Photosynthetic Efficiency in Flag Leaves and Ears of Winter Wheat during Fusarium Head Blight Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Field Experiments
2.3. Measurement and Analysis of Fast Cholophyll a Fluorescence
2.4. Statistical Analysis
3. Results
3.1. FHB Severity, Incidence and Effect on Grain Yield
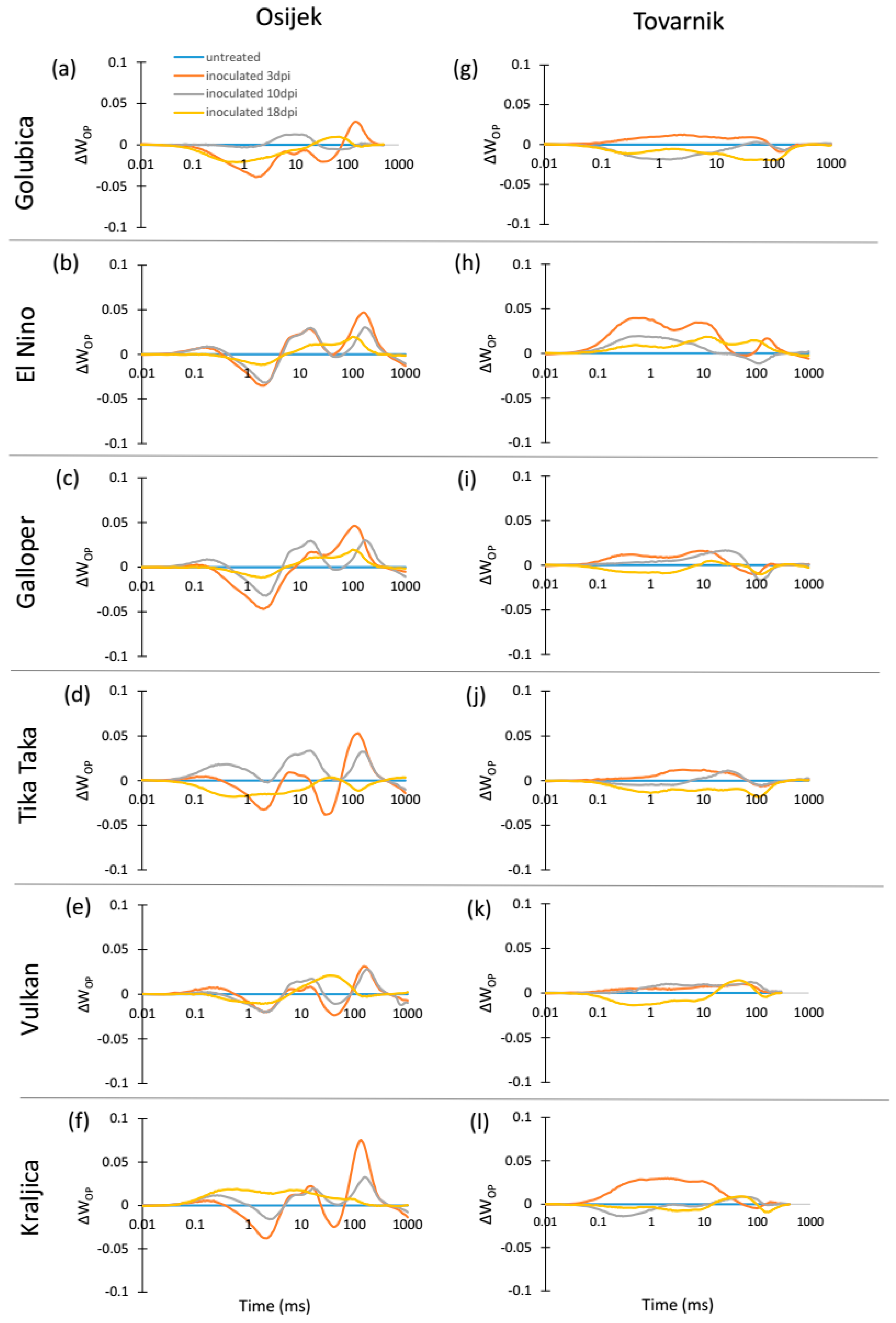
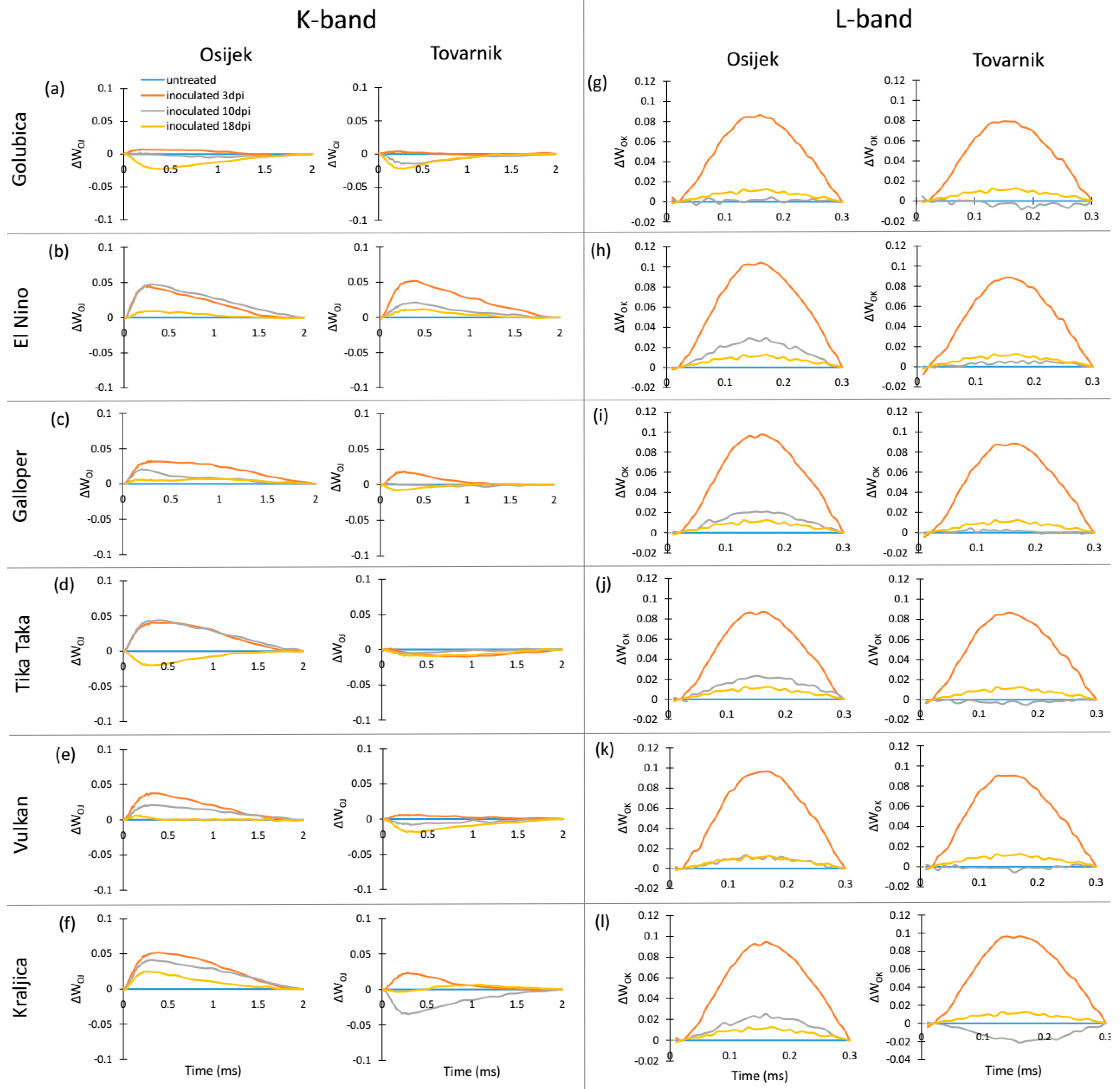
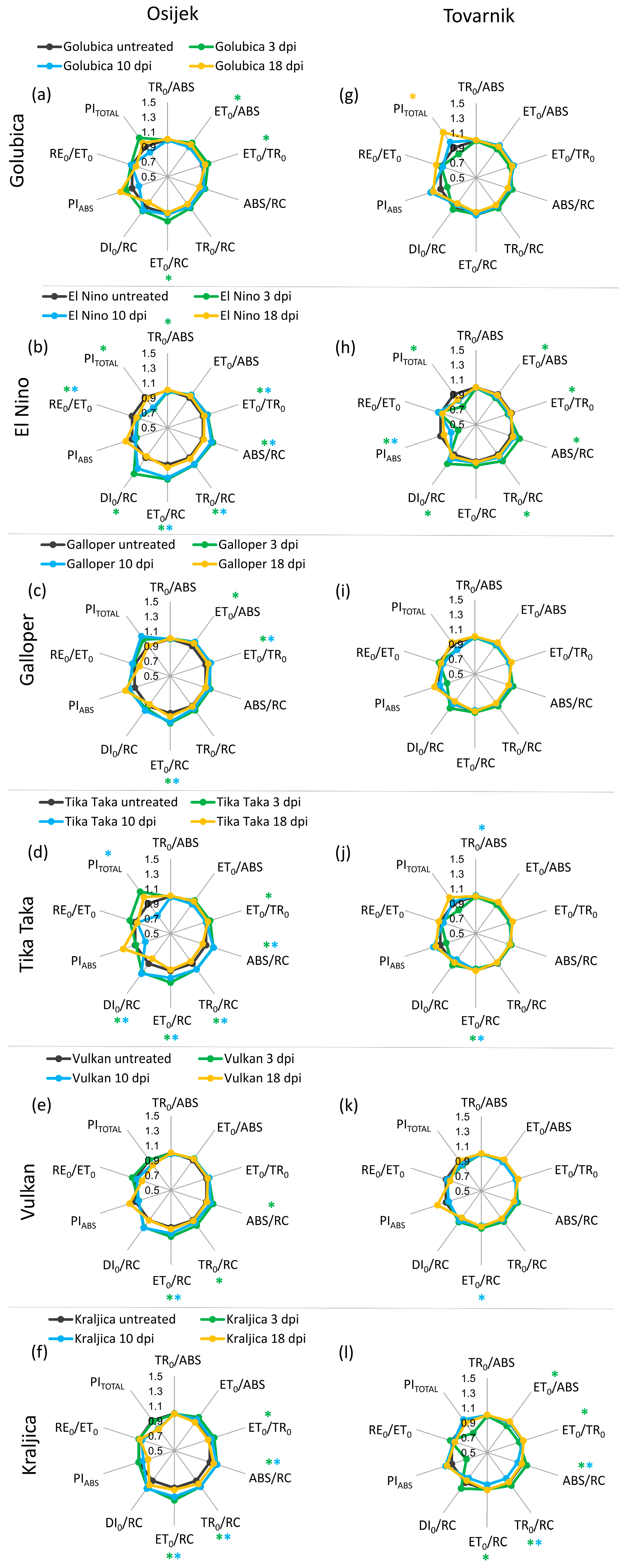
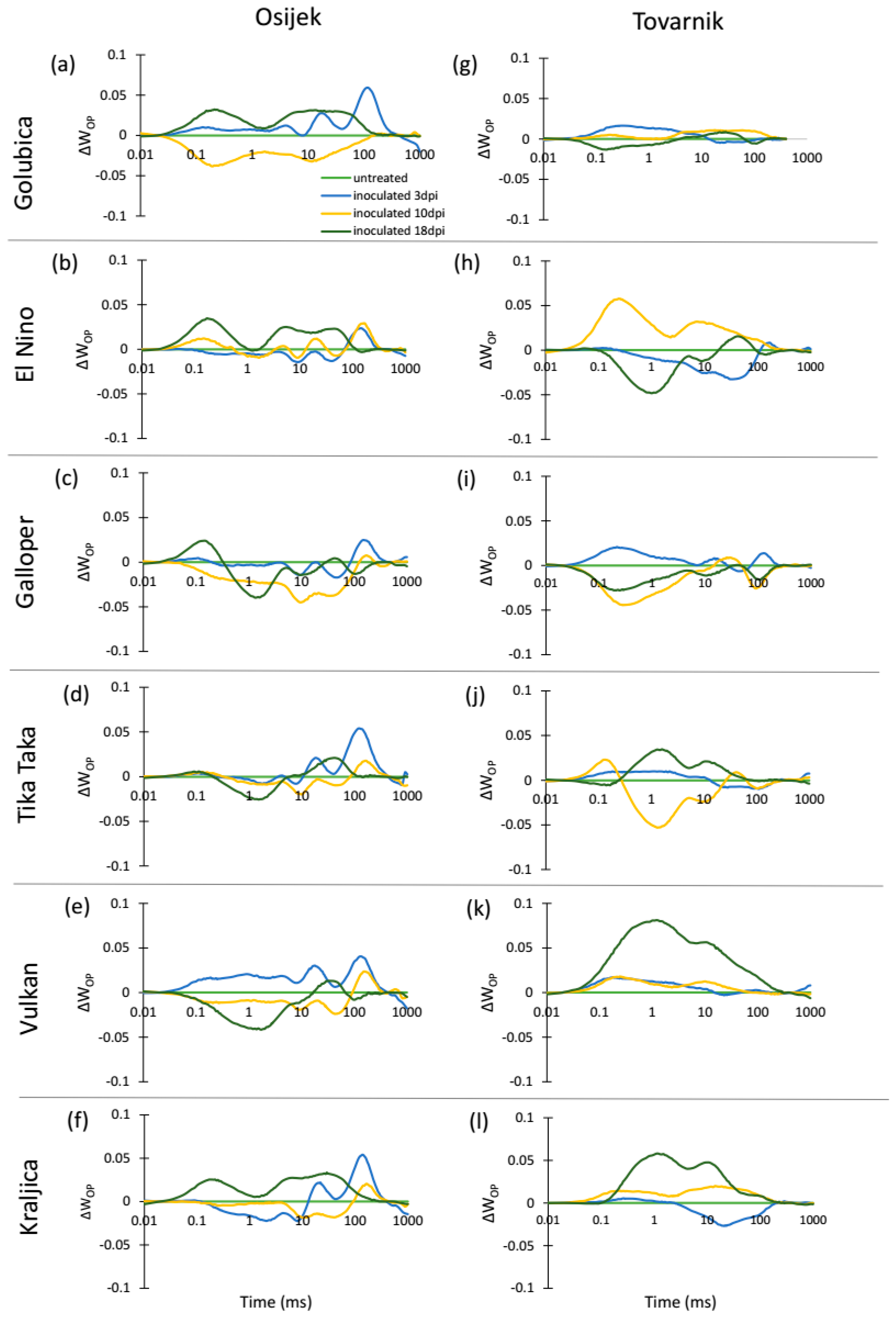
3.2. Phytosynthetic Parameters in Flag Leaves
3.3. Phytosynthetic Parameters in Ears
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hawkesford, M.J.; Araus, J.L.; Park, R.; Calderini, D.; Miralles, D.; Shen, T.; Zhang, J.; Parry, M.A.J. Prospects of doubling global wheat yields. Food Energy Secur. 2013, 2, 34–48. [Google Scholar] [CrossRef]
- Figueroa, M.; Hammond-Kosack, K.E.; Solomon, P.S. A review of wheat diseases—A field perspective. Mol. Plant Pathol. 2018, 19, 1523–1536. [Google Scholar] [CrossRef]
- Osborne, L.E.; Stein, J.M. Epidemiology of Fusarium head blight on small-grain cereals. Int. J. Food Microbiol. 2007, 119, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Parry, D.W.; Jenkinson, P.; Mcleod, L. Fusarium ear blight (scab) in small grain cereals—A review. Plant Pathol. 1995, 44, 207–238. [Google Scholar] [CrossRef]
- Dweba, C.C.; Figlan, S.; Shimelis, H.A.; Motaung, T.E.; Sydenham, S.; Mwadzingeni, L.; Tsilo, T.J. Fusarium head blight of wheat: Pathogenesis and control strategies. Crop. Prot. 2017, 91, 114–122. [Google Scholar] [CrossRef]
- Spanic, V.; Horvat, D.; Drezner, G.; Zdunic, Z. Changes in protein composition in the grain and malt after Fusarium infection dependently of wheat resistance. Pathogens 2019, 8, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Rocha, M.E.B.; da Freire, F.C.O.; Erlan Feitosa Maia, F.; Izabel Florindo Guedes, M.; Rondina, D. Mycotoxins and their effects on human and animal health. Food Control 2014, 36, 159–165. [Google Scholar] [CrossRef]
- Škrbić, B.; Malachova, A.; Živančev, J.; Veprikova, Z.; Hajšlová, J. Fusarium mycotoxins in wheat samples harvested in Serbia: A preliminary survey. Food Control 2011, 22, 1261–1267. [Google Scholar] [CrossRef]
- Spanic, V.; Katanic, Z.; Sulyok, M.; Krska, R.; Puskas, K.; Vida, G.; Drezner, G.; Šarkanj, B. Multiple fungal metabolites including mycotoxins in naturally infected and Fusarium-inoculated wheat samples. Microorganisms 2020, 8, 578. [Google Scholar] [CrossRef] [Green Version]
