Temporary Immersion System for Production of Biomass and Bioactive Compounds from Medicinal Plants
Abstract
:1. Micropropagation of Medicinal Plants
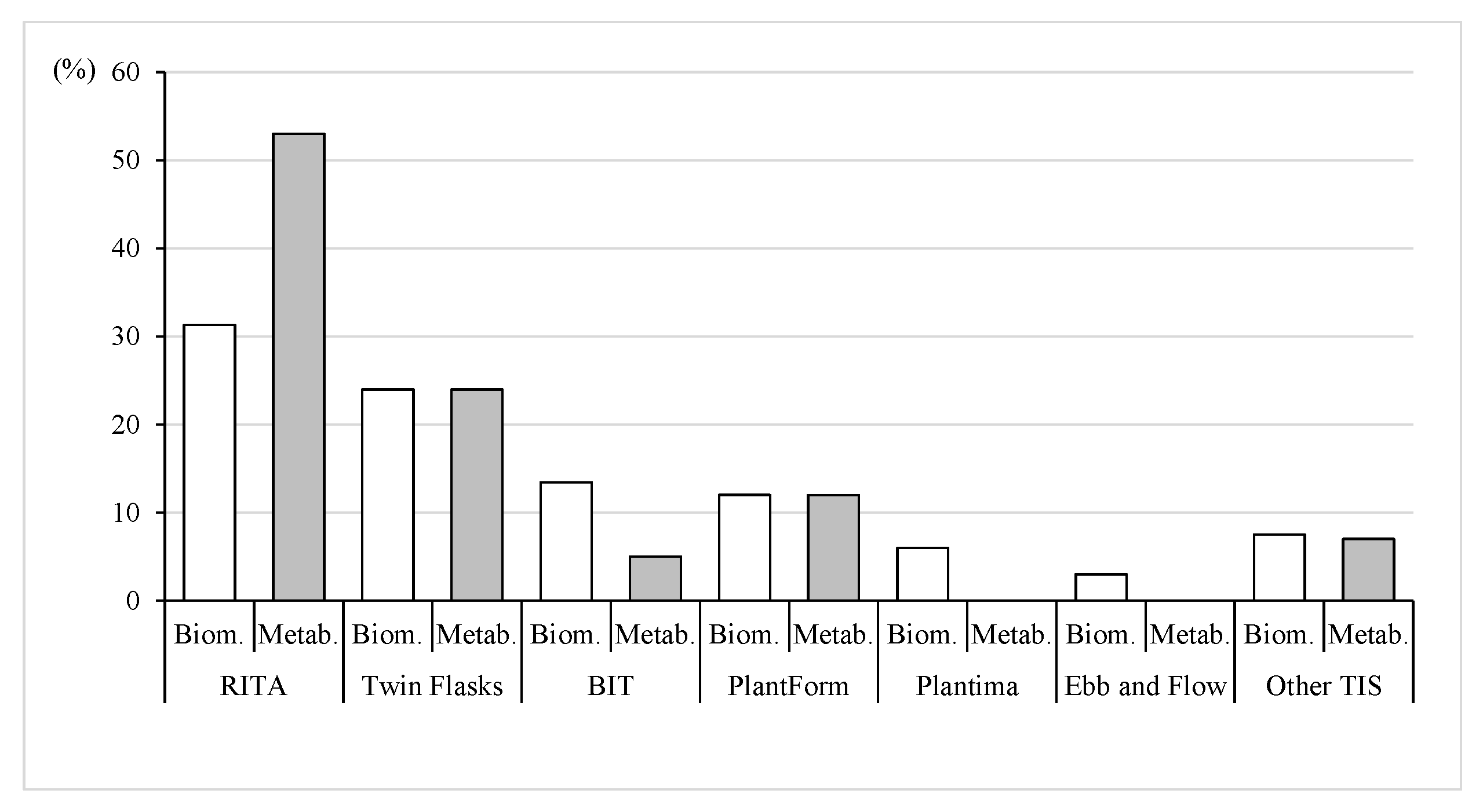
2. TIS Bioreactors for Micropropagation of Medicinal Plants
3. Use of TIS in Medicinal Plants for the Production of Biomass and Bioactive Compounds from 2000 to Today
3.1. Culture System
3.1.1. Culture System and Biomass Production
3.1.2. Culture System and Bioactive Compounds Content
| Species | Bioreactor * | Immersion Frequency (Immersion/Dry) | Bioactive Compound in TIS (vs. Semi Solid Culture System) ** | Reference |
|---|---|---|---|---|
| Arnica montana | RITA | 5 min/6 h | Sesquiterpene lactones (mg/g DW): 15.34 (7.62) | [105] |
| Aristotelia chilensis | Two Vessels | 4 min/6 h | Total phenols (g GAE/100 g DW): 2.97 (3.04 in wild plants) | [49] |
| Beta vulgaris | RITA | 15 min/1 h | Betalains (mg/g DW): 18.8 Betacyanins (mg/g DW): 9.6 Betaxanthins (mg/g DW): 9.2 | [117] |
| Camptotheca acuminata | DVS, RITA | 1 min/3 h | Camptothecin (mg/g DW): 2.5 (2.2) | [106] |
| Castilleja tenuiflora | RITA | 30 min/24 h | Total phenolics (mg GAE/g DW): 30.58 Total flavonoid (μg CE/g DW): 45.83 | [118] |
| RITA | 5 min/24 h | Total phenolics (mg GAE/g DW): 41.3 Verbascoside (mg/g DW): 113.9 Isoverbascoside (mg/g DW): 36.4 | [119] | |
| Catharanthus roseus | Bottles | 5 min/3 h | Ajmalicine (μg/g DW): 950 | [113] |
| Centaurium maritimum | RITA | 15 min/45 min | Total secoiridoid glycosides: 8 times higher vs. liquid culture | [116] |
| Centella asiatica | PlantForm | 5 min/90 min | Asiaticoside (mg/g DW): 7.91 (4.45 in agitated flasks) Madecassosid (mg/g DW): 3.81 (2.40 in agitated flasks) | [120] |
| Chlorophytum borivilianum | RITA | 15 min/1 h | Total saponin (mg diosgenin equiv./g DW): 21 (16 in mother plants) | [121] |
| Cymbopogon citratus | Twin Vessels | 5 min/4 h | α-citral (mg/g DW): 0.35 (0.27) β-citral (mg/g DW): 0.54 (0.46) | [109] |
| Digitalis lanata | Two Vessels | 2 min/4 h | Lanatoside C (µg/g DW): 316 | [122] |
| Digitalis purpurea | Two Vessels | 2 min/4 h | Digitoxin (µg): 167.6 Digoxin (µg): 119.9 | [123] |
| Dracocephalum forrestii | RITA | 10 min/80 min | Chlorogenic acid (mg/g DW): 0.99 (0.88 in NSB) Acacetin rhamnosyl-trihexoside (mg/g DW): 1.01 (0.91 in NSB) Rosmarinic acid (mg/g DW): 11.91 (18.35 in NSB) Acacetin acetylrhamnosyl-trihexoside (mg/g DW): 2.45 (2.25 in NSB) Apigenin p-coumaroylrhamnoside II (mg/g DW): 2.28 (1.76 in NSB) | [124] |
| Drosera communis | Twin Bottles | 5 min/4 h | Plumbagin content (μg/g DW): 2.44 (4.22) | [69] |
| Fabiana imbricata | Twin Vessels | 5 min/4 h | Oleanolic acid (mg/g DW): 0.01 (0.01) | [109] |
| Bottles | 10 min/4 h | Oleanolic acid (% DW): up to 0.14% (3.47 in wild plant) Rutin (% DW): 0.20 (3.35 in wild plant) | [43] | |
| Gynura procumbens | BIT | 15 min/12 h | Flavonoids content mg (CE/g DW): 32.0 | [125] |
| Harpagophytum procumbens | Two Erlenmeyer Flasks | 60 min/24 h | Harpagoside (mg/gDW): Leaves: 6.0 (3.5 in glasshouse grown plants) Stem: 3.8 (5 in glasshouse grown plants) Root: 8 (2 in glasshouse grown plants) | [114] |
| Hypericum perforatum | Twin Vessels | 5 min/4 h | Hypericin (mg/g DW): 0.18 (0.15) | [109] |
| Lavandula officinalis | Twin Vessels | 5 min/4 h | Rosmarinic acid (mg/g DW): 5.7 (55.3) | [109] |
| Leucojum aestivum | Bioreactor Vessels | 5 min/2 h | Galanthamine (mg/l): 4.64 | [126] |
| RITA | 15 min/8 h | Galanthamine (μg/RITA): 265 Lycorine (μg/RITA): 1.699 Norgalanthamine (μg/RITA): 225 | [127] | |
| Lycium barbarum | PlantForm | 6 min/24 h | Total phenol (mg GAE/g DW): 23.6 (19.4) Total flavonoid (mg RE/g DW): 1.9 (18.5) | [107] |
| Nasturtium officinale | RITA | not reported | Total polyphenols (mmol TE/100 g DW): 3.74 (2.70 in mother plants) Total flavonoids (mmol RE/100 g DW): 1.64 (1.89 in mother plants) Total glucosionolates (mg/100 g DW): 261.97 (799.47 in mother plants) | [128] |
| Panax ginseng | RITA | 5 min/1 h | Saponin (mg/g DW): 15.94 | [129] |
| RITA | 5 min/1 h | Saponin (mg/g DW): 28.51 | [130] | |
| Pancratium maritimum | RITA | 15 min/12 h | Alkaloid (μg/g DW): 3.469 Haemanthamine (μg/g): 900.1 Lycorine (μg/g): 799.9 | [131] |
| Rhododendron tomentosum | RITA | 5 min/85 min | p-cymene (%): 6.9 (0.9) Alloaromadendrene (%): 5.5 (8.1) Shyobunone (%): 8.2 (15.8) Ledene oxide (II) (%): 13.0 (14.7) | [115] |
| Rosa canina | RITA | 15 min/12 h 15 min/6 h 15 min/8 h | Total phenolics (mg/g FW): 15.8 (11.4) Soluble sugars (mg/g FW): 9.2 (8.0) Carotenoids (mg/g FW): 0.66 (0.99) | [132] |
| Rosa rubiginosa | RITA | 15 min/8 h | Total phenolics (mg/g FW): 8.03 (9.57) Carotenoids (mg/g FW): 0.10 (0.17) Soluble sugars (mg/g FW): 9.41 (7.99) | [108] |
| Rosa rugosa | BTBB | not reported | Total phenolics (mg/g DW): 10 (22) Total flavonoids (mg/g DW): 3 (3.5) | [133] |
| Rosa tomentosa | RITA | 15 min/8 h | Total phenolics (mg/g FW): 9.91 (9.66) Carotenoids (mg/g FW): 0.11 (0.09) Soluble sugars (mg/g FW): 7.34 (9.39) | [108] |
| Salvia viridis | PlantForm | 10 min/80 min | Total phenolic acid (mg/g DW): 18.3 Total phenylethanoid (mg/g DW): 11.4 Total phenol (mg/g DW): 29.7 | [134] |
