Reduction of Ammonia Emissions from Laying Hen Manure in a Closed Composting Process Using Gas-Permeable Membrane Technology
Abstract
:1. Introduction
2. Materials and Methods
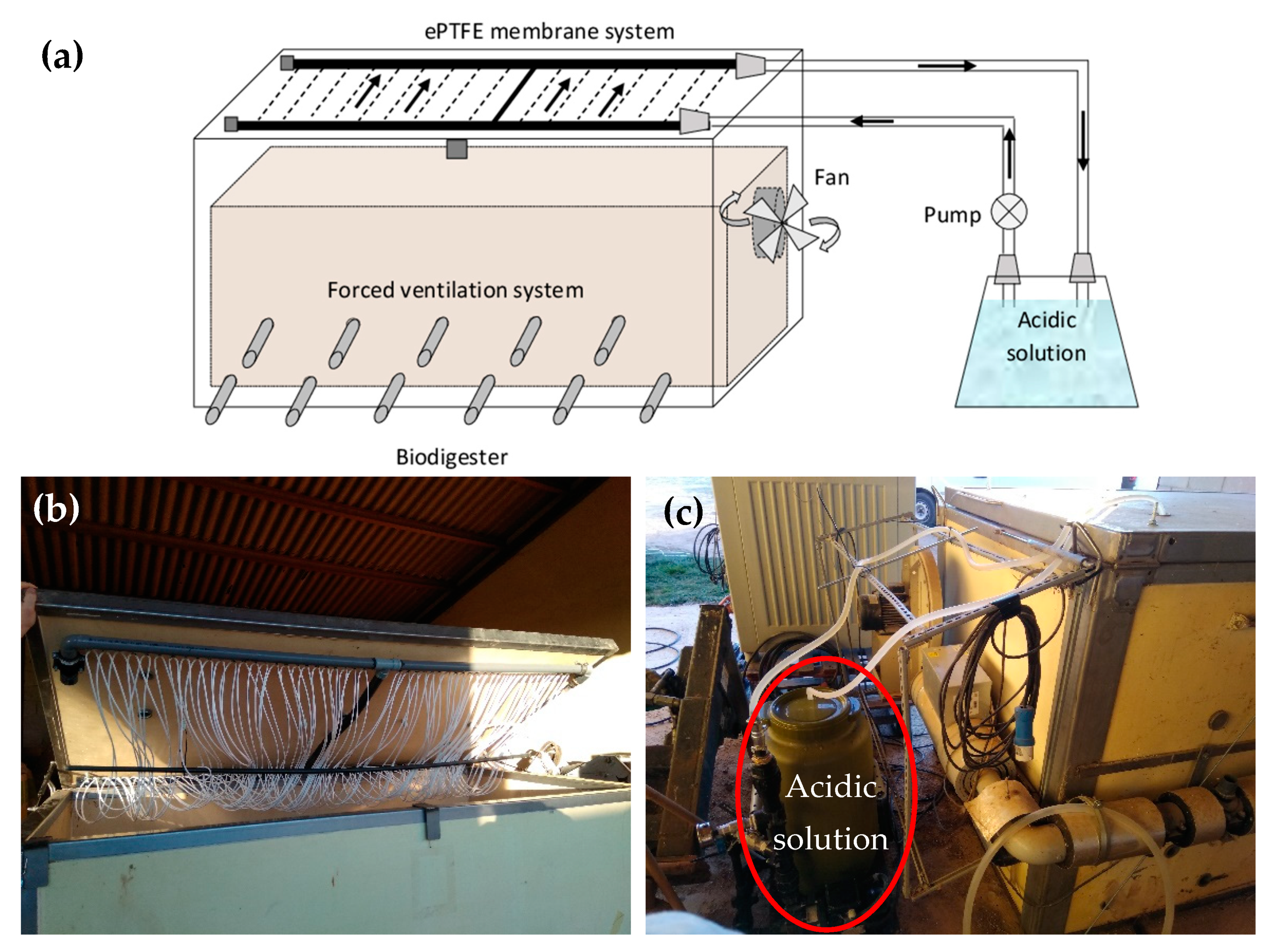
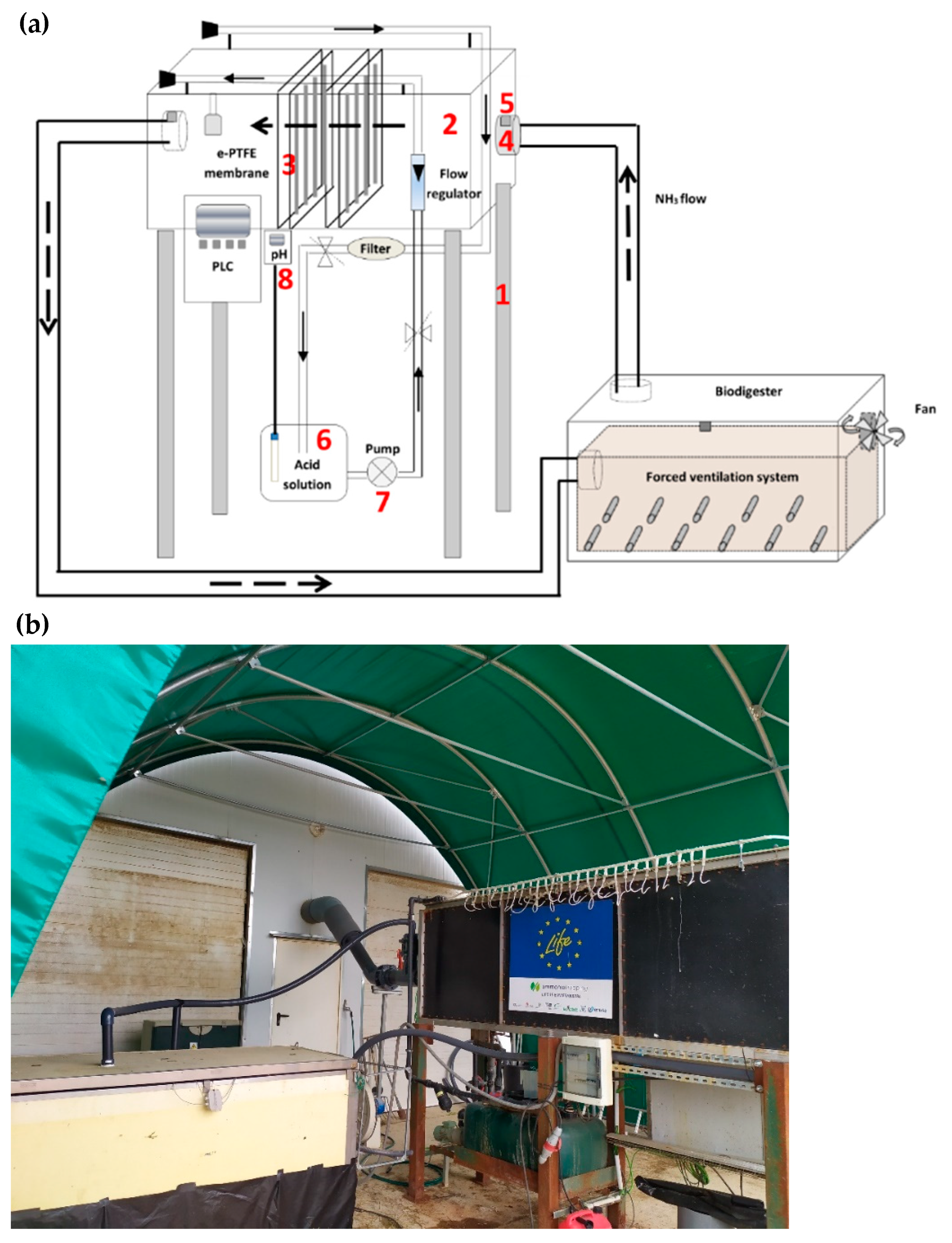
2.1. First System (S1): ePTFE Membranes Installed Inside the Portable Closed Aerobic Composter
2.2. Second System (S2): ePTFE Membranes Placed in a Compartment Outside the Portable Closed Aerobic Composter
2.3. Experimental Conditions
2.4. Physicochemical Analyses
2.5. Calculations
3. Results and Discussion
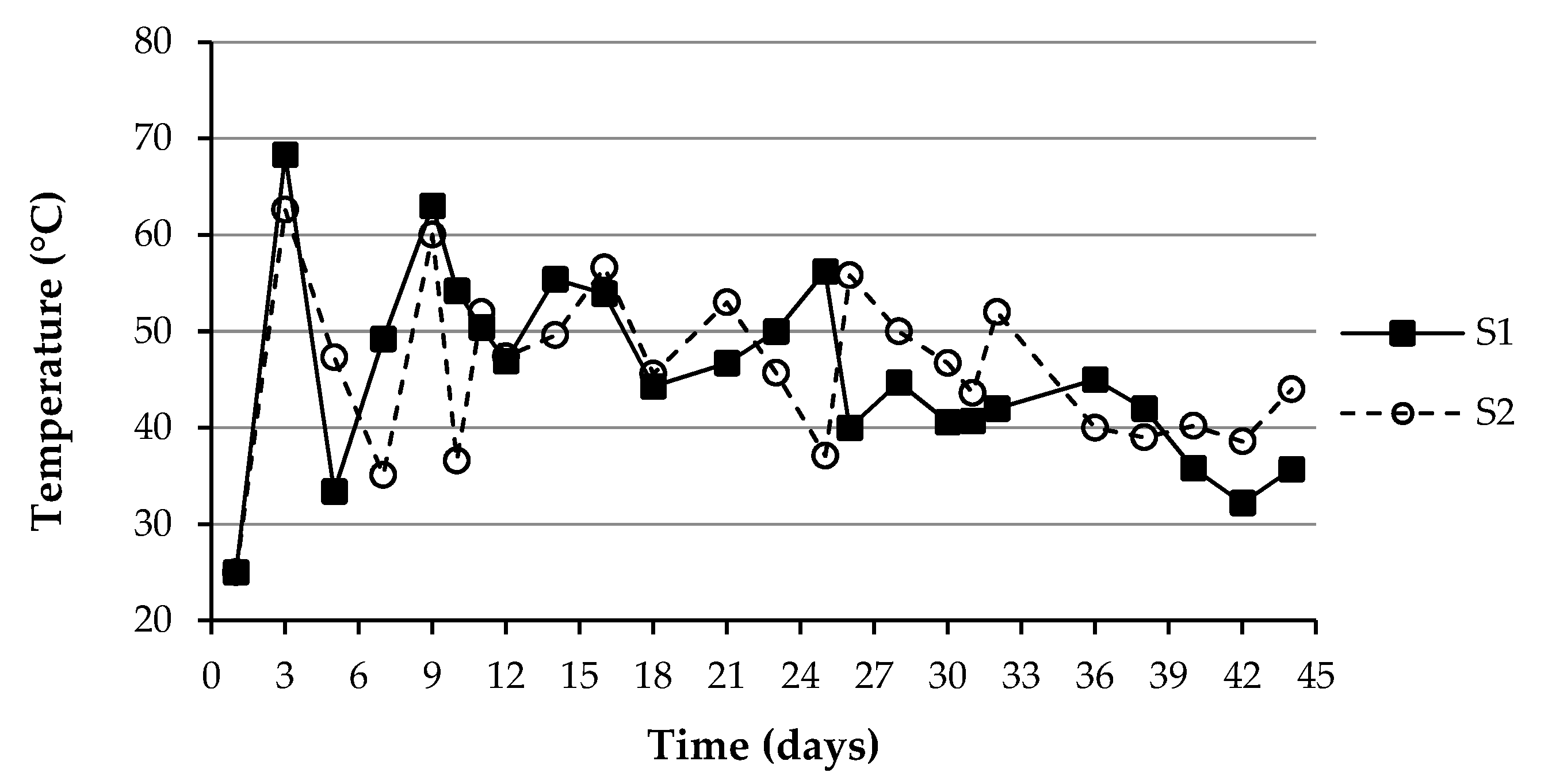
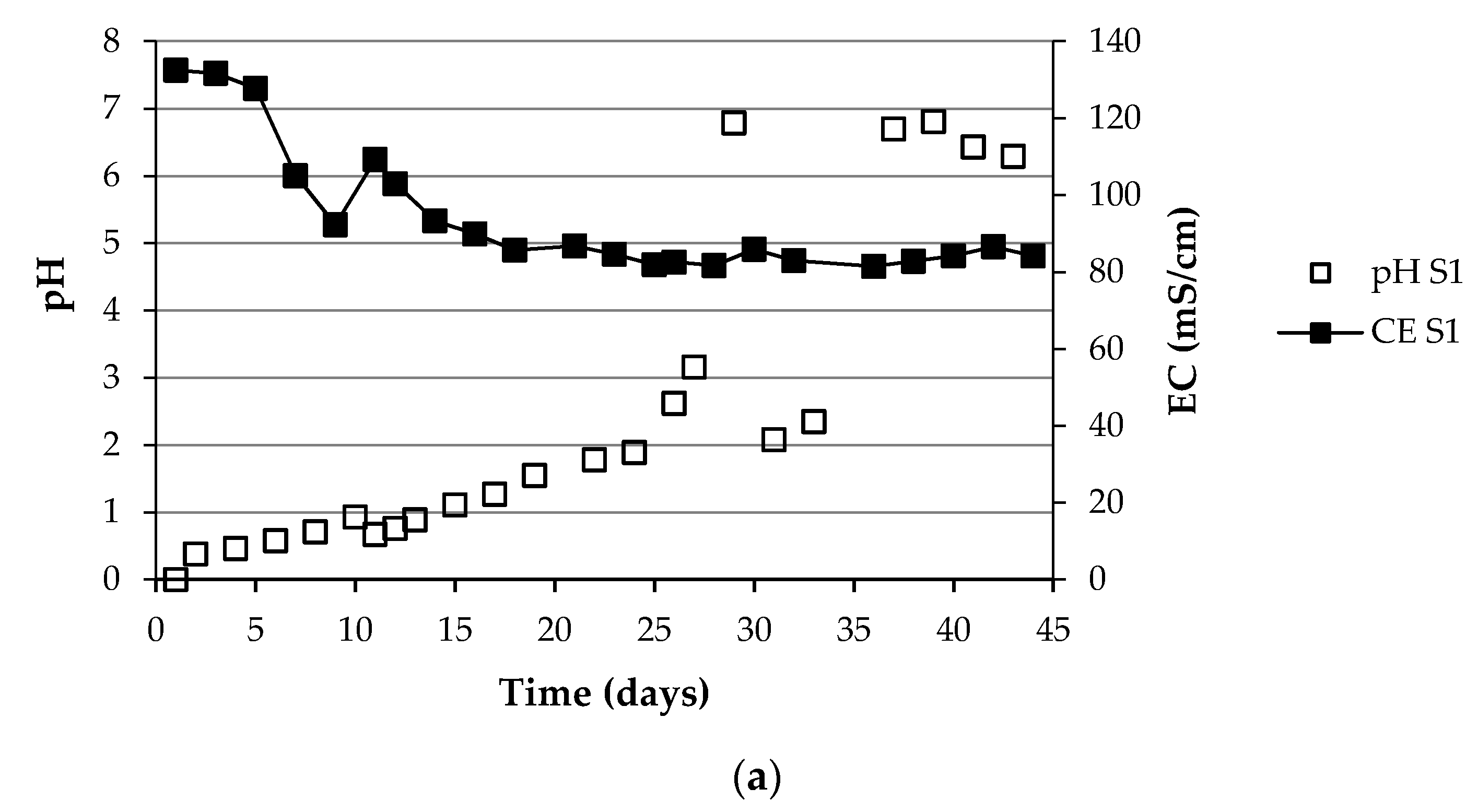
3.1. Changes in the Fundamental Physicochemical Parameters during the Composting Process
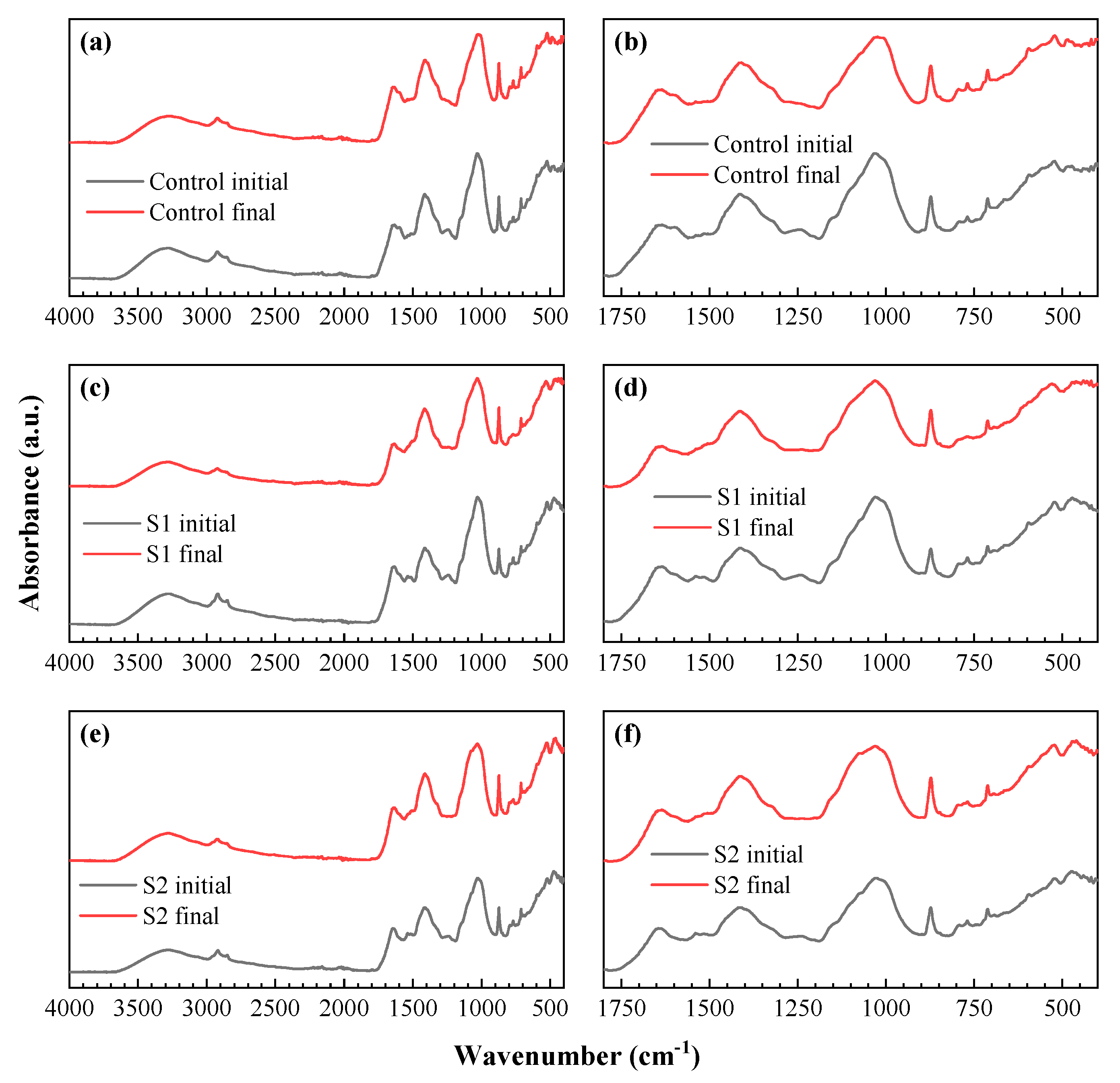
3.2. Vibrational Analysis of the Product Mixtures by Infrared Spectroscopy
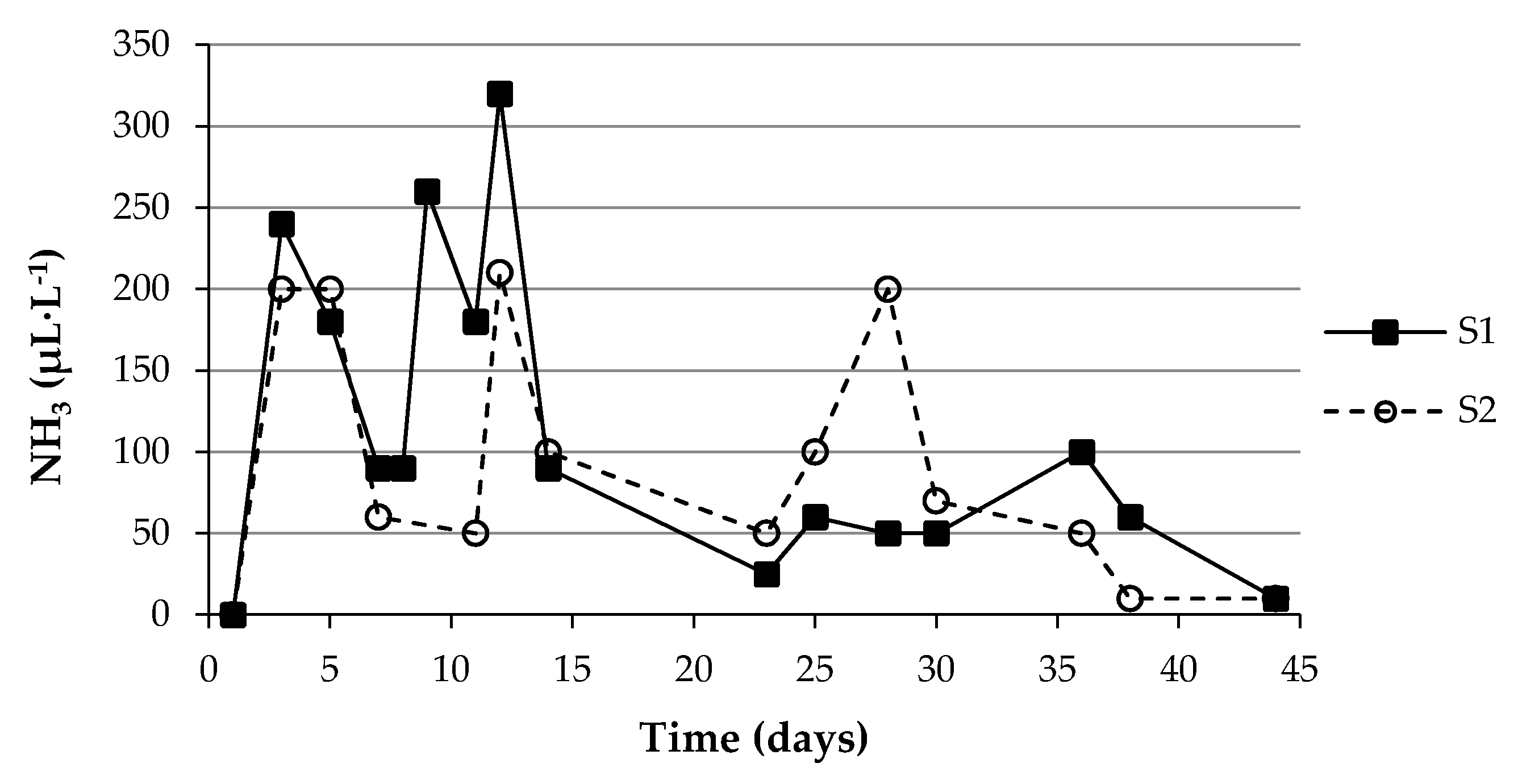