- Mesterházy, Á.; Tóth, B.; Varga, M.; Bartók, T.; Szabó-Hevér, Á.; Farády, L.; Lehoczki-Krsjak, S. Role of fungicides, application of nozzle types, and the resistance level of wheat varieties in the control of Fusarium head blight and deoxynivalenol. Toxins 2011, 3, 1453. [Google Scholar] [CrossRef] [Green Version]
- Bai, G.; Su, Z.; Cai, J. Wheat resistance to Fusarium head blight. Can. J. Plant Pathol. 2018, 40, 336–346. [Google Scholar] [CrossRef]
- Zhang, W.; Boyle, K.; Brule-Babel, A.; Fedak, G.; Gao, P.; Djama, Z.R.; Polley, B.; Cuthbert, R.; Randhawa, H.; Graf, R.; et al. Evaluation of genomic prediction for Fusarium head blight resistance with a multi-parental population. Biology 2021, 10, 756. [Google Scholar] [CrossRef]
- Li, X.; Zhong, S.; Chen, W.; Fatima, S.A.; Huang, Q.; Li, Q.; Tan, F.; Luo, P. Transcriptome analysis identifies a 140 Kb region of chromosome 3B containing genes specific to Fusarium head blight resistance in wheat. Int. J. Mol. Sci. 2018, 19, 852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroeder, H.W.; Christensen, J.J.; Christensen, J.D.; Platz-Christensen, J.; Schroeder, H.W. Factors affecting resistance of wheat to scab caused by Gibberella zeae. Phytopathology 1963, 53, 831–838. [Google Scholar]
- David Miller, J. Degradation of deoxynivalenol by suspension cultures of the Fusarium head blight resistant wheat cultivar frontana. Can. J. Plant Pathol. 1986, 8, 147–150. [Google Scholar] [CrossRef]
- Mesterhazy, A. Types and components of resistance to Fusarium head blight of wheat. Plant Breed. 1995, 114, 377–386. [Google Scholar] [CrossRef]
- Mesterházy, Á.; Bartók, T.; Mirocha, C.G.; Komoroczy, R. Nature of wheat resistance to Fusarium head blight and the role of deoxynivalenol for breeding. Plant Breed. 1999, 118, 97–110. [Google Scholar] [CrossRef]
- Berger, S.; Sinha, A.K.; Roitsch, T. Plant physiology meets phytopathology: Plant primary metabolism and plant-pathogen interactions. J. Exp. Bot. 2007, 58, 4019–4026. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, X.; Chen, W.; Liu, T.; Zhong, S.; Ma, L.; Zhang, M.; Zhang, H.; Yu, D.; Luo, P. Wheat resistance to Fusarium head blight is associated with changes in photosynthetic parameters. Plant Dis. 2016, 100, 847–852. [Google Scholar] [CrossRef]
- Spanic, V.; Vuletic, M.V.; Horvat, D.; Sarkanj, B.; Drezner, G.; Zdunic, Z. Changes in antioxidant system during grain development of wheat (Triticum aestivum l.) and relationship with protein composition under FHB stress. Pathogens 2020, 9, 17. [Google Scholar] [CrossRef] [Green Version]