| Schisandra chinensis | PlantForm | 5 min/90 min | Lignans (mg/100 g DW): 546.98 (185.77 in agitated cultures) Schisandrin (mg/100 g DW): 118.59 Deoxyschisandrin (mg/100 g DW): 77.66 Gomisin A (mg/100 g DW): 67.86 | [135] |
| PlantForm, RITA | 5 min/90 min | Total phenolic acids (mg/100 g DW): 34.56 in PlantForm (46.68 in continuous immersion) Total flavonoids (mg/100 g DW): 21.27 in RITA (29.02 in continuous immersion) | [136] | |
| Scutellaria baicalensis | RITA | 5 min/3 h | Baicalein (μg/mg DW): 2.2 (1.1) Baicalin (μg/mg DW): 3.0 (1.0) Wogonin (μg/mg DW): 0.10 (0.25) | [110] |
| Stevia rebaudiana | BIT | 3 min/6 h | Total steviol glycosides/container (g): 0.1698 (0.0239) | [111] |
| Thapsia garganica | RITA | 3 min/6 h | Thapsigargin (mg/g DW): 2.15 Nortrilobolide (mg/g DW): 17.42 | [137] |
| Vaccinium vitis-idaea ssp. minus | RITA | not reported | Carotenoids (μg/mL): 0.40 (0.30 in stationary bioreactor) | [112] |
| Zeltnera beyrichii | RITA | 15 min/6 h | Secoiridoid glycoside (mmol per 100 g DW): 7.5 (30) | [138] |
3.2. Type of TIS Bioreactors
3.2.1. Types of TIS Bioreactors and Biomass Production
3.2.2. Type of TIS Bioreactors and Bioactive Compounds Content
3.3. Immersion Frequency
3.3.1. Immersion Frequency and Biomass Production
3.3.2. Immersion Frequency and Bioactive Compounds Content
3.4. Influence of TIS on Medium Composition
3.4.1. Medium Composition and Biomass Production
3.4.2. Medium Composition and Bioactive Compounds Content
4. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chandran, H.; Meena, M.; Barupal, T.; Sharma, K. Plant tissue culture as a perpetual source for production of industrially important bioactive compounds. Biotechnol. Rep. 2020, 26, e00450. [Google Scholar] [CrossRef]
- Allen, D.; Bilz, M.; Leaman, D.J.; Miller, R.M.; Timoshyna, A.; Window, J. European Red List of Medicinal Plants; Publications Office of the European Union: Luxembourg, Germany, 2014; ISBN 9789279474507. [Google Scholar]
- Rout, G.R.; Samantaray, S.; Das, P. In vitro manipulation and propagation of medicinal plants. Biotechnol. Adv. 2000, 18, 91–120. [Google Scholar] [CrossRef]
- Chaturvedi, H.C.; Jain, M.; Kidwai, N.R. Cloning of medicinal plants through tissue culture—A review. Indian J. Exp. Biol. 2007, 45, 937–948. [Google Scholar] [PubMed]
- Gantait, S.; Mandal, N.; Nandy, S. Advances in micro propagation of selected aromatic plants: A review on vanilla and strawberry. Am. J. Biochem. Mol. Biol. 2011, 1, 1–19. [Google Scholar] [CrossRef]
- Bajaj, Y.P.S.; Furmanowa, M.; Olszowska, O. Biotechnology of the Micropropagation of Medicinal and Aromatic Plants. In Medicinal and Aromatic Plants I. Biotechnology in Agriculture and Forestry; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1988; Volume 4, pp. 60–103. [Google Scholar]
- Constabel, F. Medicinal Plant Biotechnology. Planta Med. 1989, 56, 421–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.G.; Wu, J.Y. Development and application of medicinal plant tissue cultures for production of drugs and herbal medicinals in China. Nat. Prod. Rep. 2006, 23, 789–810. [Google Scholar] [CrossRef] [PubMed]
- Yadav, K.; Singh, N.; Verma, S. Plant tissue culture: A biotechnological tool for solving the problem of propagation of multipurpose endangered medicinal plants in India. J. Agric. Technol. 2012, 8, 305–318. [Google Scholar]
- Máthé, Á.; Hassan, F.; Kader, A.A. In Vitro Micropropagation Of Medicinal and Aromatic Plants. In Medicinal and Aromatic Plants of the World. Medicinal and Aromatic Plants of the World; Máthé, Á., Ed.; Springer: Dordrecht, The Netherlands, 2015; Volume 1, pp. 305–336. [Google Scholar]
- Moraes, R.M.; Cerdeira, A.L.; Lourenço, M.V. Using micropropagation to develop medicinal plants into crops. Molecules 2021, 26, 1752. [Google Scholar] [CrossRef]
- Moraes-Cerdeira, R.M.; Burandt, C.L.; Bastos, J.K.; Nanayakkara, N.P.D.; Mc Chesney, J.D. In vitro propagation of Podophyllum peltatum. Planta Med. 1998, 64, 42–45. [Google Scholar] [CrossRef]
- Sidhu, Y. In vitro micropropagation of medicinal plants by tissue culture. Plymouth Stud. Sci. 2010, 4, 432–449. [Google Scholar]
- Khan, M.Y.; Aliabbas, S.; Kumar, V.; Rajkumar, S. Recent advances in medicinal plant biotechnology. Indian J. Biotechnol. 2009, 8, 9–22. [Google Scholar]
- Piątczak, E.; Grzegorczyk-Karolak, I.; Wysokińska, H. Micropropagation of Rehmannia glutinosa Libosch.: Production of phenolics and flavonoids and evaluation of antioxidant activity. Acta Physiol. Plant. 2014, 36, 1693–1702. [Google Scholar] [CrossRef] [Green Version]
- Takayama, S.; Akita, M. The types of bioreactors used for shoots and embryos. Plant Cell Tissue Organ Cult. 1994, 39, 147–156. [Google Scholar] [CrossRef]
- Leathers, R.R.; Smith, M.A.L.; Aitken-Christie, J. Automation of the Bioreactor Process for Mass Propagation and Secondary Metabolism. In Automation and Environmental Control in Plant Tissue Culture; Aitken-Christie, J., Kozai, T., Smith, M.A.L., Eds.; Springer: Dordrecht, The Netherlands, 1995; pp. 187–214. [Google Scholar]
- Chakrabarty, D.; Paek, K.Y. Recent Advances in In Vitro Manipulation and Propagation of Medicinal Plants, Proceedings of KSMCS International Symposium. 2001, pp. 26–61. Available online: https://kiss.kstudy.com/journal/journal-view.asp?key1=27476&key2=8017 (accessed on 20 November 2021).
