Evaluation of Kenya Stem Rust Observation Nursery Wheat Genotypes for Yield and Yield Components under Artificial Rust Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Location
2.2. Genotypes
2.3. Experimental Procedure
2.4. Data Collection
2.5. Data Analysis
3. Results
3.1. Combined Analysis of Variance
3.2. Variance Components and Broadsense Heritability (H2) Estimates
3.3. The GGE Biplot Analysis
3.4. Stability Analysis
3.5. Phenotypic and Genotypic Correlation and Trait Cluster Analysis
3.6. Stepwise Regression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Afzal, F.; Chaudhari, S.K.; Gul, A.; Farooq, A.; Ali, H.; Nisar, S.; Sarfraz, B.; Shehzadi, K.J.; Mujeeb-Kazi, A. Bread wheat (Triticum aestivum L.) under biotic and abiotic stresses: An overview. In Crop Production and Global Environmental Issues. Plant Pathology, 5th ed.; Agrios, G.N., Ed.; Elsevier Academic Press: Cambridge, MA, USA, 2015; pp. 293–317. [Google Scholar]
- Figueroa, M.; Hammond-Kosack, K.E.; Solomon, P.S. A review of wheat diseases—A field perspective. Mol. Plant Pathol. 2018, 19, 1523–1536. [Google Scholar] [CrossRef] [PubMed]
- Kogo, B.K.; Kumar, L.; Koech, R. Climate change and variability in Kenya: A review of impacts on agriculture and food security. Environ. Dev. Sustain. 2021, 23, 23–43. [Google Scholar] [CrossRef]
- CIMMYT. Sounding the Alarm on Global Stem Rust: An Assessment of Race Ug99 in Kenya and Ethiopia and the Potential for Impact in Neighboring Countries and Beyond; CIMMYT: Mexico City, Mexico, 2005. [Google Scholar]
- Macharia, J.K.; Wanyera, R. Effect of stem rust race Ug99 on grain yield and yield components of wheat cultivars in Kenya. J. Agric. Sci. Technol. A 2012, 2, 423. [Google Scholar]
- Singh, R.P.; Hodson, D.P.; Huerta-Espino, J.; Jin, Y.; Bhavani, S.; Njau, P.; Herrera-Foessel, S.; Singh, P.K.; Singh, S.; Govindan, V. The emergence of Ug99 races of the stem rust fungus is a threat to world wheat production. Annu. Rev. Phytopathol. 2011, 49, 465–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pennacchi, J.P.; Carmo-Silva, E.; Andralojc, P.J.; Lawson, T.; Allen, A.M.; Raines, C.A.; Parry, M. Stability of wheat grain yields over three field seasons in the UK. Food Energy Secur. 2019, 8, e00147. [Google Scholar] [CrossRef] [Green Version]
- Sial, M.A.; Arain, M.A.; Naqvi, M.H.; Soomro, A.M.; Laghari, S.; Nizamani, N.A.; Ali, A. Seasonal effects and genotypic responses for grain yield in semi-dwarf wheat. Asian J. Plant Sci. 2003, 2, 1097–1101. [Google Scholar] [CrossRef]
- Subira, J.; Álvaro, F.; del Moral, L.F.G.; Royo, C. Breeding effects on the cultivar×environment interaction of durum wheat yield. Eur. J. Agron. 2015, 68, 78–88. [Google Scholar] [CrossRef] [Green Version]
- Braun, H.J.; Atlin, G.; Payne, T. Multi-location testing as a tool to identify plant response to global climate change. Clim. Chang. Crop Prod. 2010, 1, 115–138. [Google Scholar] [CrossRef]
- Fischer, R.A.; Byerlee, D.; Edmeades, G. Crop Yields and Global Food Security; ACIAR: Canberra, ACT, Australia, 2014; pp. 8–11. [Google Scholar]
- Baloch, M.J.; Baloch, E.; Jatoi, W.A.; Veesar, N.F. Correlations and heritability estimates of yield and yield attributing traits in wheat (Triticum aestivum L.). Pak. J. Agric. 2013, 29, 96–105. [Google Scholar]
- Mansouri, A.; Oudjehih, B.; Benbelkacem, A.; Fellahi, Z.E.A.; Bouzerzour, H. Variation and Relationships among Agronomic Traits in Durum Wheat (Triticum turgidum (L.) Thell. ssp. turgidumconv. Durum (Desf.) MacKey) under South Mediterranean Growth Conditions: Stepwise and Path Analyses. Int. J. Agron. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Falconer, D.S.; Mackay, T.F.C. Introduction to Quantitative Genetics; Longman: London, UK, 1996. [Google Scholar]