- Walter, S.; Nicholson, P.; Doohan, F.M. Action and reaction of host and pathogen during Fusarium head blight disease. New Phytol. 2010, 185, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.; Shaner, G. Management and resistance in wheat and barley to Fusarium head blight. Annu. Rev. Phytopathol. 2004, 42, 135–161. [Google Scholar] [CrossRef] [PubMed]
- Khaledi, N.; Taheri, P.; Falahati-Rastegar, M. Evaluation of resistance and the role of some defense responses in wheat cultivars to Fusarium head blight. J. Plant Prot. Res. 2017, 57, 396–408. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Luo, P. Changes in photosynthesis could provide important insight into the interaction between wheat and fungal pathogens. Int. J. Mol. Sci. 2021, 22, 8865. [Google Scholar] [CrossRef]
- Ajigboye, O.O.; Bousquet, L.; Murchie, E.H.; Ray, R.V. Chlorophyll fluorescence parameters allow the rapid detection and differentiation of plant responses in three different wheat pathosystems. Funct. Plant Biol. 2016, 43, 356–369. [Google Scholar] [CrossRef]
- Spanic, V.; Vuletic, M.V.; Drezner, G.; Zdunic, Z.; Horvat, D. Performance indices in wheat chlorophyll a fluorescence and protein quality influenced by FHB. Pathogens 2017, 6, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tambussi, E.A.; Maydup, M.L.; Carrión, C.A.; Guiamet, J.J.; Araus, J.L. Ear photosynthesis in C3 cereals and its contribution to grain yield: Methodologies, controversies, and perspectives. J. Exp. Bot. 2021, 72, 3956–3970. [Google Scholar] [CrossRef]
- Yusuf, M.A.; Kumar, D.; Rajwanshi, R.; Strasser, R.J.; Tsimilli-Michael, M.; Govindjee; Sarin, N.B. Overexpression of γ-tocopherol methyl transferase gene in transgenic Brassica juncea plants alleviates abiotic stress: Physiological and chlorophyll a fluorescence measurements. Biochim. Et Biophys. Acta Bioenerg. 2010, 1797, 1428–1438. [Google Scholar] [CrossRef] [Green Version]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanisms, Regulation and Adaptation; Yunus, M., Pathre, U., Mohanty, P., Eds.; Taylor & Francis: London, UK, 2000; pp. 445–483. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll A Fluorescence; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar] [CrossRef]
- Begović, L.; Galić, V.; Abičić, I.; Lončarić, Z.; Lalić, A.; Mlinarić, S. Implications of intra-seasonal climate variations on chlorophyll a fluorescence and biomass in winter barley breeding program. Photosynthetica 2020, 58, 995–1008. [Google Scholar] [CrossRef]
- Begović, L.; Mlinarić, S.; Antunović Dunić, J.; Katanić, Z.; Lončarić, Z.; Lepeduš, H.; Cesar, V. Response of lemna minor L. to short-term cobalt exposure: The effect on photosynthetic electron transport chain and induction of oxidative damage. Aquat. Toxicol. 2016, 175, 117–126. [Google Scholar] [CrossRef]