- Paek, K.Y.; Chakrabarty, D.; Hahn, E.J. Application of bioreactor systems for large scale production of horticultural and medicinal plants. Plant Cell Tissue Organ Cult. 2005, 81, 287–300. [Google Scholar] [CrossRef]
- Mamun, N.H.A.; Egertsdotter, U.; Aidun, C.K. Bioreactor technology for clonal propagation of plants and metabolite production. Front. Biol. 2015, 10, 177–193. [Google Scholar] [CrossRef]
- Krol, A.; Kokotkiewicz, A.; Szopa, A.; Ekiert, H.M.; Luczkiewicz, M. Bioreactor-Grown Shoot Cultures for the Secondary Metabolite Production. In Plant Cell and Tissue Differentiation and Secondary Metabolites; Ramawat, K.G., Ekiert, H.M., Goyal, S., Eds.; Springer: Cham, Switzerland, 2021; pp. 187–247. [Google Scholar]
- Takayama, S.; Misawa, M. Mass propagation of Begonia × hiemalis plantlets by shake culture. Plant Cell Physiol. 1981, 22, 461–467. [Google Scholar]
- Debnath, S.C. Bioreactors and molecular analysis in berry crop micropropagation—A review. Can. J. Plant Sci. 2011, 91, 147–157. [Google Scholar] [CrossRef]
- Yaseen, M.; Ahmad, T.; Sablok, G.; Standardi, A.; Hafiz, I.A. Review: Role of carbon sources for in vitro plant growth and development. Mol. Biol. Rep. 2013, 40, 2837–2849. [Google Scholar] [CrossRef] [PubMed]
- Dewir, Y.H.; Indoliya, Y.; Chakrabarty, D.; Paek, K.Y. Biochemical and physiological aspects of hyperhydricity in liquid culture system. In Production of Biomass and Bioactive Compounds Using Bioreactor Technology; Paek, K.Y., Murthy, H., Zhong, J.J., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 693–709. [Google Scholar]
- Berthouly, M.; Etienne, H. Temporary Immersion System: A New Concept for Use Liquid Medium in Mass Propagation. In Liquid Culture Systems for In Vitro Plant Propagation; Hvoslef-Eide, A.K., Preil, W., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 165–195. [Google Scholar]
- Harris, R.E.; Mason, E.B.B. Two machines for in vitro propagation of plants in liquid media. Can. J. Plant Sci. 1983, 63, 311–316. [Google Scholar] [CrossRef]
- Etienne, H.; Berthouly, M. Temporary immersion systems in plant micropropagation. Plant Cell Tissue Organ Cult. 2002, 69, 215–231. [Google Scholar] [CrossRef]
- Georgiev, V.; Schumann, A.; Pavlov, A.; Bley, T. Temporary immersion systems in plant biotechnology. Eng. Life Sci. 2014, 14, 607–621. [Google Scholar] [CrossRef]
- Watt, P. The status of temporary inmersion system (TIS) technology for plant micropropagation. Afr. J. Biotechnol. 2012, 11, 14025–14035. [Google Scholar]
- Wilken, D.; Jiménez Gonzalez, E.; Gerth, A.; Gómez-Kosky, R.; Schumann, A.; Claus, D. Effect of immersion systems, lighting, and TIS designs on biomass increase in micropropagating banana (Musa spp. cv. “Grande naine” AAA). In Vitro Cell. Dev. Biol.-Plant 2014, 50, 582–589. [Google Scholar] [CrossRef]
- Akdemir, H.; Süzerer, V.; Onay, A.; Tilkat, E.; Ersali, Y.; Çiftçi, Y.O. Micropropagation of the pistachio and its rootstocks by temporary immersion system. Plant Cell Tissue Organ Cult. 2014, 117, 65–76. [Google Scholar] [CrossRef]
- Carvalho, L.S.O.; Ozudogru, E.A.; Lambardi, M.; Paiva, L.V. Temporary immersion system for micropropagation of tree species: A bibliographic and systematic review. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 269–277. [Google Scholar] [CrossRef] [Green Version]
- Steingroewer, J.; Bley, T.; Georgiev, V.; Ivanov, I.; Lenk, F.; Marchev, A.; Pavlov, A. Bioprocessing of differentiated plant in vitro systems. Eng. Life Sci. 2013, 13, 26–38. [Google Scholar] [CrossRef]
- Yancheva, S.; Georgieva, L.; Badjakov, I.; Dincheva, I.; Georgieva, M.; Georgiev, V.; Kondakova, V. Application of bioreactor technology in plant propagation and secondary metabolite production. J. Cent. Eur. Agric. 2019, 20, 321–340. [Google Scholar] [CrossRef]
- Debergh, P.; Aitken-Christie, J.; Cohen, D.; Grout, B.; von Arnold, S.; Zimmerman, R.; Ziv, M. Reconsideration of the term ‘vitrification’ as used in micropropagation. Plant Cell Tissue Organ Cult. 1992, 30, 135–140. [Google Scholar] [CrossRef]
- Gaspar, T. Vitrification in Micropropagation. In High-Tech and Micropropagation I. Biotechnology in Agriculture and Forestry; Bajaj, Y.P.S., Ed.; Springer: Berlin, Heidelberg, 1991; Volume 17, pp. 116–126. [Google Scholar]
- Ziv, M. Vitrification: Morphological and physiological Disorders of In Vitro Plants. In Micropropagation; Springer: Dordrecht, The Netherlands, 1991; pp. 45–69. [Google Scholar]
- Rojas-Martínez, L.; Visser, R.G.F.; de Klerk, G.J. The hyperhydricity syndrome: Waterlogging of plant tissues as a major cause. Propag. Ornam. Plants 2010, 10, 169–175. [Google Scholar]
- Vidensek, N.; Lim, P.; Campbell, A.; Carlson, C. Taxol content in bark, wood, root, leaf, twig, and seedling from several taxus species. J. Nat. Prod. 1990, 53, 1609–1610. [Google Scholar] [CrossRef]
- Zobayed, S.M.A.; Saxena, P.K. In vitro-grown roots: A superior explant for prolific shoot regeneration of St. John’s wort (Hypericum perforatum L. cv ‘New Stem’) in a temporary immersion bioreactor. Plant Sci. 2003, 165, 463–470. [Google Scholar] [CrossRef]
- Kaya, E.; Galatali, S.; Guldag, S.; Ozturk, B.; Ceylan, M.; Celik, O.; Aktay, I. Mass production of medicinal plants for obtaining secondary metabolite using liquid mediums via bioreactor systems: SETISTM and RITA®. Derleme 2018, 11, 5–10. [Google Scholar]
- Schmeda-Hirschmann, G.; Jordan, M.; Gerth, A.; Wilken, D.; Hormazabal, E.; Tapia, A.A. Secondary metabolite content in Fabiana imbricata plants and in vitro cultures. Z. Naturforsch. -Sect. C J. Biosci. 2004, 59, 48–54. [Google Scholar] [CrossRef]
- Yoon, Y.J.; Murthy, H.N.; Eun, J.H.; Kee, Y.P. Biomass production of Anoectochilus formosanus Hayata in a bioreactor system. J. Plant Biol. 2007, 50, 573–576. [Google Scholar] [CrossRef]
- Ruffoni, B.; Savona, M. The temporary immersion system (T.I.S.) for the improvement of micropropagation of ornamental plants. Acta Hortic. 2005, 683, 445–454. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-Mosqueda, M.A.; Cruz-Cruz, C.A.; Cano-Ricárdez, A.; Bello-Bello, J.J. Assessment of different temporary immersion systems in the micropropagation of anthurium (Anthurium andreanum). 3 Biotech 2019, 9, 307. [Google Scholar] [CrossRef]
- Martínez-Estrada, E.; Islas-Luna, B.; Pérez-Sato, J.A.; Bello-Bello, J.J. Temporary immersion improves in vitro multiplication and acclimatization of Anthurium andreanum Lind. Sci. Hortic. 2019, 249, 185–191. [Google Scholar] [CrossRef]
- Esyanti, R.R.; Fadholi, M.; Rizki, R.M.; Faizal, A. Shoot multiplication and growth rates of Aquilaria malaccensis Lamk. shoot cultures in temporary immersion system (TIS)-RITA® and bubble column bioreactors. Pak. J. Bot. 2019, 51, 1317–1321. [Google Scholar] [CrossRef]