- Daday, H.; Binet, F.E.; Grassia, A.; Peak, J.W. The effect of environment on heritability and predicted selection response in Medicago sativa. Heredity 1973, 31, 293–308. [Google Scholar] [CrossRef] [Green Version]
- Vargas, M.; Combs, E.; Alvarado, G.; Atlin, G.; Mathews, K.; Crossa, J. META: A suite of SAS programs to analyze multien-vironment breeding trials. Agron. J. 2013, 105, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Cox, T.S.; Shroyer, J.P.; Ben-Hui, L.; Sears, R.G.; Martin, T.J. Genetic improvement in agronomic traits of hard red winter wheat cultivars 1919 to 1987. Crop Sci. 1988, 28, 756–760. [Google Scholar] [CrossRef]
- Finlay, K.; Wilkinson, G. The analysis of adaptation in a plant-breeding programme. Aust. J. Agric. Res. 1963, 14, 742–754. [Google Scholar] [CrossRef] [Green Version]
- Eberhart, S.A.; Russell, W.A. Stability parameters for comparing varieties. Crop Sci. 1966, 6, 36–40. [Google Scholar] [CrossRef] [Green Version]
- Perkins, J.M.; Jinks, J.L. Environmental and genotype-environmental components of variability III. Multiple lines and crosses. Heredity 1968, 23, 339–356. [Google Scholar] [CrossRef] [Green Version]
- Shukla, G.K. Some statistical aspects of partitioning genotype-environmental components of variability. Heredity 1972, 29, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Allard, R.W.; Bradshaw, A.D. Implications of genotype-environmental interactions in applied plant breeding 1. Crop Sci. 1964, 4, 503–508. [Google Scholar] [CrossRef] [Green Version]
- Bassi, F.M.; Sanchez-Garcia, M. Adaptation and stability analysis of ICARDA durum wheat elites across 18 countries. Crop Sci. 2017, 57, 2419–2430. [Google Scholar] [CrossRef]
- Singh, C.; Gupta, A.; Gupta, V.; Kumar, P.; Sendhil, R.; Tyagi, B.; Singh, G.; Chatrath, R.; Singh, G. Genotype x environment interaction analysis of multi-environment wheat trials in India using AMMI and GGE biplot models. Crop Breed. Appl. Biotechnol. 2019, 19, 309–318. [Google Scholar] [CrossRef]
- Kun, S.; Lijuan, Y.; Xiaohang, L.; Yang, G.; Zhikai, J.; Aiwang, D. Grain quality variations from year to year among the Chinese genotypes. Cereal Res. Commun. 2020, 48, 499–505. [Google Scholar] [CrossRef]
- Jaetzold, R.; Hornetz, B.; Shisanya, C.A.; Schmidt, H. Farm Management Handbook of Kenya Vol I–IV (Western Central Eastern Nyanza Southern Rift Valley Northern Rift Valley Coast); Government Printers: Nairobi, Kenya, 2012. [Google Scholar]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Peterson, R.F.; Campbell, A.B.; Hannah, A.E. A diagrammatic scale for estimating rust intensity on leaves and stems of cereals. Can. J. Res. 1948, 26c, 496–500. [Google Scholar] [CrossRef]
- AACC (American Association of Cereal Chemists). International Approved Methods of the American Association of Cereal Chemist, 10th ed.; AACC (American Association of Cereal Chemists): St. Paul, MN, USA, 2000; Volume 1, Method 1200.56. [Google Scholar]
- CIMMYT. A Programme for Calculation of AUDPC. Mexico D. F a Software Package; CIMMYT: Mexico City, Mexico, 2008. [Google Scholar]
- SAS Institute. SAS Software, User Guide: Statistics; SAS Institute: Cary, NC, USA, 2001. [Google Scholar]
- Tukey, J.W. Comparing individual means in the analysis of variance. Biometrics 1949, 5, 99. [Google Scholar] [CrossRef]
- Pacheco, A.; Vargas, M.; Alvarado, G.; Rodríguez, F.; Crossa, J.; Burgueño, J. GEA-R (Genotype x Environment Analysis with R for Windows) Version 4.1; CIMMYT Research Data & Software Repository Network, CIMMYT: El Batan, Mexico, 2015; Volume 16, Available online: https://hdl.handle.net/11529/10203 (accessed on 1 September 2021).