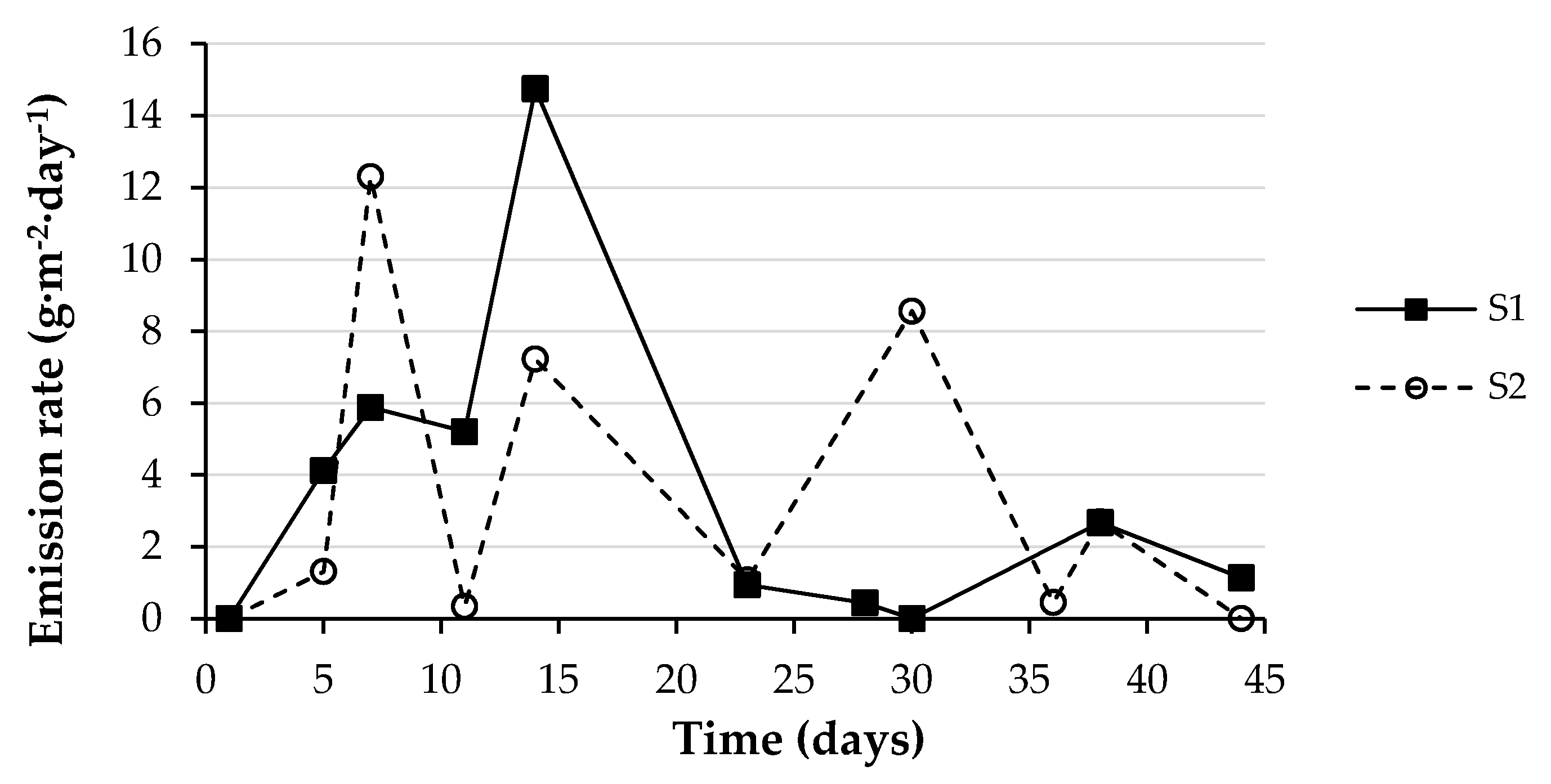
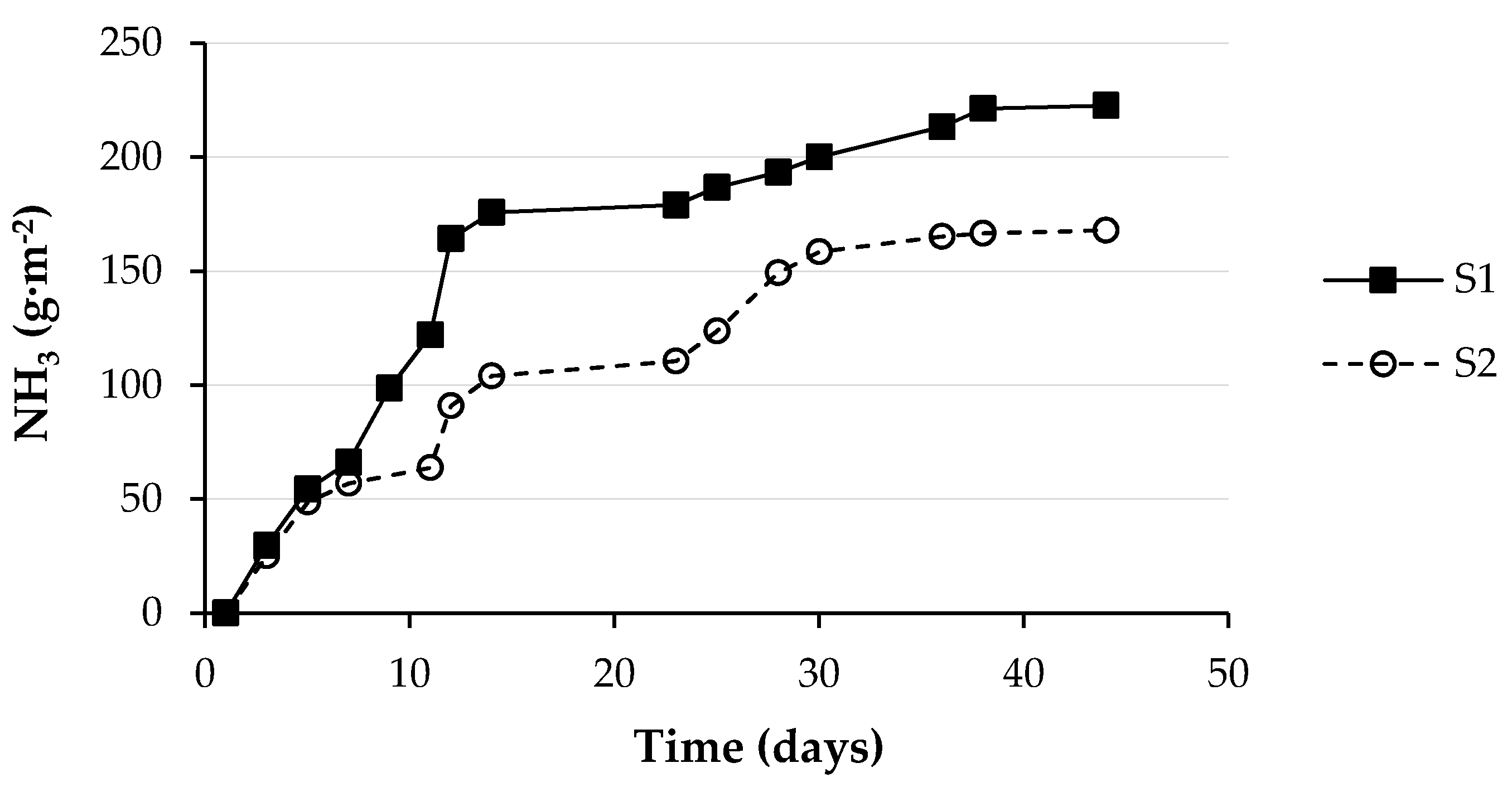
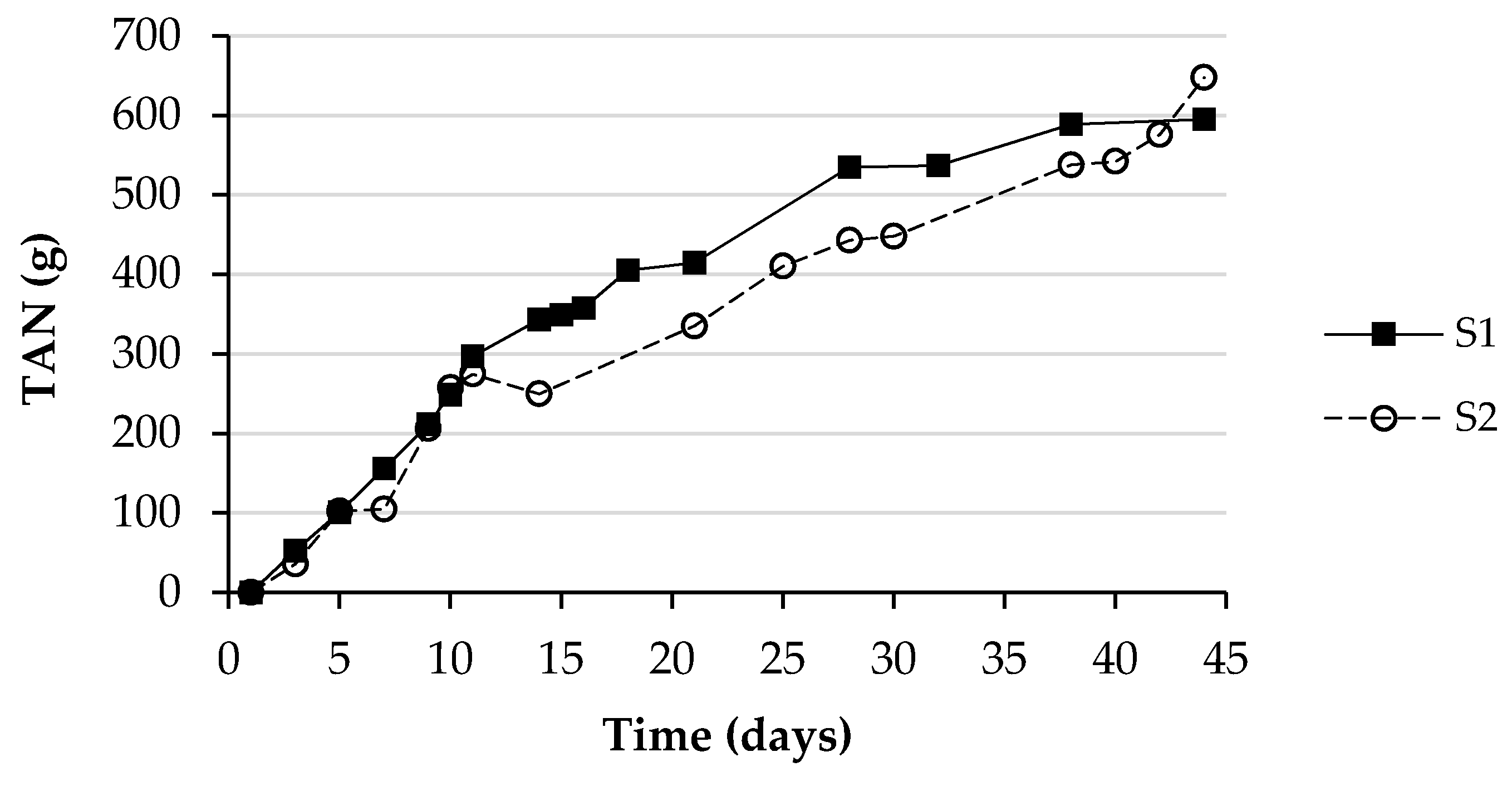
3.3. Ammonia Emissions
3.4. Operational Parameters of the GPM Systems
3.5. Comparison with Other GPM-Based Systems Used in Manure Management
3.6. Economic Assessment
3.7. Applicability of the Evaluated Membrane Composting Systems
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foged, H.; Flotats Ripoll, X.; Bonmatí Blasi, A.; Palatsi Civit, J.; Magrí Aloy, A.; Schelde, K.M. Inventory of manure processing activities in Europe. Technical Report No. I concerning “Manure Processing Activities in Europe” to the European Commission, Directorate-General Environment. 2011, p. 138. Available online: https://op.europa.eu/es/publication-detail/-/publication/d629448f-d26a-4829-a220-136aad51d1d9 (accessed on 14 November 2021).