- Mlinarić, S.; Begović, L.; Tripić, N.; Piškor, A.; Cesar, V. Evaluation of light-dependent photosynthetic reactions in reynoutria japonica houtt. leaves grown at different light conditions. Front. Plant Sci. 2021, 12, 1–13. [Google Scholar] [CrossRef]
- Pavlović, I.; Mlinarić, S.; Tarkowská, D.; Oklestkova, J.; Novák, O.; Lepeduš, H.; Bok, V.V.; Brkanac, S.R.; Strnad, M.; Salopek-Sondi, B. Early brassica crops responses to salinity stress: A comparative analysis between chinese cabbage, white cabbage, and kale. Front. Plant Sci. 2019, 10, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rapacz, M.; Sasal, M.; Kalaji, H.M.; Kościelniak, J. Is the OJIP test a reliable indicator of winter hardiness and freezing tolerance of common wheat and triticale under variable winter environments? PLoS ONE 2015, 10, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Pollastrini, M.; Desotgiu, R.; Camin, F.; Ziller, L.; Gerosa, G.; Marzuoli, R.; Bussotti, F. Severe drought events increase the sensitivity to ozone on poplar clones. Environ. Exp. Bot. 2014, 100, 94–104. [Google Scholar] [CrossRef]
- Christen, D.; Schönmann, S.; Jermini, M.; Strasser, R.J.; Défago, G. Characterization and early detection of grapevine (vitis vinifera) stress responses to esca disease by in situ chlorophyll fluorescence and comparison with drought stress. Environ. Exp. Bot. 2007, 60, 504–514. [Google Scholar] [CrossRef]
- Baghbani, F.; Lotfi, R.; Moharramnejad, S.; Bandehagh, A.; Roostaei, M.; Rastogi, A.; Kalaji, H.M. Impact of Fusarium verticillioides on chlorophyll fluorescence parameters of two maize lines. Eur. J. Plant Pathol. 2019, 154, 337–346. [Google Scholar] [CrossRef] [Green Version]
- Zhori, A.; Meco, M.; Brandl, H.; Bachofen, R. In situ chlorophyll fluorescence kinetics as a tool to quantify effects on photosynthesis in Euphorbia cyparissias by a parasitic infection of the rust fungus Uromyces pisi. BMC Res. Notes 2015, 8, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warzecha, T.; Skrzypek, E.; Adamski, T.; Surma, M.; Kaczmarek, Z.; Sutkowska, A. Chlorophyll a fluorescence parameters of hulled and hull-less barley (Hordeum vulgare l.) DH lines inoculated with Fusarium culmorum. Plant Pathol. J. 2019, 35, 112–124. [Google Scholar] [CrossRef]
- Matić, M.; Vuković, R.; Vrandečić, K.; Čamagajevac, I.Š.; Ćosić, J.; Vuković, A.; Sabljić, K.; Sabo, N.; Dvojković, K.; Novoselović, D. Oxidative status and antioxidative response to Fusarium attack and different nitrogen levels in winter wheat varieties. Plants 2021, 10, 611. [Google Scholar] [CrossRef]