- Hill, W.G.; Singh, R.K.; Chaudhary, B.D. Biometrical Methods in Quantitative Genetic Analysis; Kalyani: Ludhiana, India, 1977. [Google Scholar]
- Miller, P.A.; Williams, J.C.J.; Robinson, H.F.; Comstock, R.E. Estimates of genotypic and environmental variances and co-variances in upland cotton and their implications in selection 1. Agron. J. 1958, 50, 126–131. [Google Scholar] [CrossRef]
- Alvarado, G.; López, M.; Vargas, M.; Pacheco, A.; Rodriguez, F.; Burgueno, J.; Crossa, J. META-R (Multi-Environment Trail Analysis with R for Windows) Version 5; International Maize and Wheat Improvement Center: Mexico City, Mexico, 2015. [Google Scholar]
- Rehman, S.U.; Abid, M.A.; Bilal, M.; Ashraf, J.; Liaqat, S.; Ahmed, R.I.; Qanmber, G. Genotype by trait analysis and estimates of heritability of wheat (Triticum aestivum L.) under drought and control conditions. Basic Res. J. Agric. Sci. Rev. 2015, 4, 127–134. [Google Scholar]
- Weinig, C.; Schmitt, J. Environmental effects on the expression of quantitative trait loci and implications for phenotypic evo-lution. Bioscience 2004, 54, 627–635. [Google Scholar] [CrossRef] [Green Version]
- Balkan, A. Genetic variability, heritability and genetic advance for yield and quality traits in M2-4 generations of bread wheat (Triticum aestivum L.) genotypes. Turk. J. Field Crop 2018, 23, 173–179. [Google Scholar] [CrossRef]
- De Vita, P.; Mastrangelo, A.; Matteu, L.; Mazzucotelli, E.; Virzì, N.; Palumbo, M.; Storto, M.L.; Rizza, F.; Cattivelli, L. Genetic improvement effects on yield stability in durum wheat genotypes grown in Italy. Field Crop Res. 2010, 119, 68–77. [Google Scholar] [CrossRef]
- Kumar, N.; Markar, S.; Kumar, V. Studies on heritability and genetic advance estimates in timely sown bread wheat (Triticum aestivum L.). Biosci. Discov. 2014, 5, 64–69. [Google Scholar]
- Sgrò, C.M.; Hoffmann, A. Genetic correlations, tradeoffs and environmental variation. Heredity 2004, 93, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Becker, H.C.; Leon, J. Stability analysis in plant breeding. Plant Breed. 1988, 101, 1–23. [Google Scholar] [CrossRef]