- Toledo, M.; Gutiérrez, M.C.; Peña, A.; Siles, J.A.; Martín, M.A. Co-composting of chicken manure, alperujo, olive leaves/pruning and cereal straw at full-scale: Compost quality assessment and odour emission. Process. Saf. Environ. Prot. 2020, 139, 362–370. [Google Scholar] [CrossRef]
- Ministerio de Agricultura Pesca y Alimentación. El Sector de la Avicultura de Puesta en Cifras: Principales Indicadores Económicos; Subdirección General de Producciones Ganaderas y Cinegéticas. Dirección General de Producciones y Mercados Agrarios: Madrid, Spain, 2021.
- Bernal, M.; Bescós, B.; Burgos, L.; Bustamante, M.A.; Clemente, R.; Fabbri, C.; Flotats, X.; García-González, M.C.; Herrero, E.; Mattachini, G.; et al. Evaluación de Sistemas de Gestión de Estiércol en Europa; Sociedad Aragonesa de Gestión Ambiental: Zaragoza, Spain, 2015. [Google Scholar]
- ApSimon, H.M.; Kruse, M.; Bell, J.N.B. Ammonia emissions and their role in acid deposition. Atmos. Environ. 1987, 21, 1939–1946. [Google Scholar] [CrossRef]
- Bindra, N.; Dubey, B.; Dutta, A. Technological and life cycle assessment of organics processing odour control technologies. Sci. Total Environ. 2015, 527–528, 401–412. [Google Scholar] [CrossRef]
- Zhao, L.; Hadlocon, L.J.S.; Manuzon, R.B.; Darr, M.J.; Keener, H.M.; Heber, A.J.; Ni, J. Ammonia concentrations and emission rates at a commercial poultry manure composting facility. Biosyst. Eng. 2016, 150, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Wood, D.; Cowherd, S.; Van Heyst, B. A summary of ammonia emission factors and quality criteria for commercial poultry production in North America. Atmos. Environ. 2015, 115, 236–245. [Google Scholar] [CrossRef]
- European Environment Agency. National Emission Ceilings Directive. In NEC Directive Reporting Status; European Environment Agency: Copenhagen, Denmark, 2019; pp. 7–50. Available online: https://www.eea.europa.eu/publications/nec-directive-reporting-status-2019 (accessed on 14 November 2021).
- EC-European Commission. Directive (EU) 2016/2284 of the European Parliament and of the Council of 14 December 2016 on the reduction of national emissions of certain atmospheric pollutants, amending Directive 2003/35/EC and repealing Directive 2001/81/EC. O_. J. Eur. Commun. 2016, 344, 1–31. [Google Scholar]
- Dirección General de Calidad y Evaluación Ambiental. Proyecciones de Emisiones a la Atmósfera, Edición 2021, Resumen de Resultados; Ministerio para la Transición Ecológica y Reto Demográfico: Madrid, Spain, 2021; 61p. Available online: https://www.miteco.gob.es/es/calidad-y-evaluacion-ambiental/temas/sistema-espanol-de-inventario-sei-/210315-informeproyecciones-2021_tcm30-523951.pdf (accessed on 14 November 2021).