- Vaughan, M.; Backhouse, D.; del Ponte, E.M. Climate change impacts on the ecology of Fusarium graminearum species complex and susceptibility of wheat to Fusarium head blight: A review. World Mycotoxin J. 2016, 9, 685–700. [Google Scholar] [CrossRef] [Green Version]
- Spanic, V.; Cosic, J.; Zdunic, Z.; Drezner, G. Characterization of agronomical and quality traits of winter wheat (Triticum Aestivum L.) for Fusarium head blight pressure in different environments. Agronomy 2021, 11, 213. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Sunic, K.; Kovac, T.; Loncaric, A.; Babic, J.; Sulyok, M.; Krska, R.; Drezner, G.; Spanic, V. Fusarium secondary metabolite content in naturally produced and artificially provoked FHB pressure in winter wheat. Agronomy 2021, 11, 2239. [Google Scholar] [CrossRef]
- Torres, A.M.; Palacios, S.A.; Yerkovich, N.; Palazzini, J.M.; Battilani, P.; Leslie, J.F.; Logrieco, A.F.; Chulze, S.N. Fusarium head blight and mycotoxins in wheat: Prevention and control strategies across the food chain. World Mycotoxin J. 2019, 12, 333–355. [Google Scholar] [CrossRef]
- Francesconi, S.; Balestra, G.M. The Modulation of stomatal conductance and photosynthetic parameters is involved in Fusarium head blight resistance in wheat. PLoS ONE 2020, 15, 1–21. [Google Scholar] [CrossRef]

| Data extracted from the recorded ChlF transient | |
| Minimal fluorescence intensity at 20 ms—O step | F0 |
| Maximal fluorescence intensity—P step | Fm |
| Fluorescence intensity at 300 μs | F300 |
| Fluorescence intensity at 2 ms—I step | FI |
| Fluorescence intensity at 30 ms—J step | FJ |
| Fluorescence intensity at any time t | Ft |
| Time (in ms) to reach maximal fluorescence FM | tFM |
| Total complementary area between fluorescence induction curve and F = FM | Area |
| Fluorescence parameters derived from the extracted data | |
| Maximal variable fluorescence | FV = Fm − F0 |
| Relative variable fluorescence at 2 ms (J step) | VJ = (FJ − F0)/(Fm − F0) |
| Relative variable fluorescence at 30 ms (I step) | VI = (FI − F0)/(Fm − F0) |
| Relative variable fluorescence normalized to the amplitude at any time t | WOP = (Ft − F0)/(Fm − F0) |
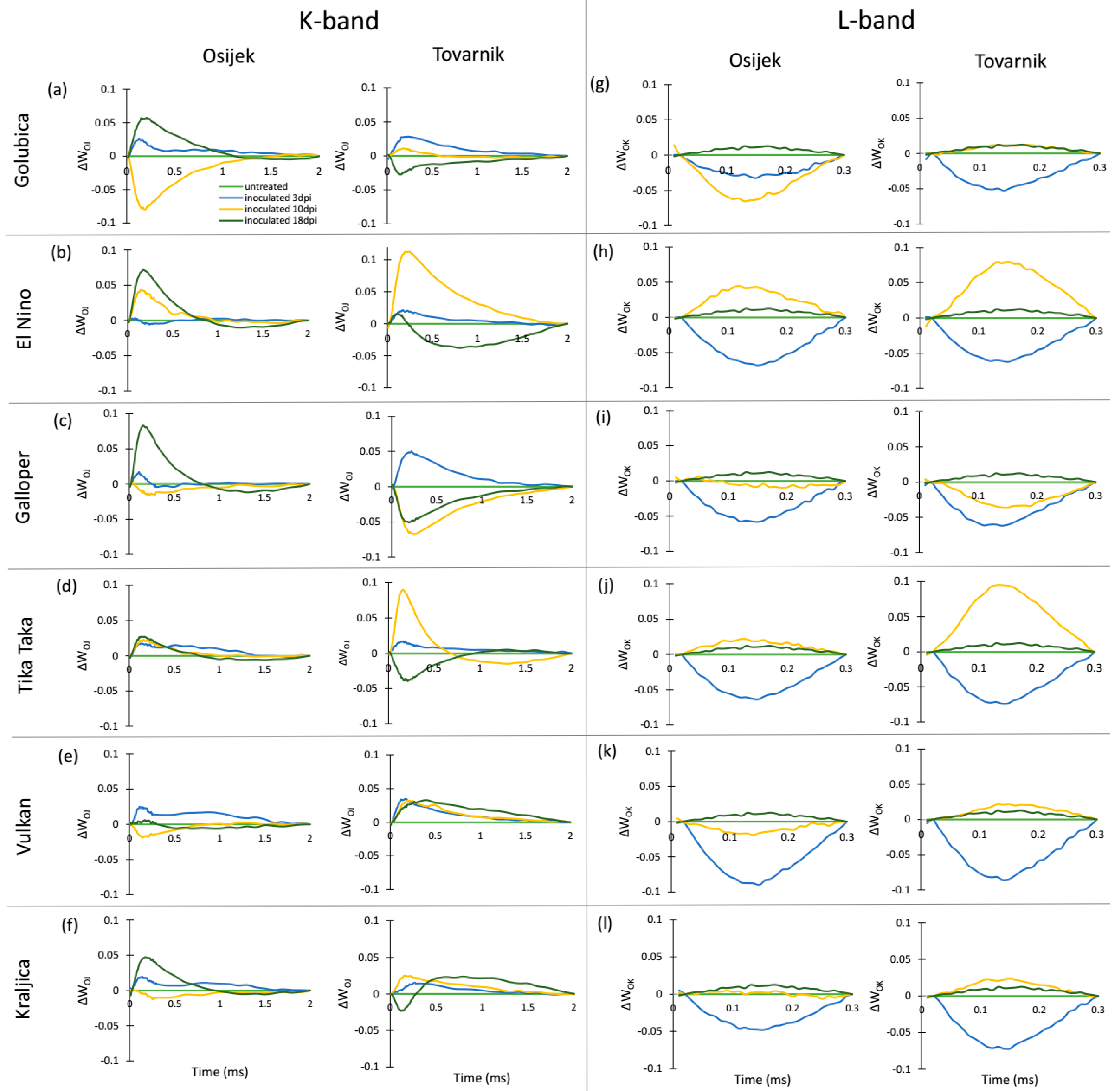
| Relative variable fluorescence normalized to the amplitude of the O–J phase (K-band) | WOJ = (Ft − F0)/(FJ − F0) |
| Relative variable fluorescence normalized to the amplitude of the O–K phase (L-band) | WOK = (Ft − F0)/(FK − F0) |
| Approximated initial slope (in ms−1) of the fluorescence transient V = f(t) | M0 = (dV/dt)0 = 4 × (F300 − F0)/(FM − F0) |