- Yan, W.; Kang, M.S. GGE Biplot Analysis: A Graphical Tool For Breeders, Geneticists And Agronomists, 1st ed.; CRC Press LLC: Boca Roton, FL, USA, 2003; p. 271. [Google Scholar]
- Powell, N.M.; Lewis, C.M.; Berry, S.T.; MacCormack, R.; Boyd, L.A. Stripe rust resistance genes in the UK winter wheat cultivar Claire. Theor. Appl. Genet. 2013, 126, 1599–1612. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.C.; Duveiller, E. Selection index for improving Helminthosporium leaf blight resistance, maturity, and kernel weight in spring wheat. Crop Sci. 2003, 43, 2031–2036. [Google Scholar] [CrossRef]
- Asmmawy, M.A.; El-Orabey, W.M.; Nazim, M.; Shahin, A.A. Effect of stem rust infection on grain yield and yield components of some wheat cultivars in Egypt. Int. J. Phytopathol. 2013, 2, 171–178. [Google Scholar] [CrossRef]
- Olivera, P.; Newcomb, M.; Szabo, L.J.; Rouse, M.; Johnson, J.; Gale, S.; Luster, D.G.; Hodson, D.; Cox, J.A.; Burgin, L.; et al. Phenotypic and genotypic characterization of race TKTTF of Puccinia graminis f. sp. tritici that caused a wheat stem rust epidemic in Southern Ethiopia in 2013–2014. Phytopathology 2015, 105, 917–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdulbagiyeva, S.; Zamanov, A.; Talai, J.; Allahverdiyev, T. Effect of rust disease on photosynthetic rate of wheat plant. J. Agric. Sci. Technol. B 2015, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.E.; Cui, J.M.; Su, Y.Q.; Yuan, S.; Yuan, M.; Zhang, H.Y. Influence of stripe rust infection on the photosynthetic char-acteristics and antioxidant system of susceptible and resistant wheat cultivars at the adult plant stage. Front. Plant Sci. 2015, 6, 779. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.K.M. Yield penalties of disease resistance in crops. Curr. Opin. Plant Biol. 2002, 5, 339–344. [Google Scholar] [CrossRef]
- Summers, R.W.; Brown, J.K.M. Constraints on breeding for disease resistance in commercially competitive wheat cultivars. Plant Pathol. 2013, 62, 115–121. [Google Scholar] [CrossRef]
- Dundas, I.S.; Anugrahwati, D.R.; Verlin, D.C.; Park, R.F.; Bariana, H.S.; Mago, R.; Islam, A.K.M.R. New Sources of rust re-sistance from alien species: Meliorating linked defects and discovery. Aust. J. Agric. Res. 2007, 58, 545–549. [Google Scholar] [CrossRef]