- Trentini, G.E.; Rojas, M.; Gajardo, D.; Alburquenque, D.; Villagra, E.; Gómez, A.; Arru, L.; Arencibia, A.D. Elicitation of phenylpropanoids in maqui (Aristotelia chilensis [Mol.] Stuntz) plants micropropagated in photomixotrophic temporary immersion bioreactors (TIBs). Plant Cell Tissue Organ Cult. 2021, 146, 607–619. [Google Scholar] [CrossRef]
- Liu, C.Z.; Murch, S.J.; El-Demerdash, M.; Saxena, P.K. Artemisia judaica L.: Micropropagation and antioxidant activity. J. Biotechnol. 2004, 110, 63–71. [Google Scholar] [CrossRef]
- García-Ramírez, Y.; Barrera, G.P.; Freire-Seijo, M.; Barbón, R.; Concepción-Hernández, M.; Mendoza-Rodríguez, M.F.; Torres-García, S. Effect of sucrose on physiological and biochemical changes of proliferated shoots of Bambusa vulgaris Schrad. Ex Wendl in temporary immersion. Plant Cell Tissue Organ Cult. 2019, 137, 239–247. [Google Scholar] [CrossRef]
- Zhang, B.; Sarsaiya, S.; Pan, X.; Jin, L.; Xu, D.; Zhang, B.; Duns, G.J.; Shi, J.; Chen, J. Optimization of nutritional conditions using a temporary immersion bioreactor system for the growth of Bletilla striata pseudobulbs and accumulation of polysaccharides. Sci. Hortic. 2018, 240, 155–161. [Google Scholar] [CrossRef]
- Yang, S.H.; Yeh, D.M. In vitro leaf anatomy, ex vitro photosynthetic behaviors and growth of Calathea orbifolia (Linden) Kennedy plants obtained from semi-solid medium and temporary immersion systems. Plant Cell Tissue Organ Cult. 2008, 93, 201–207. [Google Scholar] [CrossRef]
- Gianguzzi, V.; Inglese, P.; Barone, E.; Sottile, F. In vitro regeneration of Capparis spinosa L. by using a temporary immersion system. Plants 2019, 8, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreira, A.L.; da Silva, A.B.; Santos, A.; dos Reis, C.O.; Landgraf, P.R.C. Crescimento de Cattleya walkeriana em diferentes sistemas de micropropagação. Cienc. Rural 2013, 43, 1804–1810. [Google Scholar] [CrossRef] [Green Version]
- Wawrosch, C.; Kongbangkerd, A.; Köpf, A.; Kopp, B. Shoot regeneration from nodules of Charybdis sp.: A comparison of semisolid, liquid and temporary immersion culture systems. In Liquid Culture Systems for In Vitro Plant Propagation; Hvoslef-Eide, A.K., Preil, W., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 275–280. [Google Scholar]
- Mancilla-álvarez, E.; Pérez-Sato, J.A.; Núñez-Pastrana, R.; Spinoso-Castillo, J.L.; Bello-Bello, J.J. Comparison of different semi-automated bioreactors for in vitro propagation of taro (Colocasia esculenta L. Schott). Plants 2021, 10, 1010. [Google Scholar] [CrossRef] [PubMed]
- Murch, S.J.; Liu, C.; Romero, R.M.; Saxena, P.K. In Vitro culture and temporary immersion bioreactor production of Crescentia cujete. Plant Cell Tissue Organ Cult. 2004, 78, 63–68. [Google Scholar] [CrossRef]
- Quiala, E.; Barbón, R.; Jiménez, E.; De Feria, M.; Chávez, M.; Capote, A.; Pérez, N. Biomass production of Cymbopogon citratus (D.C.) Stapf., a medicinal plant, in temporary immersion systems. In Vitro Cell. Dev. Biol. -Plant 2006, 42, 298–300. [Google Scholar] [CrossRef]
- Quiala, E.; Barbón, R.; Capote, A.; Pérez, N.; Jiménez, E. In Vitro Mass Propagation of Cymbopogon citratus Stapf., A medicinal Gramineae. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2016; Volume 1391. [Google Scholar]
- Marchant, M.J.; Molina, P.; Montecinos, M.; Guzmán, L.; Balada, C.; Fassio, C.; Castro, M. In vitro propagation of Easter Island Curcuma longa from rhizome explants using temporary immersion system. Agronomy 2021, 11, 2121. [Google Scholar] [CrossRef]
- Stanly, C.; Bhatt, A.; Keng, C.L. A comparative study of Curcuma zedoaria and Zingiber zerumbet plantlet production using different micropropagation systems. Afr. J. Biotechnol. 2010, 9, 4326–4333. [Google Scholar] [CrossRef]
- Ahmadian, M.; Babaei, A.; Shokri, S.; Hessami, S. Micropropagation of carnation (Dianthus caryophyllus L.) in liquid medium by temporary immersion bioreactor in comparison with solid culture. J. Genet. Eng. Biotechnol. 2017, 15, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Thi, L.T.; Park, Y.G.; Jeong, B.R. Growth and development of carnation ‘Dreambyul’ plantlets in a temporary immersion system and comparisons with conventional solid culture methods. In Vitro Cell. Dev. Biol. -Plant 2019, 55, 539–548. [Google Scholar] [CrossRef]
- Pérez-Alonso, N.; Capote, A.; Pérez, A.; Gerth, A.; Chong-Pérez, B.; Jiménez, E. Efecto de la densidad de inóculo y la renovación de la atmósfera gaseosa en el cultivo de brotes de Digitalis purpurea L. en Sistemas de Inmersión Temporal. Biotecnol. Veg. 2015, 15, 35–45. [Google Scholar]
- Pérez-Alonso, N.; Chong-Pérez, B.; Capote, A.; Pérez, A.; Gerth, A.; Angenon, G.; Jiménez, E. Biotechnological Approaches for Biomass and Cardenolide Production in Digitalis purpurea L. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2016; Volume 1391, pp. 81–102. [Google Scholar]
- Yan, H.; Yang, L.; Li, Y. Improved growth and quality of Dioscorea fordii Prain et Burk and Dioscorea alata plantlets using a temporary immersion system. Afr. J. Biotechnol. 2011, 10, 19444–19448. [Google Scholar] [CrossRef]
- Lata, H.; Bedir, E.; Moraes, R.M.; Andrade, Z. Mass Propagation of Echinacea Angustifolia: A Protocol Refinement Using Shoot Encapsulation and Temporary Immersion Liquid System. In Proceedings of the Acta Horticulturae; Canadian International Development Agency: Toronto, Canada, 2004; Volume 629. [Google Scholar]
- Kunakhonnuruk, B.; Inthima, P.; Kongbangkerd, A. In vitro propagation of rheophytic orchid, Epipactis flava Seidenf.—A comparison of semi-solid, continuous immersion and temporary immersion systems. Biology 2019, 8, 72. [Google Scholar] [CrossRef] [Green Version]
- Souza, D.M.S.C.; Avelar, M.L.M.; Fernandes, S.B.; Silva, E.O.; Duarte, V.P.; Molinari, L.V.; Brondani, G.E. Spectral quality and temporary immersion bioreactor for in vitro multiplication of Eucalytpus grandis × Eucalyptus urophylla. 3 Biotech 2020, 10, 457. [Google Scholar] [CrossRef]
- Mohd, N.M.; Ja’afar, H.; Zawawi, D.D.; Alias, N. In vitro somatic embryos multiplication of Eurycoma longifolia Jack using temporary immersion system RITA®. Sains Malays. 2017, 46, 897–902. [Google Scholar] [CrossRef]
- Wawrosch, C. Temporary Immersion Systems for Efficient Mass Propagation of Medicinal and Aromatic Plants. In Proceedings of the Acta Horticulturae; ISHS: Angers, France, 2015; Volume 1099. [Google Scholar]
- Manuhara, Y.S.W.; Kusuma, D.Y.; Sari, R.L.K.; Kristanti, A.N. Biomass production of Gynura procumbens adventitious roots in different type of liquid culture. Biosaintifika J. Biol. Biol. Educ. 2017, 9, 523. [Google Scholar] [CrossRef]
- Martre, P.; Lacan, D.; Just, D.; Teisson, C. Physiological effects of temporary immersion on Hevea brasiliensis callus. Plant Cell Tissue Organ Cult. 2001, 67, 25–35. [Google Scholar] [CrossRef]
- Ilczuk, A.; Winkelmann, T.; Richartz, S.; Witomska, M.; Serek, M. In vitro propagation of Hippeastrum × chmielii Chm.—Influence of flurprimidol and the culture in solid or liquid medium and in temporary immersion systems. Plant Cell Tissue Organ Cult. 2005, 83, 339–346. [Google Scholar] [CrossRef]