| Quantum yields and efficiencies | |
| Maximum quantum yield for primary photochemistry | TR0/ABS ≡ φP0 = [1 − (F0/FM)] = Fv/FM |
| Quantum yield for electron transport (ET) | ET0/ABS ≡ φE0 = (1 − F0/FM) × (1 − VJ) |
| Efficiency/probability for electron transport (ET), i.e., efficiency/probability that an electron moves further than QA− | ET0/TR0 ≡ ψE0 = (1 − VJ) |
| Efficiency/probability with which an electron from the intersystem electron carriers moves to reduce end electron acceptors at the photosystem I (PSI acceptor) side (RE) | RE0/ET0 ≡ δR0 = (1 − VI)/(1 − VJ) |
| Probability that PSII chlorophyll (Chl) molecule functions as RC | γRC = ChlRC/Chltotal = RC/(ABS + RC) |
| QA-reducing RCs per PSII antenna Chl (reciprocal of ABS/RC) | RC/ABS = γRC/(1 − γRC) = φP0 (VJ/M0) |
| Specific energy fluxes per active (QA− reducing) photosystem II (PSII) reaction center (RC) | |
| Absorption flux (of antenna Chls) per active RC | ABS/RC = M0 × (1/VJ) × (1/φP0) |
| Trapping flux (leading to QA reduction) per active RC | TR0/RC = M0 × (1/VJ) |
| Electron transport flux (further than QA−) per active RC | ET0/RC = M0 × (1/VJ) × ψE0 |
| Dissipation flux per active RC | DI0/RC = (ABS/RC) − (TR0/RC) |
| Performance indexes | |
| Performance index (potential) for energy conservation from exciton to the reduction of intersystem electron acceptors | PIABS = [γRC/(1 − γRC)] × [φP0/(1 − φP0)] × [ψE0/(1 − ψE0)] |
| Performance index (potential) for energy conservation from exciton to the reduction of PSI end acceptors | PITOTAL = PIABS × (δR0/1−δR0) |
| Variety | FHB Severity at 10 dpi | FHB Severity at 18 dpi | AUDPC for FHB Severity | FHB Incidence at 10 dpi | FHB Incidence at 18 dpi | AUDPC for Type I Resistance | GY Decrease (%) |
|---|---|---|---|---|---|---|---|
| Osijek | |||||||
| Golubica | 1.5 | 7.5 | 104 | 4.85 | 22 | 222 | 43 |
| El Nino | 0.5 | 9 | 137 | 3 | 23.5 | 244 | 39 |
| Galloper | 0 | 0 | 1 | 1.5 | 3 | 33 | 8 |
| Tika Taka | 0.5 | 3 | 43 | 3.5 | 18.5 | 215 | 25 |
| Vulkan | 1 | 2 | 36 | 4.85 | 8.35 | 120 | 16 |
| Kraljica | 2 | 4.5 | 72 | 6.85 | 18.5 | 217 | 9 |
| Tovarnik | |||||||
| Golubica | 0 | 3 | 93 | 0 | 4.85 | 111 | 59 |
| El Nino | 2.5 | 22.5 | 213 | 11.5 | 42 | 421 | 54 |
| Galloper | 0 | 1 | 18 | 0 | 4.85 | 87 | 13 |
| Tika Taka | 0 | 2 | 70 | 0 | 11.85 | 138 | 34 |
| Vulkan | 0 | 1.5 | 34 | 0 | 3 | 51 | 29 |
| Kraljica | 0 | 0.5 | 18 | 1.5 | 3 | 80 | 14 |
| MS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Source of Variation | df | TR0/ABS | ET0/ABS | ET0/TR0 | ABS/RC | TR0/RC | ET0/RC | DI0/RC | PIABS | RE0/ET0 | PITOTAL |