- Knott, D.R. The inheritance of rust resistance. VI. The transfer of stem rust resistance from agropyron elongatum to common wheat. Can. J. Plant Sci. 1961, 41, 109–123. [Google Scholar] [CrossRef]
- Daspute, A.; Fakrudin, B. Identification of coupling and repulsion phase DNA marker associated with an allele of a gene conferring host plant resistance to pigeon pea sterility mosaic virus (PPSMV) in pigeon pea (Cajanus cajan L. Millsp.). Plant Pathol. J. 2015, 31, 33–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molero, G.; Reynolds, M.P. Spike photosynthesis measured at high throughput indicates genetic variation independent of flag leaf photosynthesis. Field Crop Res. 2020, 255, 107866. [Google Scholar] [CrossRef]
- Monpara, B.A. Grain filling period as a measure of yield improvement in bread wheat. Crop Improv. 2011, 38, 1–5. [Google Scholar]
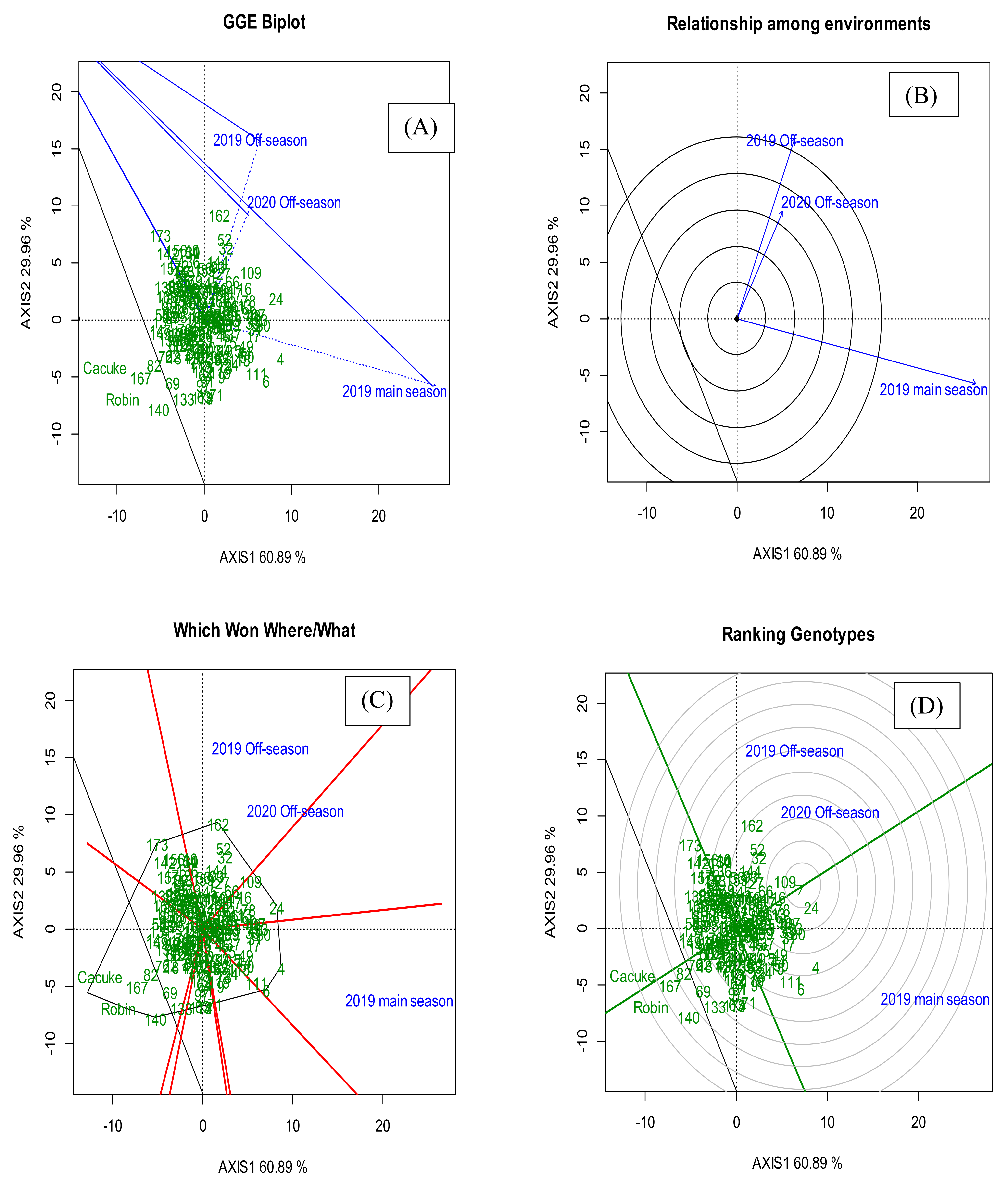
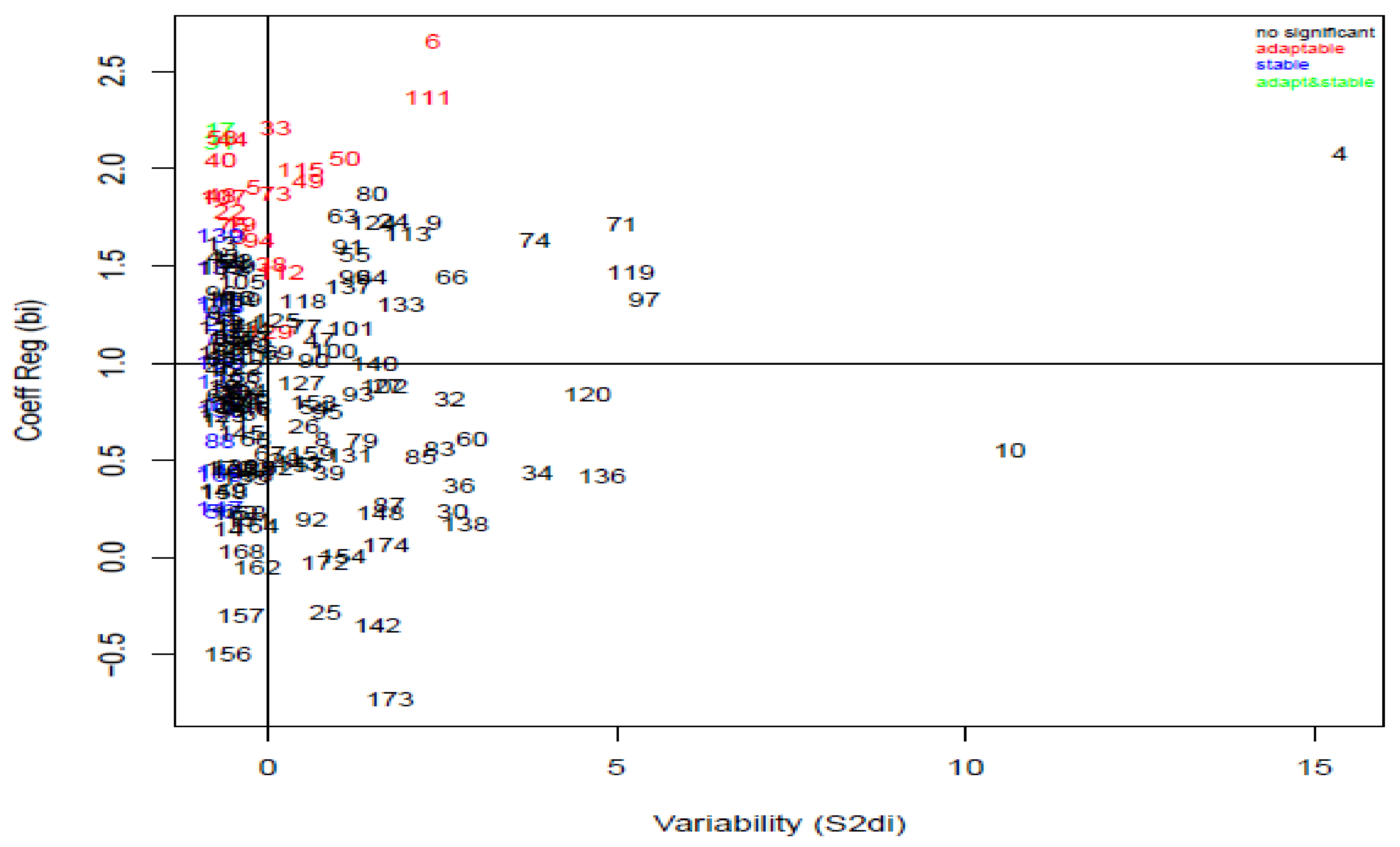
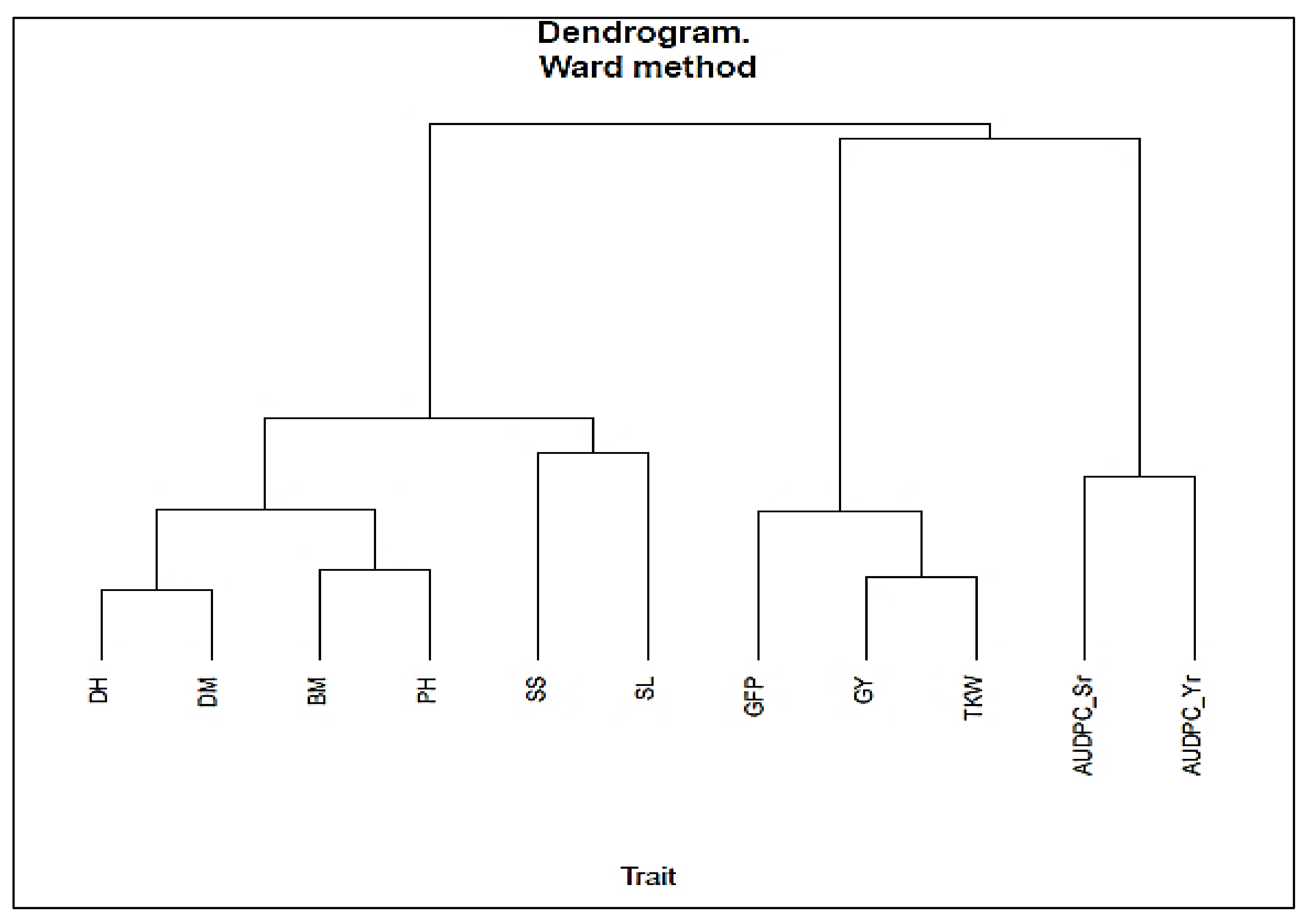
| Source of Variation | df | PH | SL | DH | DM | GFP | GY | SS | TKW | BM | AUDPC_Sr | AUDPC_Yr |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Season (S) | 2 | 1021.21 *** | 8.99 *** | 3834.73 *** | 5663.84 *** | 1185.57 *** | 1760.11 *** | 17982.55 *** | 13944.19 *** | 8028.24 *** | 12641.26 *** | 6269.00 *** |
| Rep (R)/S | 6 | 186.48 | 10.51 | 17.44 | 271.16 | 199.03 | 80.16 | 1201.15 | 260.91 | 2647.53 | 104.19 | 124.11 |
| Block/(R × S) | 216 | 43.47 | 0.68 | 3.50 | 16.30 | 18.67 | 2.90 | 47.40 | 9.89 | 162.28 | 14.22 | 6.62 |
| Genotype (G) | 174 | 98.65 *** | 2.11 *** | 52.19 *** | 49.66 *** | 42.88 *** | 9.11 *** | 98.12 *** | 95.88 *** | 249.91 *** | 144.12 *** | 75.37 *** |
| G × S | 348 | 26.33 *** | 0.47 * | 3.36 | 15.93 *** | 18.95 *** | 4.52 *** | 42.16 | 16.24 *** | 121.83 * | 22.75 *** | 8.36 *** |
| Error | 828 | 19.79 | 0.39 | 2.98 | 7.99 | 10.08 | 2.09 | 36.45 | 7.81 | 104.91 | 6.97 | 3.94 |
| CV (%) | 4.79 | 6.13 | 2.55 | 2.27 | 5.62 | 22.44 | 13.62 | 9.44 | 66.97 | 18.00 | 28.06 | |
| R2 | 0.73 | 0.72 | 0.89 | 0.84 | 0.75 | 0.83 | 0.75 | 0.90 | 26.73 | 0.92 | 0.91 |
| Season | PH | SL | DH | DM | GFP | SS | TKW | GY | BM | AUDP_Sr | AUDPC_Yr |