- Wing, S.; Wolf, S. Intensive livestock operations, health, and quality of life among residents of eastern North Carolina. Environ. Health Perspect. 2000, 108, 233–238. [Google Scholar] [CrossRef]
- Spencer, J.L.; Van Heyst, B.J. Effect of different intermediate amendments on pH and ammonia emissions of composted poultry mortalities. J. Appl. Poult. Res. 2013, 22, 700–714. [Google Scholar] [CrossRef]
- Erisman, J.W. The Nanjing declaration on management of reactive nitrogen. Bioscience 2004, 54, 286–287. [Google Scholar] [CrossRef] [Green Version]
- Bustamante, M.A.; Restrepo, A.P.; Alburquerque, J.A.; Pérez-Murcia, M.D.; Paredes, C.; Moral, R.; Bernal, M.P. Recycling of anaerobic digestates by composting: Effect of the bulking agent used. J. Clean. Prod. 2013, 47, 61–69. [Google Scholar] [CrossRef]
- Roman, P.; Martinez, M.M.; Pantoja, A. Farmer’s Compost Handbook Experiences in Latin America; Food and Agriculture Organization: Santiago, Chile, 2015. [Google Scholar]
- Bolan, N.S.; Szogi, A.A.; Chuasavathi, T.; Seshadri, B.; Rothrock, M.J.; Panneerselvam, P. Uses and management of poultry litter. Worlds. Poult. Sci. J. 2010, 66, 673–698. [Google Scholar] [CrossRef] [Green Version]
- Agyarko-Mintah, E.; Cowie, A.; Van Zwieten, L.; Singh, B.P.; Smillie, R.; Harden, S.; Fornasier, F. Biochar lowers ammonia emission and improves nitrogen retention in poultry litter composting. Waste Manag. 2017, 61, 129–137. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, X. Microbiological Safety of Chicken Litter or Chicken Litter-Based Organic Fertilizers: A Review. Agriculture 2014, 4, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Ravindran, B.; Mupambwa, H.A.; Silwana, S.; Mnkeni, P.N.S. Assessment of nutrient quality, heavy metals and phytotoxic properties of chicken manure on selected commercial vegetable crops. Heliyon 2017, 3, e00493. [Google Scholar] [CrossRef] [PubMed]
- Martins, O.; Dewes, T. Loss of nitrogenous compounds during composting of animal wastes. Bioresour. Technol. 1992, 42, 103–111. [Google Scholar] [CrossRef]
- Beck-Friis, B.; Smårs, S.; Jönsson, H.; Kirchmann, H. Gaseous emissions of carbon dioxide, ammonia and nitrous oxide from organic household waste in a compost reactor under different temperature regimes. J. Agric. Eng. Res. 2001, 78, 423–430. [Google Scholar] [CrossRef]
- Wang, S.; Zeng, Y. Ammonia emission mitigation in food waste composting: A review. Bioresour. Technol. 2018, 248, 13–19. [Google Scholar] [CrossRef]
- Ouatmane, A.; Provenzano, M.R.; Hafidi, M.; Senesi, N. Compost maturity assessment using calorimetry, spectroscopy and chemical analysis. Compost Sci. Util. 2000, 8, 124–134. [Google Scholar] [CrossRef]
- Yang, Y.; Awasthi, M.K.; Ren, X.; Guo, H.; Lv, J. Effect of bean dregs on nitrogen transformation and bacterial dynamics during pig manure composting. Bioresour. Technol. 2019, 288, 121430. [Google Scholar] [CrossRef]
- Adani, F.; Genevini, P.L.; Gasperi, F.; Zorzi, G. Organic matter evolution index (omei) as a measure of composting efficiency. Compost Sci. Util. 1997, 5, 53–62. [Google Scholar] [CrossRef]
- Inbar, Y.; Chen, Y.; Hadar, Y. Humic substances formed during composting of organic matter. J. Soil Sci. Soc. Am. 1990, 54, 1316–1323. [Google Scholar] [CrossRef]
- Pagans, E.; Barrena, R.; Font, X.; Sánchez, A. Ammonia emissions from the composting of different organic wastes. Dependency on process temperature. Chemosphere 2006, 62, 1534–1542. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.; Qiu, X.; Chen, L.; Zhang, C.; Ma, D.; Zhang, J. Succession of organics metabolic function of bacterial community in response to addition of earthworm casts and zeolite in maize straw composting. Bioresour. Technol. 2019, 280, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, M.K.; Wang, M.; Chen, H.; Wang, Q.; Zhao, J.; Ren, X.; Li, D.S.; Awasthi, S.K.; Shen, F.; Li, R.; et al. Heterogeneity of biochar amendment to improve the carbon and nitrogen sequestration through reduce the greenhouse gases emissions during sewage sludge composting. Bioresour. Technol. 2017, 224, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Jürgen Hellebrand, H.; Kalk, W.-D. Emission of methane, nitrous oxide and ammonia from manure rows. Nutr. Cycles Agroec. Syst. 2001, 60, 83–87. [Google Scholar] [CrossRef]
- Li, S.; Huang, G.H.; An, C.J.; Yu, H. Effect of different buffer agents on in-vessel composting of food waste: Performance analysis and comparative study. J. Environ. Sci. Heal. Part A Toxic/Hazardous Subst. Environ. Eng. 2013, 48, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Huang, G.H. Effects of sodium acetate as a pH control amendment on the composting of food waste. Bioresour. Technol. 2009, 100, 2005–2011. [Google Scholar] [CrossRef]
- Velasco-Velasco, J.; Parkinson, R.; Kuri, V. Ammonia emissions during vermicomposting of sheep manure. Bioresour. Technol. 2011, 102, 10959–10964. [Google Scholar] [CrossRef]
- Andersson, M. Performance of bedding materials in affecting ammonia emissions from pig manure. J. Agric. Eng. Res. 1996, 65, 213–222. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, X.; Bai, Z.; Chadwick, D.; Misselbrook, T.; Sommer, S.G.; Qin, W.; Ma, L. Mitigation of ammonia, nitrous oxide and methane emissions during solid waste composting with different additives: A meta-analysis. J. Clean. Prod. 2019, 235, 626–635. [Google Scholar] [CrossRef]
- Shan, G.; Li, W.; Gao, Y.; Tan, W.; Xi, B. Additives for reducing nitrogen loss during composting: A review. J. Clean. Prod. 2021, 307, 127308. [Google Scholar] [CrossRef]
- Jiang, T.; Ma, X.; Tang, Q.; Yang, J.; Li, G.; Schuchardt, F. Combined use of nitrification inhibitor and struvite crystallization to reduce the NH3 and N2O emissions during composting. Bioresour. Technol. 2016, 217, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, W.; Chen, L.; Meng, L.; Zheng, Z. Effect of enriched thermotolerant nitrifying bacteria inoculation on reducing nitrogen loss during sewage sludge composting. Bioresour. Technol. 2020, 311, 123461. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Akdeniz, N.; Yi, S. Biochar-amended poultry mortality composting to increase compost temperatures, reduce ammonia emissions, and decrease leachate’s chemical oxygen demand. Agric. Ecosyst. Environ. 2021, 315, 107451. [Google Scholar] [CrossRef]
- Janczak, D.; Malińska, K.; Czekała, W.; Cáceres, R.; Lewicki, A.; Dach, J. Biochar to reduce ammonia emissions in gaseous and liquid phase during composting of poultry manure with wheat straw. Waste Manag. 2017, 66, 36–45. [Google Scholar] [CrossRef]
- Bei Li, Y.; Ting Liu, T.; Li Song, J.; Hua Lv, J.; Shao Jiang, J. Effects of chemical additives on emissions of ammonia and greenhouse gas during sewage sludge composting. Process. Saf. Environ. Prot. 2020, 143, 129–137. [Google Scholar] [CrossRef]
- Ren, L.; Schuchardt, F.; Shen, Y.; Li, G.; Li, C. Impact of struvite crystallization on nitrogen losses during composting of pig manure and cornstalk. Waste Manag. 2010, 30, 885–892. [Google Scholar] [CrossRef]
- Chen, H.; Awasthi, M.K.; Liu, T.; Zhao, J.; Ren, X.; Wang, M.; Duan, Y.; Awasthi, S.K.; Zhang, Z. Influence of clay as additive on greenhouse gases emission and maturity evaluation during chicken manure composting. Bioresour. Technol. 2018, 266, 82–88. [Google Scholar] [CrossRef]
- Masse, L.; Massé, D.I.; Pellerin, Y.; Dubreuil, J. Osmotic pressure and substrate resistance during the concentration of manure nutrients by reverse osmosis membranes. J. Memb. Sci. 2010, 348, 28–33. [Google Scholar] [CrossRef]
- Bonmatí, A.; Flotats, X. Air stripping of ammonia from pig slurry: Characterisation and feasibility as a pre- or post-treatment to mesophilic anaerobic digestion. Waste Manag. 2003, 23, 261–272. [Google Scholar] [CrossRef]
- Montégut, G.; Michelin, L.; Brendlé, J.; Lebeau, B.; Patarin, J. Ammonium and potassium removal from swine liquid manure using clinoptilolite, chabazite and faujasite zeolites. J. Environ. Manag. 2016, 167, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Uludag-Demirer, S.; Demirer, G.N.; Chen, S. Ammonia removal from anaerobically digested dairy manure by struvite precipitation. Process. Biochem. 2005, 40, 3667–3674. [Google Scholar] [CrossRef]
- Bellahsen, N.; Varga, G.; Halyag, N.; Kertész, S.; Tombácz, E.; Hodúr, C. Pomegranate peel as a new low-cost adsorbent for ammonium removal. Int. J. Environ. Sci. Technol. 2021, 18, 711–722. [Google Scholar] [CrossRef]
- Garcia-González, M.C.; Vanotti, M.B. Recovery of ammonia from swine manure using gas-permeable membranes: Effect of waste strength and pH. Waste Manag. 2015, 38, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Vanotti, M.B.; Szogi, A.A. Removal and Recovery of Ammonia from Liquid Manure Using Gas-Permeable Membranes. In Proceedings of the ASABE Annual International Meeting, Pittsburgh, PA, USA, 20–23 July 2010. [Google Scholar] [CrossRef]