| Location (L) | 1 | 0.0164 * | 0.0844 * | 0.0695 * | 0.2011 * | 0.4948 * | 0.8105 * | 0.0650 * | 15.371 * | 0.0016 ns | 11.838 * |
| Variety (V) | 5 | 0.0008 * | 0.0100 * | 0.0116 * | 0.4886 * | 0.2943 * | 0.0417 * | 0.0283 * | 6.5287 * | 0.0675 * | 7.4672 * |
| Treatment (T) | 1 | 0.0014 * | 0.0120 * | 0.0257 * | 1.1816 * | 0.6566 * | 0.6015 * | 0.0765 * | 0.1362 ns | 0.0163 * | 2.2237 * |
| Measurement point (M) | 2 | 0.0010 * | 0.0013 ns | 0.0043 * | 2.5874 * | 1.5735 * | 0.6717 * | 0.1263 * | 3.5233 * | 0.3737 * | 52.807 * |
| L × V | 5 | 0.0003 ns | 0.0009 ns | 0.0009 ns | 0.0406 ns | 0.0226 ns | 0.0108 ns | 0.0042 ns | 0.4499 ns | 0.0121 * | 1.5749 * |
| L × T | 1 | 0.0008 * | 0.0286 * | 0.0512 * | 1.1390 * | 0.6550 * | 0.8227 * | 0.0665 * | 0.6890 ns | 0.0014 ns | 0.2751 ns |
| V × T | 5 | 0.0002 ns | 0.0014 ns | 0.0014 ns | 0.2623 * | 0.1551 * | 0.0378 * | 0.0149 * | 1.3500 * | 0.0015 ns | 1.5473 * |
| L × M | 2 | 0.0005 * | 0.0243 * | 0.0399 * | 1.3478 * | 0.9452 * | 0.7080 * | 0.0401 * | 2.1542 * | 0.0398 * | 0.3673 ns |
| V × M | 10 | 0.0004 * | 0.0021 * | 0.0020 * | 0.1023 * | 0.0587 * | 0.0206 * | 0.0081 * | 1.1179 * | 0.0028 * | 0.5757 * |
| T × M | 2 | 0.0023 * | 0.0007 ns | 0.0000 ns | 0.8547 * | 0.4265 * | 0.1553 * | 0.0748 * | 4.6259 * | 0.0105 * | 1.2659 * |
| L × V × T | 5 | 0.0001 ns | 0.0018 * | 0.0022 * | 0.0632 ns | 0.0415 * | 0.0112 ns | 0.0028 ns | 0.8940 * | 0.0044 * | 0.3705 ns |
| L × V × M | 10 | 0.0003 ns | 0.0021 * | 0.0024 * | 0.0865 * | 0.0448 * | 0.0169 * | 0.0077 * | 0.9259 * | 0.0015 ns | 0.3350 ns |
| L × T × M | 2 | 0.0009 * | 0.0107 * | 0.0159 * | 0.1900 * | 0.0870 * | 0.1127 * | 0.0199 * | 3.4890 * | 0.0046 * | 4.1613 * |
| V × T × M | 10 | 0.0001 ns | 0.0002 ns | 0.0003 ns | 0.0283 ns | 0.0164 ns | 0.0036 ns | 0.0024 ns | 0.2175 ns | 0.0018 ns | 0.3449 ns |
| L × V × T × M | 10 | 0.0002 ns | 0.0015 * | 0.0018 * | 0.0339 ns | 0.0199 ns | 0.0092 ns | 0.0033 ns | 0.6381 * | 0.0033 * | 0.5676 * |
| MS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Source of Variation | df | TR0/ABS | ET0/ABS | ET0/TR0 | ABS/RC | TR0/RC | ET0/RC | DI0/RC | PIABS | RE0/ET0 | PITOTAL |
| Location (L) | 1 | 0.1965 * | 0.0047 * | 0.1776 * | 11.822 * | 1.6843 * | 3.0867 * | 4.5816 * | 10.468 * | 0.0003 ns | 14.352 * |
| Variety (V) | 5 | 0.0101 * | 0.0523 * | 0.0491 * | 1.8584 * | 0.6258 * | 0.0928 * | 0.3545 * | 11.416 * | 0.0894 * | 8.8653 * |
| Treatment (T) | 1 | 0.0375 * | 0.0107 * | 0.0006 ns | 3.9920 * | 0.8694 * | 0.3737 * | 1.1355 * | 10.060 * | 0.0367 * | 12.178 * |