|---|---|---|---|---|---|---|---|---|---|---|---|
| cm | Days | (g) | t ha−1 | ||||||||
| OS 2019 | 92.75b | 10.27a | 68.40b | 123.19b | 54.79c | 38.65c | 32.66a | 6.16b | 33.88c | 414.61a | 23.11c |
| MS 2019 | 94.43a | 10.35a | 70.28a | 128.02a | 57.74a | 50.34a | 32.53a | 8.41a | 39.78b | 259.61b | 59.04b |
| OS 2020 | 91.66c | 10.09b | 64.95c | 121.74c | 56.79b | 44.03b | 24.03b | 4.78c | 41.28a | 108.61c | 133.21a |
| Mean | 92.95 | 10.24 | 67.88 | 124.32 | 56.44 | 44.34 | 29.74 | 6.45 | 38.32 | 228.28 | 71.79 |
| Tukey MSD(0.05) | 0.64 | 0.09 | 0.25 | 0.41 | 0.46 | 0.88 | 0.41 | 0.21 | 1.48 | 13.91 | 4.79 |
| Source of Variation | df | SS | MS | F Gollop | Probability Value | % Explained | G × E Explained |
|---|---|---|---|---|---|---|---|
| Season (S) | 2 | 3520.21 | 1760.11 | 650.56 *** | 0 | 48.20 | |
| Genotype (G) | 174 | 1842.95 | 10.59 | 3.91 *** | 0 | 25.23 | |
| S × G | 348 | 1940.50 | 5.58 | 2.06 *** | 0 | 26.57 | |
| Residual | 1050 | 2840.82 | 2.71 | 0 | |||
| PCA 1 | 60.89% | 60.89% | |||||
| PCA 2 | 29.96% | 90.85% |
| Traits | Mean ± Se | Range | PCV% | GCV% | H2 | ||
|---|---|---|---|---|---|---|---|
| PH | 92.95 ± 0.34 | 84.01–102.02 | 12.49 | 36.66 | 9.49 | 31.96 | 75.99 |
| DH | 67.88 ± 0.13 | 61.89–74.11 | 6.88 | 31.83 | 6.47 | 30.88 | 94.11 |
| DM | 124.31 ± 0.21 | 116.56–132.67 | 6.84 | 23.46 | 4.88 | 19.82 | 71.40 |
| TKW | 29.62 ± 0.21 | 14.50–39.84 | 12.85 | 65.87 | 10.83 | 60.46 | 84.25 |
| SL | 10.24 ± 0.05 | 8.74–11.72 | 0.28 | 16.49 | 0.23 | 14.92 | 81.91 |
| GFP | 56.44 ± 0.24 | 48.44–64.11 | 5.80 | 32.06 | 3.52 | 24.96 | 60.62 |
| SS | 44.34 ± 0.46 | 31.87–53.50 | 12.09 | 52.21 | 7.27 | 40.48 | 60.11 |
| GY | 6.45 ± 0.11 | 1.76–9.27 | 1.15 | 42.22 | 0.57 | 29.73 | 49.58 |
| BM | 38.32 ± 0.77 | 15.62–56.96 | 44.38 | 104.64 | 31.20 | 87.73 | 61.12 |
| Best Performing Genotypes in Both MS 2019 and OS 2020 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stem Rust Severity and Response | Yellow Rust Severity and Response | |||||||||||||
| Genotype | Rank | 2019 OS | 2019 MS | 2020 OS | Mean FDS + se | Response | AUDPC_Sr | 2019 OS | 2019 MS | 2020 OS | Mean FDS + se | Response | AUDPC_Yr | GY (t ha−1) |