- Daguerre-Martini, S.; Vanotti, M.B.; Rodriguez-Pastor, M.; Rosal, A.; Moral, R. Nitrogen recovery from wastewater using gas-permeable membranes: Impact of inorganic carbon content and natural organic matter. Water Res. 2018, 137, 201–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Kankanamge, N.R.; Chow, C.; Welsh, D.T.; Li, T.; Teasdale, P.R. Removing ammonium from water and wastewater using cost-effective adsorbents: A review. J. Environ. Sci. 2018, 63, 174–197. [Google Scholar] [CrossRef]
- Zarebska, A.; Romero Nieto, D.; Christensen, K.V.; Fjerbæk Søtoft, L.; Norddahl, B. Ammonium fertilizers production from manure: A critical review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1469–1521. [Google Scholar] [CrossRef]
- Soto-Herranz, M.; Sánchez-Báscones, M.; Antolín-Rodríguez, J.M.; Vanotti, M.B.; Martín-Ramos, P. Effect of acid flow rate, membrane surface area, and capture solution on the effectiveness of suspended gpm systems to recover ammonia. Membranes 2021, 11, 538. [Google Scholar] [CrossRef]
- Sun, X.; Ma, S.; Han, L.; Li, R.; Schlick, U.; Chen, P.; Huang, G. The effect of a semi-permeable membrane-covered composting system on greenhouse gas and ammonia emissions in the Tibetan Plateau. J. Clean. Prod. 2018, 204, 778–787. [Google Scholar] [CrossRef]
- Ma, S.; Xiong, J.; Cui, R.; Sun, X.; Han, L.; Xu, Y.; Kan, Z.; Gong, X.; Huang, G. Effects of intermittent aeration on greenhouse gas emissions and bacterial community succession during large-scale membrane-covered aerobic composting. J. Clean. Prod. 2020, 266, 121551. [Google Scholar] [CrossRef]
- Watanabe, F.S.; Olsen, S.R. Test of an ascorbic acid method for the determination of phosphorus in water and NaHCO3 extracts from the soil. J. Soil Sci. Soc. Am. 1965, 29, 677–678. [Google Scholar] [CrossRef]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties, 9.2.2, 2nd ed.; Page, A.L., Ed.; American Society for Soil Sciences: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar] [CrossRef]
- Rice, E.W.; Baird, R.B.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2017. [Google Scholar]
- Schneider, M.; Marison, I.W.; Von Stockar, U. Principles of an efficient new method for the removal of ammonia from animal cell cultures using hydrophobic membranes. Enzym. Microb. Technol. 1994, 16, 957–963. [Google Scholar] [CrossRef]
- Blet, V.; Pons, M.N.; Greffe, J.L. Separation of ammonia with a gas-permeable tubular membrane. Anal. Chim. Acta 1989, 219, 309–311. [Google Scholar] [CrossRef]
- Sommer, S.G.; Møller, H.B. Emission of greenhouse gases during composting of deep litter from pig production—Effect of straw content. J. Agric. Sci. 2000, 134, 327–335. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Liu, Y.; Yong, X.; Wu, X.; Jia, H.; Wong, J.W.C.; Wu, H.; Zhou, J. Odor emission and microbial community succession during biogas residue composting covered with a molecular membrane. Bioresour. Technol. 2020, 297, 122518. [Google Scholar] [CrossRef]
- Guo, R.; Li, G.; Jiang, T.; Schuchardt, F.; Chen, T.; Zhao, Y.; Shen, Y. Effect of aeration rate, C/N ratio and moisture content on the stability and maturity of compost. Bioresour. Technol. 2012, 112, 171–178. [Google Scholar] [CrossRef]
- Chowdhury, M.A.; de Neergaard, A.; Jensen, L.S. Potential of aeration flow rate and bio-char addition to reduce greenhouse gas and ammonia emissions during manure composting. Chemosphere 2014, 97, 16–25. [Google Scholar] [CrossRef]
- Sánchez-Monedero, M.A.; Roig, A.; Paredes, C.; Bernal, M.P. Nitrogen transformation during organic waste composting by the Rutgers system and its effects on pH, EC and maturity of the composting mixtures. Bioresour. Technol. 2001, 78, 301–308. [Google Scholar] [CrossRef]
- Tang, H.; Zheng, Y.; Chen, Y. Materials Chemistry of Nanoultrasonic Biomedicine. Adv. Mater. 2017, 29, 1–22. [Google Scholar] [CrossRef]
- Osada, T.; Kuroda, K.; Yonaga, M. Determination of nitrous oxide, methane, and ammonia emissions from a swine waste composting process. J. Mater. Cycle. Waste 2000, 2, 51–56. [Google Scholar]
- Calderón, F.; Haddix, M.; Conant, R.; Magrini-Bair, K.; Paul, E. Diffuse-Reflectance Fourier-Transform Mid-Infrared Spectroscopy as a Method of Characterizing Changes in Soil Organic Matter. Soil Sci. Soc. Am. J. 2013, 77, 1591–1600. [Google Scholar] [CrossRef] [Green Version]
- Bernabé, G.A.; Almeida, S.; Ribeiro, C.A.; Crespi, M.S. Evaluation of organic molecules originated during composting process. J. Therm. Anal. Calorim. 2011, 106, 773–778. [Google Scholar] [CrossRef]
- Razali, W.; Aizuddin, W.; Baharuddin, A.S.; Tarmezeetalib, A.; Sulaiman, A.; Naim, M.N.; Hassan, M.A.; Shirai, Y. Degradation of oil palm empty fruit bunches (OPEFB) fibre during composting process using in vessel composter. Bioresources 2012, 7, 4786–4805. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, S.B.; Portela, R.; Bazin, P.; Ávila, P.; Bañares, M.A.; Daturi, M. Transient operando study on the NH3/NH4+ interplay in V-SCR monolithic catalysts. Appl. Catal. B Environ. 2018, 224, 109–115. [Google Scholar] [CrossRef]
- Smidt, E.; Eckhardt, K.-U.; Lechner, P.; Schulten, H.-R.; Leinweber, P. Characterization of different decomposition stages of biowaste using FT-IR spectroscopy and pyrolysis-field ionisation mass spectrometry. Biodegradation 2005, 16, 67–79. [Google Scholar] [CrossRef]
- Farah Nadia, O.; Xiang, L.Y.; Lie, L.Y.; Chairil Anuar, D.; Mohd Afandi, M.P.; Azhari Baharuddin, S. Investigation of physico-chemical properties and microbial community during poultry manure co-composting process. J. Environ. Sci. 2015, 28, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Jouraiphy, A.; Amir, S.; El Gharous, M.; Revel, J.C.; Hafidi, M. Chemical and spectroscopic analysis of organic matter transformation during composting of sewage sludge and green plant waste. Int. Biodeterior. Biodegrad. 2005, 56, 101–108. [Google Scholar] [CrossRef]
- Castaldi, P.; Alberti, G.; Merella, R.; Melis, P. Study of the organic matter evolution during municipal solid waste composting aimed at identifying suitable parameters for the evaluation of compost maturity. Waste Manag. 2005, 25, 209–213. [Google Scholar] [CrossRef]
- Jarvis, Å.; Sundberg, C.; Milenkovski, S.; Pell, M.; Smårs, S.; Lindgren, P.E.; Hallin, S. Activity and composition of ammonia oxidizing bacterial communities and emission dynamics of NH3 and N2O in a compost reactor treating organic household waste. J. Appl. Microbiol. 2009, 106, 1502–1511. [Google Scholar] [CrossRef]
- Torres-Climent, A.; Gomis, P.; Martín-Mata, J.; Bustamante, M.A.; Marhuenda-Egea, F.C.; Pérez-Murcia, M.D.; Pérez-Espinosa, A.; Paredes, C.; Moral, R. Chemical, thermal and spectroscopic methods to assess biodegradation of winery-distillery wastes during composting. PLoS ONE 2015, 10, e0138925. [Google Scholar] [CrossRef] [Green Version]
- Schnitzer, M.I.; Monreal, C.M.; Facey, G.A.; Fransham, P.B. The conversion of chicken manure to biooil by fast pyrolysis I. Analyses of chicken manure, biooils and char by 13C and 1H NMR and FTIR spectrophotometry. J. Environ. Sci. Heal. Part B Pestic. Food Contam. Agric. Wastes 2007, 42, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, Y.; Suzuki, K.; Kuroda, K.; Waki, M.; Yasuda, T. Effects of struvite formation and nitratation promotion on nitrogenous emissions such as NH3, N2O and NO during swine manure composting. Bioresour. Technol. 2011, 102, 1468–1474. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Yin, H.; Han, L.; Ma, S.; He, X.; Huang, G. Effects of semi-permeable membrane covering coupled with intermittent aeration on gas emissions during aerobic composting from the solid fraction of dairy manure at industrial scale. Waste Manag. 2021, 131, 1–9. [Google Scholar] [CrossRef]
- Molinuevo-Salces, B.; Riaño, B.; Vanotti, M.B.; Hernández-González, D.; García-González, M.C. Pilot-scale demonstration of membrane-based nitrogen recovery from swine manure. Membranes 2020, 10, 270. [Google Scholar] [CrossRef]
- Rothrock, M.J.; Szögi, A.A.; Vanotti, M.B. Recovery of ammonia from poultry litter using gas-permeable membranes. Trans. ASABE 2010, 53, 1267–1275. [Google Scholar] [CrossRef]
- Lahav, O.; Mor, T.; Heber, A.J.; Molchanov, S.; Ramirez, J.C.; Li, C.; Broday, D.M. A new approach for minimizing ammonia emissions from poultry houses. Water Air Soil Pollut. 2008, 191, 183–197. [Google Scholar] [CrossRef] [Green Version]
- Rothrock, M.J.; Szögi, A.A.; Vanotti, M.B. Recovery of ammonia from poultry litter using flat gas permeable membranes. Waste Manag. 2013, 33, 1531–1538. [Google Scholar] [CrossRef]
- Moral, R.; Perez-Murcia, M.D.; Perez-Espinosa, A.; Moreno-Caselles, J.; Paredes, C. Estimation of nutrient values of pig slurries in Southeast Spain using easily determined properties. Waste Manag. 2005, 25, 719–725. [Google Scholar] [CrossRef]
- CREA. 2005. Available online: http://crea.uclm.es/siar/publicaciones/files/HOJA11.pdf (accessed on 14 November 2021).