| Measurement point (M) | 2 | 0.0217 * | 1.0873 * | 1.9154 * | 4.4020 * | 1.3606 * | 14.373 * | 0.8684 * | 89.485 * | 0.0273 * | 99.214 * |
| L × V | 5 | 0.0046 * | 0.0045 * | 0.0033 * | 0.4963 * | 0.1504 * | 0.0404 ns | 0.1309 * | 1.5515 * | 0.0061 * | 3.2458 * |
| L × T | 1 | 0.0134 * | 0.0014 ns | 0.0204 * | 0.2670 ns | 0.0000 ns | 0.1007 * | 0.2613 * | 0.0584 ns | 0.0040 ns | 0.1454 ns |
| V × T | 5 | 0.0021 * | 0.0036 * | 0.0092 * | 0.3110 * | 0.1135 ns | 0.0825 * | 0.0627 * | 0.6261 * | 0.0218 * | 6.2946 * |
| L × M | 2 | 0.0028 * | 0.2210 * | 0.3933 * | 0.3258 * | 0.3597 * | 1.5361 * | 0.0008 ns | 22.316 * | 0.0954 * | 38.717 * |
| V × M | 10 | 0.0024 * | 0.0093 * | 0.0104 * | 0.4299 * | 0.2365 * | 0.1653 * | 0.0604 * | 0.8278 * | 0.0235 * | 4.4150 * |
| T × M | 2 | 0.0060 * | 0.0091 * | 0.0065 * | 0.1906 ns | 0.0117 ns | 0.0203 ns | 0.1246 * | 2.8933 * | 0.0367 * | 2.1364 * |
| L × V × T | 5 | 0.0026 * | 0.0053 * | 0.0060 * | 0.2133 ns | 0.1064 ns | 0.0394 ns | 0.0572 * | 0.6986 * | 0.0089 * | 4.0147 * |
| L × V × M | 10 | 0.0022 * | 0.0039 * | 0.0077 * | 0.2696 * | 0.1467 * | 0.0460 * | 0.0489 * | 0.6680 * | 0.0115 * | 2.8763 * |
| L × T × M | 2 | 0.0009 ns | 0.0073 * | 0.0069 * | 1.1995 * | 0.9113 * | 0.5624 * | 0.0199 ns | 0.0269 ns | 0.0609 * | 0.9325 ns |
| V × T × M | 10 | 0.0023 * | 0.0036 * | 0.0030 * | 0.4347 * | 0.1987 * | 0.0663 * | 0.0678 * | 1.3925 * | 0.0229 * | 3.1128 * |
| L × V × T × M | 10 | 0.0023 * | 0.0058 * | 0.0079 * | 0.3705 * | 0.1362 * | 0.0561 * | 0.0738 * | 1.0498 * | 0.0157 * | 3.7578 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katanić, Z.; Mlinarić, S.; Katanić, N.; Ćosić, J.; Španić, V. Photosynthetic Efficiency in Flag Leaves and Ears of Winter Wheat during Fusarium Head Blight Infection. Agronomy 2021, 11, 2415. https://doi.org/10.3390/agronomy11122415
Katanić Z, Mlinarić S, Katanić N, Ćosić J, Španić V. Photosynthetic Efficiency in Flag Leaves and Ears of Winter Wheat during Fusarium Head Blight Infection. Agronomy. 2021; 11(12):2415. https://doi.org/10.3390/agronomy11122415
Chicago/Turabian StyleKatanić, Zorana, Selma Mlinarić, Nataša Katanić, Josipa Ćosić, and Valentina Španić. 2021. "Photosynthetic Efficiency in Flag Leaves and Ears of Winter Wheat during Fusarium Head Blight Infection" Agronomy 11, no. 12: 2415. https://doi.org/10.3390/agronomy11122415
APA StyleKatanić, Z., Mlinarić, S., Katanić, N., Ćosić, J., & Španić, V. (2021). Photosynthetic Efficiency in Flag Leaves and Ears of Winter Wheat during Fusarium Head Blight Infection. Agronomy, 11(12), 2415. https://doi.org/10.3390/agronomy11122415