| KSRON 32 | 3 | 60.00 | 46.67 | 3.33 | 46.67 ± 1.94 | S | 503.61 | 0 | 3.33 | 5.33 | 2.89 ± 2.09 | M | 37.72 | 8.08 |
| KSRON 52 | 2 | 13.33 | 13.33 | 5.00 | 10.57 ± 1.80 | M | 99.00 | 3.33 | 6.67 | 8.33 | 6.11 ± 1.97 | M | 57.17 | 8.26 |
| KSRON 162 | 1 | 30.00 | 28.33 | 13.33 | 23.89 ± 2.33 | MS | 212.33 | 13.33 | 16.67 | 30.00 | 20.00 ± 1.85 | M | 187.06 | 8.50 |
| Best Performing Genotypes Across the Seasons | ||||||||||||||
| KSRON 4 | 2 | 30.00 | 23.33 | 15.00 | 22.78 ± 1.90 | MS | 194.39 | 0.33 | 11.67 | 11.67 | 7.89 ± 2.84 | MS | 73.89 | 8.74 |
| KSRON 24 | 1 | 26.67 | 23.33 | 10.00 | 20.00 ± 2.17 | M | 214.39 | 0.33 | 0.33 | 3.67 | 1.44 ± 1.72 | MR | 6.61 | 9.27 |
| KSRON 52 | 6 | 13.33 | 13.22 | 5.00 | 10.56 ± 1.80 | M | 99.00 | 3.33 | 6.67 | 8.33 | 6.11 ± 1.97 | M | 57.17 | 8.26 |
| KSRON 53 | 8 | 21.67 | 8.33 | 5.00 | 11.67 ± 3.27 | MR | 127.89 | 0.33 | 6.67 | 10.00 | 5.67 ± 2.16 | M | 45.11 | 8.24 |
| KSRON 78 | 7 | 16.67 | 6.67 | 5.00 | 9.44 ± 1.90 | RMR | 100.00 | 5.33 | 5.33 | 20.00 | 10.22 ± 2.50 | M | 107.72 | 8.25 |
| KSRON 80 | 5 | 20.00 | 18.33 | 8.33 | 15.56 ± 2.32 | M | 144.44 | 0.00 | 3.33 | 5.00 | 2.78 ± 1.58 | M | 18.67 | 8.43 |
| KSRON 99 | 9 | 26.67 | 30.00 | 5.00 | 20.56 ± 3.09 | M | 206.67 | 0.00 | 2.00 | 11.67 | 4.56 ± 2.94 | M | 45.11 | 8.21 |
| KSRON 107 | 10 | 30.00 | 30.00 | 11.67 | 23.89 ± 239 | MSS | 208.44 | 1.67 | 5.00 | 8.33 | 5.00 ± 1.94 | M | 43.17 | 8.18 |
| KSRON 109 | 3 | 43.33 | 43.33 | 11.67 | 32.78 ± 3.00 | MSS | 329.17 | 0.00 | 0.33 | 10.00 | 4.44 ± 2.50 | MR | 38.89 | 8.63 |
| KSRON 162 | 4 | 30.00 | 28.33 | 13.33 | 23.89 ± 2.33 | M | 212.33 | 13.33 | 16.67 | 30.00 | 20.00 ± 1.85 | M | 187.06 | 8.5 |
| * Cacuke | 175 | 100.00 | 73.33 | 100.00 | 91.11 ± 1.92 | S | 1156.11 | 8.67 | 30.00 | 33.33 | 24.00 ± 2.54 | M | 282.72 | 1.76 |
| * Robin | 174 | 100.00 | 100.00 | 90.00 | 96.67 ± 1.02 | S | 1265.00 | 6.67 | 5.00 | 13.00 | 8.22 ± 1.90 | M | 91.39 | 2.82 |
| Tukey MSD0.05 | 18.47 | 206.43 | 7.96 | 71.05 | 3.11 | |||||||||
| Traits | AUDPC_Sr | AUDPC_Yr | DH | BM | GY | TKW | DM | GFP | |
|---|---|---|---|---|---|---|---|---|---|
| AUDPC_Yr | G P | 0.22 ** 0.16 * | |||||||