- Be, N.; Zhu, L. Developing an in vitro propagation method for mass production of medicinal Hypoxis species using bioreactors. Asian J. Plant Sci. Res. 2017, 7, 1–8. [Google Scholar]
- Malosso, M.G.; Bertoni, B.W.; da Silva Coppede, J.; de Castro Franca, S.; Pereira, A.M.S. Micropropagation and in vitro conservation of Jacaranda decurrens Cham. J. Med. Plants Res. 2012, 6, 1147–1154. [Google Scholar] [CrossRef]
- Jeong, B.R.; Sivanesan, I. Micropropagation, berberine content and antitumor activity of Jeffersonia dubia (Maxim.) Benth et Hook. Plant Cell Tissue Organ Cult. 2016, 124, 453–458. [Google Scholar] [CrossRef]
- Shaik, S.; Dewir, Y.H.; Singh, N.; Nicholas, A. Micropropagation and bioreactor studies of the medicinally important plant Lessertia (Sutherlandia) frutescens L. S. Afr. J. Bot. 2010, 76, 180–186. [Google Scholar] [CrossRef] [Green Version]
- Ptak, A. Leucojum aestivum L. in vitro bulbs induction and acclimatization. Cent. Eur. J. Biol. 2014, 9, 1011–1021. [Google Scholar] [CrossRef]
- Jiménez, E.; Reyes, C.; Machado, P.; Pérez-Alonso, N.; Capote, A.; Pérez, A.; Eichler-Loebermann, B. Multiplicación in vitro de Morinda royoc L. en Sistemas de Inmersión Temporal. Biotecnol. Veg. 2011, 11, 115–118. [Google Scholar]
- De Carlo, A.; Cencetti, G.; Michelozzi, M. Impiego dell’innovativo bioreattore “Plantform” ad immersione temporanea per la coltura in vitro di Myrtus communis e per la produzione di metaboliti secondari. Acta Italus Hortus 2013, 12, 146. [Google Scholar]
- Kaçar, Y.A.; Biçen, B.; Şimşek, Ö.; Dönmez, D.; Erol, M.H. Evaluation and comparison of a new type of temporary immersion system (TIS) bioreactors for myrtle (Myrtus communis L.). Appl. Ecol. Environ. Res. 2020, 18, 1611–1620. [Google Scholar] [CrossRef]
- Benelli, C.; De Carlo, A. In vitro multiplication and growth improvement of Olea europaea L. cv Canino with temporary immersion system (PlantformTM). 3 Biotech 2018, 8, 1–5. [Google Scholar] [CrossRef]
- Välimäki, S.; Paavilainen, L.; Tikkinen, M.; Salonen, F.; Varis, S.; Aronen, T. Production of Norway spruce embryos in a temporary immersion system (TIS). In Vitro Cell. Dev. Biol.-Plant 2020, 56, 430–439. [Google Scholar] [CrossRef]
- Jia, M.L.; Zhang, B.H.; Gao, W.P.; Chen, J.S.; Ouyang, P. Micropropagation of Pinellia Ternata (Thunb.) Breit in a bioreactor using temporary immersion system. China Biotechnol. 2012, 32, 49–54. [Google Scholar]
- De Sousa, P.C.A.; Souza, S.S.S.E.; Meira, F.S.; Meira, R.D.O.; Gomes, H.T.; Silva-Cardoso, I.M.D.A.; Scherwinski-Pereira, J.E. Somatic embryogenesis and plant regeneration in Piper aduncum L. In Vitro Cell. Dev. Biol. -Plant 2020, 56, 618–633. [Google Scholar] [CrossRef]
- Meimand, M.J.M.; Ruffoni, B.; Mascarello, C.; Shamshiri, M.H.; Malekzadeh, K. Micropropagation of Pistacia lentiscus L.—Optimization of the surface sterilization protocol and forced ventilation in temporary immersion culture. Fruit Grow. Res. 2020, 36, 75–82. [Google Scholar] [CrossRef]
- Vilchez, J.; Albany, N. Multiplicación in vitro de Psidium guajava L. en sistemas de inmersión temporal In vitro multiplication of Psidium guajava L. in temporary immersion systems. Rev. Colomb. Biotecnol. Diciembre 2014, 16, 96–103. [Google Scholar] [CrossRef]
- Gatti, E.; Sgarbi, E.; Ozudogru, E.A.; Lambardi, M. The effect of PlantformTM bioreactor on micropropagation of Quercus robur in comparison to a conventional in vitro culture system on gelled medium, and assessment of the microenvironment influence on leaf structure. Plant Biosyst. 2017, 151, 1129–1136. [Google Scholar] [CrossRef]
- Debnath, S.C. Zeatin and TDZ-induced shoot proliferation and use of bioreactor in clonal propagation of medicinal herb, roseroot (Rhodiola rosea L). J. Plant Biochem. Biotechnol. 2009, 18, 245–248. [Google Scholar] [CrossRef]
- Yan, H.; Liang, C.; Li, Y. Improved growth and quality of Siraitia grosvenorii plantlets using a temporary immersion system. Plant Cell Tissue Organ Cult. 2010, 103, 131–135. [Google Scholar] [CrossRef]
- Alvarenga-Venutolo, S.; Salazar-Aguilar, T. Micropropagación masiva de Stevia Rebaudiana Bertoni en sistemas de inmersión temporal. Rev. Cultiv. Trop. 2015, 36, 50–57. [Google Scholar]
- Sacco, E.; Mascarello, C.; Pamato, M.; Musso, V.; Ruffoni, B. Evaluation of Temporary Immersion System for in vitro propagation of Stevia rebaudiana Bertoni. Acta Hortic. 2015, 1083, 327–333. [Google Scholar] [CrossRef]
- Ramírez-Mosqueda, M.A.; Iglesias-Andreu, L.G. Evaluation of different temporary immersion systems (BIT®, BIG, and RITA®) in the micropropagation of Vanilla planifolia Jacks. In Vitro Cell. Dev. Biol. -Plant 2016, 52, 154–160. [Google Scholar] [CrossRef]
- Rosales, C.; Brenes, J.; Salas, K.; Arce-Solano, S.; Abdelnour-Esquivel, A. Micropropagación de Stevia rebaudiana en sistemas de inmersión temporal para incursionar en la producción hortícola. Rev. Chapingo Ser. Hortic. 2018, 24, 69–84. [Google Scholar] [CrossRef]
- Bayraktar, M. Micropropagation of Stevia rebaudiana Bertoni using RITA® bioreactor. HortScience 2019, 54, 725–731. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Kaushal, S.; Sharma, S.; Sood, H.; Sharma, D.R. Novel method of bioreactor to propagate Swertia chirayita: A critically endangered medicinal plant. Wkly. Sci. Res. J. 2013, 1, 7–8. [Google Scholar] [CrossRef]
- Peña-Rojas, G.; Carhuaz-Condori, R.; Andía-Ayme, V.; Dávalos-Prado, J.Z. Use of RITA® temporary immersion system to obtain microtubers of several mashua (Tropaeolum tuberosum Ruiz & Pavón) morphotypes. Trop. Subtrop. Agroecosystems 2020, 23, 1–10. [Google Scholar]
- Arencibia, A.D.; Vergara, C.; Quiroz, K.; Carrasco, B.; Bravo, C.; Garcia-Gonzales, R. An approach for micropropagation of blueberry (Vaccinium corymbosum L.) plants mediated by Temporary Immersion Bioreactors (TIBs). Am. J. Plant Sci. 2013, 4, 1022–1028. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Castellá, A.; Iglesias-Andreu, L.G.; Bello-Bello, J.J.; Lee-Espinosa, H. Improved propagation of vanilla (Vanilla planifolia Jacks. ex Andrews) using a temporary immersion system. In Vitro Cell. Dev. Biol. -Plant 2014, 50, 576–581. [Google Scholar] [CrossRef]
- Spinoso-Castillo, J.L.; Chavez-Santoscoy, R.A.; Bogdanchikova, N.; Pérez-Sato, J.A.; Morales-Ramos, V.; Bello-Bello, J.J. Antimicrobial and hormetic effects of silver nanoparticles on in vitro regeneration of vanilla (Vanilla planifolia Jacks. ex Andrews) using a temporary immersion system. Plant Cell Tissue Organ Cult. 2017, 129, 195–207. [Google Scholar] [CrossRef]
- Ramírez-Mosqueda, M.A.; Bello-Bello, J.J. SETISTM bioreactor increases in vitro multiplication and shoot length in vanilla (Vanilla planifolia Jacks. Ex Andrews). Acta Physiol. Plant. 2021, 43, 1–8. [Google Scholar] [CrossRef]
