version]
- Grzegorczyk-Karolak, I.; Grąbkowska, R.; Piątczak, E. Plant Liquid Cultures as a Source of Bioactive Metabolites. Plant Cell Tissue Differ. Second. Metab. Fundam. Appl. 2021, 743–771. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- McCree, K. The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. J. Agric. Meteorol. 1971, 9, 191–216. [Google Scholar] [CrossRef]
- Adelberg, J.; Cousins, M. Thin Films of Liquid Media for Heterotrophic Growth and Storage Organ Development: Turmeric (Curcuma longa) as a Model Plant. HortScience 2006, 41, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Stanly, C.; Bhatt, A.; Keng, C.L. A comparative study of Curcuma zedoaria and Zingiber zerumbet plantlet production using different micropropagation systems. Afr. J. Biotechnol. 2010, 9, 4326–4333. [Google Scholar]
- Schaer, J.A.; Mandoli, D.F.; Briggs, W.R. Phytochrome-Mediated Cellular Photomorphogenesis. Plant Physiol. 1983, 72, 706–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boss, P.K.; Bastow, R.M.; Mylne, J.S.; Dean, C. Multiple Pathways in the Decision to Flower: Enabling, Promoting, and Resetting. Plant Cell 2004, 16, S18–S31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Runkle, E.; Heins, R.D. Specific Functions of Red, Far Red, and Blue Light in Flowering and Stem Extension of Long-day Plants. J. Am. Soc. Hortic. Sci. 2001, 126, 275–282. [Google Scholar] [CrossRef] [Green Version]
- Lin, C. Plant blue-light receptors. Trends Plant Sci. 2000, 5, 337–342. [Google Scholar] [CrossRef]
- Banerjee, R.; Batschauer, A. Plant blue-light receptors. Planta 2005, 20, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Fraikin, G.Y.; Strakhovskaya, M.G.; Rubin, A.B. Biological photoreceptors of light-dependent regulatory processes. Biochemistry 2013, 78, 1238–1253. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gu, M.; Cui, J.; Shi, K.; Zhou, Y.; Yu, J. Effects of light quality on CO2 assimilation, chlorophyll-fluorescence quenching, expression of Calvin cycle genes and carbohydrate accumulation in Cucumis sativus. J. Photochem. Photobiol. B Biol. 2009, 96, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Kopsell, D.; Sams, C.E.; Morrow, R.C. Blue Wavelengths from LED Lighting Increase Nutritionally Important Metabolites in Specialty Crops. HortScience 2015, 50, 1285–1288. [Google Scholar] [CrossRef] [Green Version]
- Brazaitytė, A.; Miliauskienė, J.; Vaštakaitė-Kairienė, V.; Sutulienė, R.; Laužikė, K.; Duchovskis, P.; Małek, S. Effect of Different Ratios of Blue and Red LED Light on Brassicaceae Microgreens under a Controlled Environment. Plants 2021, 10, 801. [Google Scholar] [CrossRef]



| LED Source | YPFD (μmol m−2 s−1) | Ratio Red: Blue | DLI (mol m−2) | |
|---|---|---|---|---|
| Red | Blue | |||
| White | 2.2092 | 2.7178 | ≈1:1 | 0.8609 |
| RB1 | 25.531 | 5.4207 | ≈5:1 | 3.0226 |
| RB2 | 25.975 | 11.733 | ≈2:1 | 3.8047 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchant, M.J.; Molina, P.; Montecinos, M.; Guzmán, L.; Balada, C.; Fassio, C.; Castro, M. In Vitro Propagation of Easter Island Curcuma longa from Rhizome Explants Using Temporary Immersion System. Agronomy 2021, 11, 2121. https://doi.org/10.3390/agronomy11112121
Marchant MJ, Molina P, Montecinos M, Guzmán L, Balada C, Fassio C, Castro M. In Vitro Propagation of Easter Island Curcuma longa from Rhizome Explants Using Temporary Immersion System. Agronomy. 2021; 11(11):2121. https://doi.org/10.3390/agronomy11112121
Chicago/Turabian StyleMarchant, María José, Paula Molina, Miriam Montecinos, Leda Guzmán, Cristobal Balada, Claudia Fassio, and Mónica Castro. 2021. "In Vitro Propagation of Easter Island Curcuma longa from Rhizome Explants Using Temporary Immersion System" Agronomy 11, no. 11: 2121. https://doi.org/10.3390/agronomy11112121
APA StyleMarchant, M. J., Molina, P., Montecinos, M., Guzmán, L., Balada, C., Fassio, C., & Castro, M. (2021). In Vitro Propagation of Easter Island Curcuma longa from Rhizome Explants Using Temporary Immersion System. Agronomy, 11(11), 2121. https://doi.org/10.3390/agronomy11112121