| DH | G P | −0.21 ** −0.20 ** | −0.16 * −0.16 * | ||||||
| BM | G P | −0.52 *** −0.38 *** | −0.38 *** −0.28 *** | 0.51 *** 0.35 *** | |||||
| GY | G P | −0.53 *** −0.38 *** | −0.28 *** −0.19 * | −0.13 −0.09 | 0.55 *** 0.45 *** | ||||
| TKW | G P | −0.51 *** −0.46 *** | −0.15 * −0.12 | −0.27 *** −0.24 ** | 0.37 *** 0.30 *** | 0.65 *** 0.49 *** | |||
| DM | G P | −0.52 *** −0.42 *** | −0.09 −0.11 | 0.70 *** 0.56 *** | 0.68 *** 0.52 *** | 0.16 * 0.13 | 0.13 0.14 | ||
| GFP | G P | −0.33 *** −0.25 ** | 0.11 0.06 | −0.53 *** −0.45 *** | 0.10 0.18 * | 0.35 *** 0.23 ** | 0.52 *** 0.40 *** | 0.22 ** 0.48 *** | |
| SS | G P | −0.23 ** −0.16 * | −0.29 *** −0.19 * | 0.28 *** 0.19 * | 0.32 *** 0.23 ** | 0.25 *** 0.20 ** | −0.24 ** −0.15 | 0.26 *** 0.11 | −0.88 −0.08 |
| Dependable Variable | Independent Variable | Intercept | Parameter Estimate | Standard Error | Partial R2 | Model R2 | C(P) |
|---|---|---|---|---|---|---|---|
| TKW | AUDPC_Sr | 32.31 | −0.01029 | 0.00148 | 0.22 | 0.22 | 1.44 |
| GY | AUDPC_Sr | 7.26 | −0.00238 | 0.00047 | 0.14 | 0.14 | 4.18 |
| AUDPC_Yr | −0.00260 | 0.00146 | 0.02 | 0.16 | 3.00 | ||
| BM | AUDPC_Sr | 43.26 | −0.01227 | 0.00258 | 0.14 | 0.14 | 10.25 |
| AUDPC_Yr | −0.02422 | 0.00796 | 0.04 | 0.18 | 3.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madahana, S.L.; Owuoche, J.O.; Oyoo, M.E.; Macharia, G.K.; Randhawa, M.S. Evaluation of Kenya Stem Rust Observation Nursery Wheat Genotypes for Yield and Yield Components under Artificial Rust Conditions. Agronomy 2021, 11, 2394. https://doi.org/10.3390/agronomy11122394
Madahana SL, Owuoche JO, Oyoo ME, Macharia GK, Randhawa MS. Evaluation of Kenya Stem Rust Observation Nursery Wheat Genotypes for Yield and Yield Components under Artificial Rust Conditions. Agronomy. 2021; 11(12):2394. https://doi.org/10.3390/agronomy11122394
Chicago/Turabian StyleMadahana, Sammy Larry, James Otieno Owuoche, Maurice Edwards Oyoo, Godwin Kamau Macharia, and Mandeep Singh Randhawa. 2021. "Evaluation of Kenya Stem Rust Observation Nursery Wheat Genotypes for Yield and Yield Components under Artificial Rust Conditions" Agronomy 11, no. 12: 2394. https://doi.org/10.3390/agronomy11122394