- Hempfling, T.; Preil, W. Application of a Temporary Immersion System in Mass Propagation of Phalaenopsis. In Liquid Culture Systems for In Vitro Plant Propagation; Hvoslef-Eide, A.K., Preil, W., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 231–242. [Google Scholar] [CrossRef]
- Petrova, M.; Zayova, E.; Todorova, M.; Stanilova, M. Enhancement of Arnica montana in-vitro shoot multiplication and sesquiterpene lactones production using temporary immersion system. Int. J. Pharm. Sci. Res. 2014, 5, 5170–5176. [Google Scholar]
- Sankar-Thomas, Y.D.; Lieberei, R. Camptothecin accumulation in various organ cultures of Camptotheca acuminata Decne grown in different culture systems. Plant Cell Tissue Organ Cult. 2011, 106, 445–454. [Google Scholar] [CrossRef]
- Ruta, C.; De Mastro, G.; Ancona, S.; Tagarelli, A.; De Cillis, F.; Benelli, C.; Lambardi, M. Large-scale plant production of Lycium barbarum L. by liquid culture in temporary immersion system and possible application to the synthesis of bioactive substance. Plants 2020, 9, 844. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Warchoł, M.; Kwaśniewska, E.; Pawłowska, B. Biochemical and morphometric analysis of Rosa tomentosa and Rosa rubiginosa during application of liquid culture systems for in vitro shoot production. J. Hortic. Sci. Biotechnol. 2017, 92, 606–613. [Google Scholar] [CrossRef]
- Wilken, D.; González, E.J.; Hohe, A.; Jordan, M.; Gomez Kosky, R.; Schmeda Hirschmann, G.; Gerth, A. Comparison of Secondary Plant Metabolite Production in Cell Suspension, Callus Culture and Temporary Immersion System. In Liquid Culture Systems for In Vitro Plant Propagation; Hvoslef-Eide, A.K., Preil, W., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 525–537. [Google Scholar] [CrossRef]
- Zobayed, S.M.A.; Murch, S.J.; Rupasinghe, H.P.V.; De Boer, J.G.; Glickman, B.W.; Saxena, P.K. Optimized system for biomass production, chemical characterization and evaluation of chemo-preventive properties of Scutellaria baicalensis Georgi. Plant Sci. 2004, 167, 439–446. [Google Scholar] [CrossRef]
- Vives, K.; Andújar, I.; Lorenzo, J.C.; Concepción, O.; Hernández, M.; Escalona, M. Comparison of different in vitro micropropagation methods of Stevia rebaudiana B. including temporary immersion bioreactor (BIT®). Plant Cell Tissue Organ Cult. 2017, 131, 195–199. [Google Scholar] [CrossRef]
- Arigundam, U.; Variyath, A.M.; Siow, Y.L.; Marshall, D.; Debnath, S.C. Liquid culture for efficient in vitro propagation of adventitious shoots in wild Vaccinium vitis-idaea ssp. minus (lingonberry) using temporary immersion and stationary bioreactors. Sci. Hortic. 2020, 264, 109199. [Google Scholar] [CrossRef]
- Phuc, V.T.; Trung, N.M.; Thien, H.T.; Tien, L.T.T. Proliferation and ajmalicine biosynthesis of Catharanthus roseus (L). G. Don adventitious roots in self-built temporary immersion system. In Proceedings of the AIP Conference Proceedings, Ho Chi Minh City, Vietnam, 12–13 October 2017; Volume 1878. [Google Scholar]
- Leveille, G.; Rai, D.; Cahill, E.; Caffrey, E.; Tennyson, E.; Wilson, G. Application of the temporary immersion system for the in vitro production of bioactive compounds in harpagophytum (Devil’s claw). Acta Hortic. 2006, 725, 597–604. [Google Scholar] [CrossRef]
- Jesionek, A.; Kokotkiewicz, A.; Wlodarska, P.; Zabiegala, B.; Bucinski, A.; Luczkiewicz, M. Bioreactor shoot cultures of Rhododendron tomentosum (Ledum palustre) for a large-scale production of bioactive volatile compounds. Plant Cell Tissue Organ Cult. 2017, 131, 51–64. [Google Scholar] [CrossRef] [Green Version]
- Mišić, D.; Šiler, B.; Skorić, M.; Djurickovic, M.S.; Nestorović Živković, J.; Jovanović, V.; Giba, Z. Secoiridoid glycosides production by Centaurium maritimum (L.) Fritch hairy root cultures in temporary immersion bioreactor. Process Biochem. 2013, 48, 1587–1591. [Google Scholar] [CrossRef]
- Pavlov, A.; Bley, T. Betalains biosynthesis by Beta vulgaris L. hairy root culture in a temporary immersion cultivation system. Process Biochem. 2006, 41, 848–852. [Google Scholar] [CrossRef]
- Valdez-Tapia, R.; Capataz-Tafur, J.; López-Laredo, A.R.; Trejo-Espino, J.L.; Trejo-Tapia, G. Effect of immersion cycles on growth, phenolics content, and antioxidant properties of Castilleja tenuiflora shoots. In Vitro Cell. Dev. Biol. -Plant 2014, 50, 471–477. [Google Scholar] [CrossRef]
- Medina-Pérez, V.; López-Laredo, A.R.; Sepúlveda-Jiménez, G.; Zamilpa, A.; Trejo-Tapia, G. Nitrogen deficiency stimulates biosynthesis of bioactive phenylethanoid glycosides in the medicinal plant Castilleja tenuiflora Benth. Acta Physiol. Plant. 2015, 37, 1–8. [Google Scholar] [CrossRef]
- Skrzypczak-Pietraszek, E.; Urbańska, A.; Żmudzki, P.; Pietraszek, J. Elicitation with methyl jasmonate combined with cultivation in the PlantformTM temporary immersion bioreactor highly increases the accumulation of selected centellosides and phenolics in Centella asiatica (L.) Urban shoot culture. Eng. Life Sci. 2019, 19, 931–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashraf, M.F.; Aziz, M.A.; Stanslas, J.; Kadir, M.A. Optimization of immersion frequency and medium substitution on microtuberization of Chlorophytum borivilianum in RITA system on production of saponins. Process Biochem. 2013, 48, 73–77. [Google Scholar] [CrossRef]
- Pérez-Alonso, N.; Capote, A.; Gerth, A.; Jiménez, E. Increased cardenolides production by elicitation of Digitalis lanata shoots cultured in temporary immersion systems. Plant Cell Tissue Organ Cult. 2012, 110, 153–162. [Google Scholar] [CrossRef]
- Pérez-Alonso, N.; Wilken, D.; Gerth, A.; Jähn, A.; Nitzsche, H.M.; Kerns, G.; Capote-Perez, A.; Jiménez, E. Cardiotonic glycosides from biomass of Digitalis purpurea L. cultured in temporary immersion systems. Plant Cell Tissue Organ Cult. 2009, 99, 151–156. [Google Scholar] [CrossRef]
- Weremczuk-Jezyna, I.; Lisiecki, P.; Gonciarz, W.; Kuzma, Ł.; Szemraj, M.; Chmiela, M.; Grzegorczyk-Karolak, I. Transformed shoots of Dracocephalum forrestii W.W. smith from different bioreactor systems as a rich source of natural phenolic compounds. Molecules 2020, 25, 4533. [Google Scholar] [CrossRef]
- Pramita, A.D.; Kristanti, A.N.; Utami, E.S.W.; Manuhara, Y.S.W. Production of biomass and flavonoid of Gynura procumbens (Lour.) Merr shoots culture in temporary immersion system. J. Genet. Eng. Biotechnol. 2018, 16, 639–643. [Google Scholar] [CrossRef]
- Schumann, A.; Torras-Claveria, L.; Berkov, S.; Claus, D.; Gerth, A.; Bastida, J.; Codina, C. Elicitation of galanthamine production by Leucojum aestivum shoots grown in temporary immersion system. Biotechnol. Prog. 2013, 29, 311–318. [Google Scholar] [CrossRef]
- Ivanov, I.; Georgiev, V.; Georgiev, M.; Ilieva, M.; Pavlov, A. Galanthamine and related alkaloids production by Leucojum aestivum L. shoot culture using a temporary immersion technology. Appl. Biochem. Biotechnol. 2011, 163, 268–277. [Google Scholar] [CrossRef]