- Miles, C.; Jonathan, R.; Elizabeth, M.; Coolong, T. Fertigation in Organic Vegetable Production Systems. Available online: https://eorganic.org/node/4937 (accessed on 14 November 2021).
- Chen, T.L.; Chen, L.H.; Lin, Y.J.; Yu, C.P.; Ma, H.W.; Chiang, P.C. Advanced ammonia nitrogen removal and recovery technology using electrokinetic and stripping process towards a sustainable nitrogen cycle: A review. J. Clean. Prod. 2021, 309, 127369. [Google Scholar] [CrossRef]
- González-García, I.; Riaño, B.; Molinuevo-Salces, B.; Vanotti, M.B.; García-González, M.C. Improved anaerobic digestion of swine manure by simultaneous ammonia recovery using gas-permeable membranes. Water Res. 2021, 190, 116789. [Google Scholar] [CrossRef]
- Ma, S.; Sun, X.; Han, L.; Li, R.; Schlick, U.W.E.; Huang, G. Reduction of ammonia emission during membrane-covered aerobic composting. Trans. Chinese Soc. Agric. 2017, 48, 334–349. [Google Scholar] [CrossRef]
- Sun, X.; Ma, S.; Han, L.; Huang, G. Design and test on lab-scale intelligent membrane-covered aerobic composting reactor. Trans. Chin. Soc. Agric. Mach. 2016, 47, 240–245. [Google Scholar] [CrossRef]
- Kim, J.K.; Lee, D.J.; Ravindran, B.; Jeong, K.-H.; Wong, J.W.-C.; Selvam, A.; Karthikeyan, O.P.; Kwag, J.-H. Evaluation of integrated ammonia recovery technology and nutrient status with an in-vessel composting process for swine manure. Bioresour. Technol. 2017, 245, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Dube, P.; Vanotti, M.; Szogi, A.; González, M.C.G. Enhancing recovery of ammonia from swine manure anaerobic digester effluent using gas-permeable membrane technology. Waste Manag. 2016, 49, 372–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melse, R.W.; Ogink, N.W.M. Air scrubbing techniques for ammonia and odor reduction at livestock operations: Review of on-farm research in the Netherlands. Trans. ASAE 2005, 48, 2303–2313. [Google Scholar] [CrossRef]
- Lais, S.; Buscher, W. Reduzierung von Ammoniak- und Geruchsemissionen a us der Tierhaltung durch biologische Abluftwasche Reduction of Ammonia and Odour Emission from Livestock Buildings by Bioscrubbers. Agrartechnische Forschung 1995, 1, 102–108. [Google Scholar]
- Van’t Klooster, C.E.; Roelofs, P.F.M.M.; den Hartog, L.A. Effects of filtration, vacuum cleaning and washing in pighouses on aerosol levels and pig performance. Livest. Prod. Sci. 1993, 33, 171–182. [Google Scholar] [CrossRef]
- United Nations Economic and Social Council. Guidance Document on Preventing and Abating Ammonia Emissions from Agricultural Sources, ECE/EB.AIR/120; United Nations: Geneva, Switzerland, 2014; p. 100. Available online: https://www.unece.org/fileadmin/DAM/env/documents/2012/EB/ECE_EB.AIR_120_ENG.pdf (accessed on 14 November 2021).

| Characteristics | System 1 (S1) | System 2 (S2) |
|---|---|---|
| Length (m) | 120 | 474 |
| Absorption surface (m2) | 1.96 | 7.74 |
| Internal diameter (mm) | 4.56 | 4.56 |
| Wall thickness (mm) | 0.64 | 0.64 |
| Density of polymer (g/cm3) | 0.95 | 0.95 |
| Porosity (%) | <60 | <60 |
| Average pore size length (µm) | 12.7 ± 5.9 | 12.7 ± 5.9 |
| Average pore size width (µm) | 1.3 ± 0.9 | 1.3 ± 0.9 |
| Parameters | System 1 (S1) | System 2 (S2) | ||
|---|---|---|---|---|
| Initial | Final | Initial | Final | |
| Compost weight (kg) | 470 | 450 | 470 | 420 |
| Dry weight (‰) | 0.410 | 0.396 | 0.429 | 0.437 |
| TAN content (‰) | 0.029 | 0.027 | 0.032 | 0.030 |
| Total TAN content of compost (kg) | 5.65 | 4.85 | 6.35 | 5.51 |
| TAN emitted (kg) | 0.80 | 0.85 | ||
| TAN concentration in acidic solution (g·L−1) | 13.6 | 4.6 | ||
| Volume of acidic solution (L) | 45 | 45 | 150 | 150 |
| Total TAN content in acidic solution (kg) | 0.61 | 0.68 | ||
| TAN recovery rate (g TAN·m−2·day−1) | 6.9 | 1.9 | ||
| NH3 Recovery Technology | NH3 Source | Net Cost (€·Place−1·Year−1) | Reference |
|---|---|---|---|
| Biotrickling filter/biofilters | Air from animal houses | 0.43 (broilers) 13.2 (pigs) | [96] |
| Acid scrubbing | 0.43 (broilers) 13.69 (pigs) | ||
| Bioscrubbers | 8.23–15.55 (pigs) | [97] | |
| Air filtration | 1.39 (pigs) | [98] | |
| Air scrubbing | 22–50 (sows) 4–15 (pigs) | [99] | |
| GPM | Air from a closed aerobic composting reactor | 3.55 (free-range laying hen) | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto-Herranz, M.; Sánchez-Báscones, M.; Antolín-Rodríguez, J.M.; Martín-Ramos, P. Reduction of Ammonia Emissions from Laying Hen Manure in a Closed Composting Process Using Gas-Permeable Membrane Technology. Agronomy 2021, 11, 2384. https://doi.org/10.3390/agronomy11122384
Soto-Herranz M, Sánchez-Báscones M, Antolín-Rodríguez JM, Martín-Ramos P. Reduction of Ammonia Emissions from Laying Hen Manure in a Closed Composting Process Using Gas-Permeable Membrane Technology. Agronomy. 2021; 11(12):2384. https://doi.org/10.3390/agronomy11122384
Chicago/Turabian StyleSoto-Herranz, María, Mercedes Sánchez-Báscones, Juan Manuel Antolín-Rodríguez, and Pablo Martín-Ramos. 2021. "Reduction of Ammonia Emissions from Laying Hen Manure in a Closed Composting Process Using Gas-Permeable Membrane Technology" Agronomy 11, no. 12: 2384. https://doi.org/10.3390/agronomy11122384
APA StyleSoto-Herranz, M., Sánchez-Báscones, M., Antolín-Rodríguez, J. M., & Martín-Ramos, P. (2021). Reduction of Ammonia Emissions from Laying Hen Manure in a Closed Composting Process Using Gas-Permeable Membrane Technology. Agronomy, 11(12), 2384. https://doi.org/10.3390/agronomy11122384