- Klimek-Szczykutowicz, M.; Dziurka, M.; Blažević, I.; Ðulović, A.; Granica, S.; Korona-Glowniak, I.; Ekiert, H.; Szopa, A. Phytochemical and biological activity studies on Nasturtium officinale (Watercress) microshoot cultures grown in RITA® temporary immersion systems. Molecules 2020, 25, 5257. [Google Scholar] [CrossRef]
- Langhansova, L.; Marsik, P.; Vanek, T. Regulation of tissue differentiation by plant growth regulators on tTCLs of Panax ginseng adventitious roots. Ind. Crops Prod. 2012, 35, 154–159. [Google Scholar] [CrossRef]
- Vaněk, T.; Langhansová, L.; MaršÍk, P. Cultivation of Root Cultures of Panax Ginseng in Different Bioreactors and in Temporary Immersion—Comparison of Growth and Saponin Production. In Liquid Culture Systems for In Vitro Plant Propagation; Hvoslef-Eide, A.K., Preil, W., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 539–546. [Google Scholar]
- Georgiev, V.; Ivanov, I.; Berkov, S.; Pavlov, A. Temporary immersion systems for Amaryllidaceae alkaloids biosynthesis by Pancratium maritimum L. shoot culture. J. Plant Biochem. Biotechnol. 2014, 23, 389–398. [Google Scholar] [CrossRef]
- Malik, M.; Warchoł, M.; Pawłowska, B. Liquid culture systems affect morphological and biochemical parameters during Rosa canina plantlets in vitro production. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 58–64. [Google Scholar] [CrossRef] [Green Version]
- Jang, H.-R.; Lee, H.-J.; Shohael, A.M.; Park, B.-J.; Paek, K.-Y.; Park, S.-Y. Production of biomass and bioactive compounds from shoot cultures of Rosa rugosa using a bioreactor culture system. Hortic. Environ. Biotechnol. 2016, 57, 79–87. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Staniewska, P.; Lebelt, L.; Piotrowska, D.G. Optimization of cultivation conditions of Salvia viridis L. shoots in the Plantform bioreactor to increase polyphenol production. Plant Cell Tissue Organ Cult. 2021, 1–12. [Google Scholar] [CrossRef]
- Szopa, A.; Kokotkiewicz, A.; Luczkiewicz, M.; Ekiert, H. Schisandra lignans production regulated by different bioreactor type. J. Biotechnol. 2017, 247, 11–17. [Google Scholar] [CrossRef]
- Szopa, A.; Kokotkiewicz, A.; Bednarz, M.; Jafernik, K.; Luczkiewicz, M.; Ekiert, H. Bioreactor type affects the accumulation of phenolic acids and flavonoids in microshoot cultures of Schisandra chinensis (Turcz.) Baill. Plant Cell Tissue Organ Cult. 2019, 139, 199–206. [Google Scholar] [CrossRef] [Green Version]
- López, C.Q.; Corral, P.; Lorrain-Lorrette, B.; Martinez-Swatson, K.; Michoux, F.; Simonsen, H.T. Use of a temporary immersion bioreactor system for the sustainable production of thapsigargin in shoot cultures of Thapsia garganica. Plant Methods 2018, 14, 79. [Google Scholar] [CrossRef]
- Radović, M.; Šiler, B.; Živković, J.N.; Banjanac, T.; Živković, S.; Nikolić, M.; Soković, M.; Mišić, D. Bioreactor cultivation of Zeltnera beyrichii (Torr. & A. Gray) Mans.: A novel source of biologically active compounds. Rec. Nat. Prod. 2013, 7, 266–280. [Google Scholar]
- Pérez, M.; Bueno, M.A.; Escalona, M.; Toorop, P.; Rodríguez, R.; Cañal, M.J. Temporary immersion systems (RITA®) for the improvement of cork oak somatic embryogenic culture proliferation and somatic embryo production. Trees -Struct. Funct. 2013, 27, 1277–1284. [Google Scholar] [CrossRef]
- Escalona, M.; Lorenzo, J.C.; González, B.; Daquinta, M.; González, J.L.; Desjardins, Y.; Borroto, C.G. Pineapple (Ananas comosus L. Merr) micropropagation in temporary immersion systems. Plant Cell Rep. 1999, 18, 743–748. [Google Scholar] [CrossRef]
- Bello-Bello, J.J.; Cruz-Cruz, C.A.; Pérez-Guerra, J.C. A new temporary immersion system for commercial micropropagation of banana (Musa AAA cv. Grand Naine). In Vitro Cell. Dev. Biol. -Plant 2019, 55, 313–320. [Google Scholar] [CrossRef]
- Da Silva, J.A.; Solis-Gracia, N.; Jifon, J.; Souza, S.C.; Mandadi, K.K. Use of bioreactors for large-scale multiplication of sugarcane (Saccharum spp.), energy cane (Saccharum spp.), and related species. In Vitro Cell. Dev. Biol. -Plant 2020, 56, 366–376. [Google Scholar] [CrossRef]
- Lotfi, M.; Bayoudh, C.; Werbrouck, S.; Mars, M. Effects of meta–topolin derivatives and temporary immersion on hyperhydricity and in vitro shoot proliferation in Pyrus communis. Plant Cell Tissue Organ Cult. 2020, 143, 499–505. [Google Scholar] [CrossRef]
- Silva, S.T.; Bertolucci, S.K.V.; da Cunha, S.H.B.; Lazzarini, L.E.S.; Tavares, M.C.; Pinto, J.E.B.P. Effect of light and natural ventilation systems on the growth parameters and carvacrol content in the in vitro cultures of Plectranthus amboinicus (Lour.) Spreng. Plant Cell Tissue Organ Cult. 2017, 129, 501–510. [Google Scholar] [CrossRef]
- Aragón, C.E.; Sánchez, C.; Gonzalez-Olmedo, J.; Escalona, M.; Carvalho, L.; Amâncio, S. Comparison of plantain plantlets propagated in temporary immersion bioreactors and gelled medium during in vitro growth and acclimatization. Biol. Plant. 2014, 58, 29–38. [Google Scholar] [CrossRef]
- Liu, C.Z.; Guo, C.; Wang, Y.C.; Ouyang, F. Comparison of various bioreactors on growth and artemisinin biosynthesis of Artemisia annua L. shoot cultures. Process Biochem. 2003, 39, 45–49. [Google Scholar] [CrossRef]
- Young, I.S.; Woodside, J.V. Antioxidants in health and disease. J. Clin. Pathol. 2001, 54, 176–186. [Google Scholar] [CrossRef] [Green Version]
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011, 89, 217–233. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Roleira, F.M.F.; Tavares-Da-Silva, E.J.; Varela, C.L.; Costa, S.C.; Silva, T.; Garrido, J.; Borges, F. Plant derived and dietary phenolic antioxidants: Anticancer properties. Food Chem. 2015, 183, 235–258. [Google Scholar] [CrossRef]
- Petersen, M. Rosmarinic acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef]
- Jova, M.C.; Kosky, R.G.; Morales, S.R.; Torres, J.L.; Cabrera, A.R.; Pérez, M.B.; Pino, A.S.; Vega, V.M.; Rodríguez, G.R. Blanco de Guinea (Dioscorea cayenensis-D. rotundata) yam clone in vitro nodal segment multiplication in a temporary immersion system. Rev. Colomb. Biotecnol. 2008, 10, 97–103. [Google Scholar]
- Preil, W. General introduction: A personal reflection on the use of liquid media for in vitro culture. In Liquid Culture Systems for In Vitro Plant Propagation; Hvoslef-Eide, A.K., Preil, W., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 1–18. [Google Scholar]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]



| Species | Bioreactor * | Immersion Frequency (Immersion/Dry) | Biomass in TIS (vs. Semi-Solid Culture System) ** | Reference |
|---|---|---|---|---|
| Anoectochilus formosanus | Ebb and Flood | 30 min/6 h | Fresh biomass (g/L): ~100 (919.2 in continuous immersion) | [44] |
| Anthurium andreanum | RITA | 3 min/3 h | Multiplication rate: 5.17 | [45] |
| TIB | 2 min/12 h | No. shoots/explant: 50.83 FW (g): 0.61 DW (g): 0.031 g | [46] | |
| Ebb and Flow | 2 min/4 h | No. shoots/explant: 31.50 (4.50) | [47] | |
| Aquilaria malaccensis | RITA | 15 min/4 h | Shoots/initial explant: 5.12 (2.14 in continuous immersion) FW (g): 3.12, increases of about 1.8-fold | [48] |
| Aristotelia chilensis | Two Vessels | 4 min/6 h | Multiplication rate: 6.38 FW (mg) per cluster: 37.42 | [49] |
| Artemisia judaica | RITA | 3 min/3 h | Multiplication rate: 65 (35 in continuous immersion) | [50] |
| Bambusa vulgaris | Twin Flasks | 2 min/6 h | No. shoots/explant: 13.05 | [51] |
| Bletilla striata | BioF-V | 3 min/6 h | not reported | [52] |
| Calathea orbifolia | Plantima | 2 min/3 h | FW (g): 13.8 (8.2) | [53] |
| Capparis spinosa | PlantForm | 2 min/12 h | RGR (mg/g d): 7.89 (0.84) No. shoots/explant: 7.32 (5.24) | [54] |
| Cattleya walkeriana | BIT | 3 min/90 min | FW (g): 0.32 (0.24) DW (g): 0.032 (0.008) | [55] |
| Charybdis numidica | Two Bottles | 5 min/12 h | Shoot per gram inoculum: 36.6 (194.2) | [56] |
| Colocasia esculenta | SETIS | 2 min/4 h | Multiplication rate: 36 (6.10) | [57] |
| Crescentia cujete | RITA | 3 min/3 h | FW (mg): 950 (200) | [58] |
| Cymbopogon citratus | Two Flasks | 6 immersions/24 h | Multiplication coefficient: 12.3 (4.8) FW (g): 66.2 (10.1) | [59] |
| RITA | 1 min/4 h | not reported | [60] | |
| Curcuma longa | Two Flasks | 4 min/4h | Proliferation rate: 4.2 | [61] |
| Curcuma zedoaria | Nalgene® filtration system | 15 min/24 h | No. shoots/explant: 5 (3) FW (g): 4.91 (9.94 in continuous) | [62] |
| Dianthus caryophyllus | Two Containers | 2 min/6 h | No. new shoots: 14.33 (5.7) | [63] |
| RITA | 90 s/8 h | No. shoots/plantlet: 2.0 (1.3) | [64] | |
| Digitalis purpurea | Two Bottles | 2 min/4 h | FW (g): 104.03 DW (g): 5.74 | [65] |
| Vessels | 2 min/4 h | not reported | [66] | |
| Dioscorea fordii | Plantima | 3 min/4 h | Proliferation rate: 5.0 (2.4) FW (mg): 423.3 (72.2) DW (mg): 39.4 (6.3) | [67] |
| Echinacea angustifolia | RITA | not reported | Multiplication rate: highest in RITA | [68] |
| Epipactis flava | Twin Bottles | 5 min/4 h | No. shoots/explant: 1.5 (1.0) | [69] |
| Eucalyptus grandis × E. urophylla | TIB | 30 s/3 h | No. shoots/explant: 8 | [70] |
| Eurycoma longifolia | RITA | 5 min/4 h | Multiplication rate: 69.6 (26.6) | [71] |
| Glycyrrhiza glabra | Twin Flasks | 5 min/4 h | Multiplication rate: 9.47 (6.16) | [72] |
| Gynura procumbens | BIT | 15 min/12 h | FW (g): 10.24 | [73] |
| Helichrysum italicum | Twin Flasks | 5 min/24 h | Multiplication rate: 16.4 (11.6) | [72] |
| Hevea brasiliensis | RITA | 1 min/24 h | RGR: 0.084 (0.085) | [74] |
| Hippeastrum × chmielii | Twin Flasks | 32 min/24 h | Propagation rate: 6.44 (3.85) | [75] |
| Hypericum perforatum | TI Bioreactor | 5 min/3h for 15 days and then 5 min/24 h | No. regenerants/explants: 209 (106, in continuous immersion) | [41] |
| Hypoxis argentea | PlantForm | 6 min/12 h | No. shoots/explant: 12 | [76] |
| Jacaranda decurrens | RITA | 15 min/4 h | Multiplication rate: 9.61 | [77] |
| Jeffersonia dubia | Plantima | 30 s/30 min | No. shoots/explant: 13.6 (7.3) | [78] |
| Lessertia (Sutherlandia) frutescens | BTBB | 30 min/4 h | Shoot multiplication: 12.9 (7.8) FW (mg): 3109 (104.2) DW (mg): 351.8 (10.3) | [79] |
| Leucojum aestivum | RITA | 5 min/2 h | No. bulbs (%): 74.6 (71.7) | [80] |
| Morinda royoc | TIS Bioreactor | 2 min/4 h | Multiplication coefficient: 6.0 | [81] |
| Myrtus communis | PlantForm | 8 min/16 h | RGR: 6.2 (5.3) | [82] |
| PlantForm | 15 min/8 h | Micropropagation rate: 11.40 (6.25) FW (g): 1.00 (0.17) DW (g): 0.26 (0.02) | [83] | |
| Olea europaea | PlantForm | 8 min/16h | No. shoots/explant: 0.77 (0.36) | [84] |
| Picea abies | PlantForm | 20 min/4 h | Embryo production per g pro-embryogenic mass: 696 (174) | [85] |
| Picrorhiza kurroa | Twin Flasks | 5 min/8 h | Multiplication rate: 8.20 (4.68) | [72] |
| Pinellia ternata | not reported | not reported | No. tubers/plantlet: 24.73 (14.75) | [86] |
| Piper aduncum | RITA | 3 min/6 h | Germination of somatic embryos (%): 100 (100) | [87] |
| Pistacia lentiscus | RITA | 3 h/24 h | No. shoot/explant: 6.18 (5.8) | [88] |
| Psidium guajava | RITA | 2 min/6 h | Multiplication coeff.: 4.90 (4.36) No. shoots: 2.17 (1.78) | [89] |
| Quercus robur | PlantForm | 8 min/16 h | RGR: 6 (4) No. shoots/explant: 3 (2.5) | [90] |
| Rhodiola rosea | RITA | 15 min/4 h | No. shoots/explant: 16 | [91] |
| Saponaria officinalis | Twin Flasks | 5 min/8 h | Multiplication rate: 4.36 (2.39) | [72] |
| Siraitia grosvenorii | Plantima | 4 min/4 h | Proliferation rate: 8.75 (3.72) FW (mg): 501.25 (168.89) DW (mg): 60.83 (21.94) | [92] |
| Stevia rebaudiana | BIT | 10 min/12 h | FW (mg): 774.39 DW (mg): 83.41 | [93] |
| RITA PlantForm | 3 min/3 h 3 min/8 h | No. shoots/explants: 14 | [94] | |
| RITA | 2 min/4 h | No. shoots/explant: 11.8 (4.6) | [95] | |
| BIT | 2 min/12 h | Multiplication rate: 8 shoots/plant | [96] | |
| RITA | 10 s/1 h | No. shoots/explant: 8.47 (2.00) | [97] | |
| Swertia chirayita | Twin Flasks | not reported | No. shoots/explants: 28 | [98] |
| Tropaeolum tuberosum | RITA | 2 min/3 h | Microtubers quantity: 56 Weight of microtubers (g): 0.2 Size of microtuber (cm): 1.9 | [99] |
| Tussilago farfara | Two Flasks | 5 min/8 h | Multiplication rate: 5.65 (4.15) | [72] |
| Vaccinium corymbosum | Two Vessels | 3 min/6 or 8 h | Multiplication rate: 26.2 (12.7) | [100] |
| Vanilla planifolia | BIT | 2 min/4 h | No. shoots/explants: 18.06 FW (g): 6.02 DW (g): 0.41 | [95] |
| TIB | 2 min/8 h | No. shoots/plantlet: 9.15 (1.3) | [46] | |
| RITA | 2 min/4 h | Multiplication rate: 14.27 (5.80) | [101] | |
| RITA | 2 min/6 h | No. shoots/explant: 14.89 FW (g): 5829.00 DW (g): 447.20 | [102] | |
| SETIS | 2 min/4 h | No. shoots/explant: 11.41 (3.76) FW (g): 6.65 (1.71) DW (g): 0.42 (0.15) | [103] | |
| Zingiber zerumbet | Nalgene® filtration system | 15 min/24 h | No. shoots/explant: 4 (3) FW (g): 2.31 (9.94 in continuous immersion) | [62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Carlo, A.; Tarraf, W.; Lambardi, M.; Benelli, C. Temporary Immersion System for Production of Biomass and Bioactive Compounds from Medicinal Plants. Agronomy 2021, 11, 2414. https://doi.org/10.3390/agronomy11122414
De Carlo A, Tarraf W, Lambardi M, Benelli C. Temporary Immersion System for Production of Biomass and Bioactive Compounds from Medicinal Plants. Agronomy. 2021; 11(12):2414. https://doi.org/10.3390/agronomy11122414
Chicago/Turabian StyleDe Carlo, Anna, Waed Tarraf, Maurizio Lambardi, and Carla Benelli. 2021. "Temporary Immersion System for Production of Biomass and Bioactive Compounds from Medicinal Plants" Agronomy 11, no. 12: 2414. https://doi.org/10.3390/agronomy11122414
APA StyleDe Carlo, A., Tarraf, W., Lambardi, M., & Benelli, C. (2021). Temporary Immersion System for Production of Biomass and Bioactive Compounds from Medicinal Plants. Agronomy, 11(12), 2414. https://doi.org/10.3390/agronomy11122414









