Comparative Efficiency of Mineral, Chelated and Nano Forms of Zinc and Iron for Improvement of Zinc and Iron in Chickpea (Cicer arietinum L.) through Biofortification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Specification
2.2. Synthesis of Nanoparticles
2.3. Plant Analysis for the Estimation of DTPA-Zn and Fe
2.4. Economic Analysis
2.5. Statistical Analysis
3. Results
3.1. Impact of Foliar Application of Zn and Fe on Grain and Straw Yield of Chickpeas
3.2. Impact of Foliar Application of Zn and Fe on Grain Zn and Fe Concentration of Chickpeas
3.3. Impact of Foliar Application of Zn and Fe on Straw Zn and Fe Concentration of Chickpea
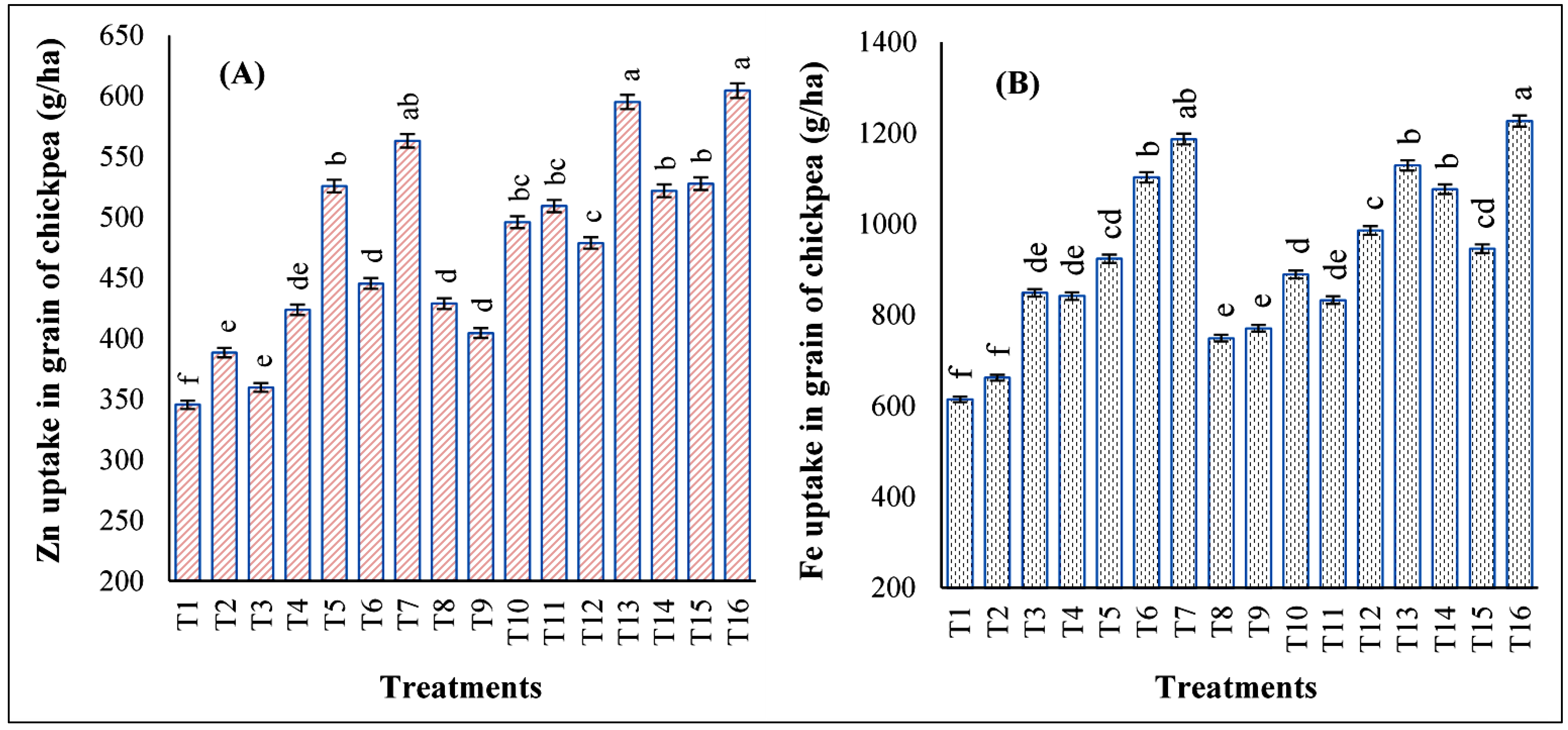
3.4. Impact of Foliar Application of Zn and Fe on Grain Zn and Fe Uptake of Chickpeas
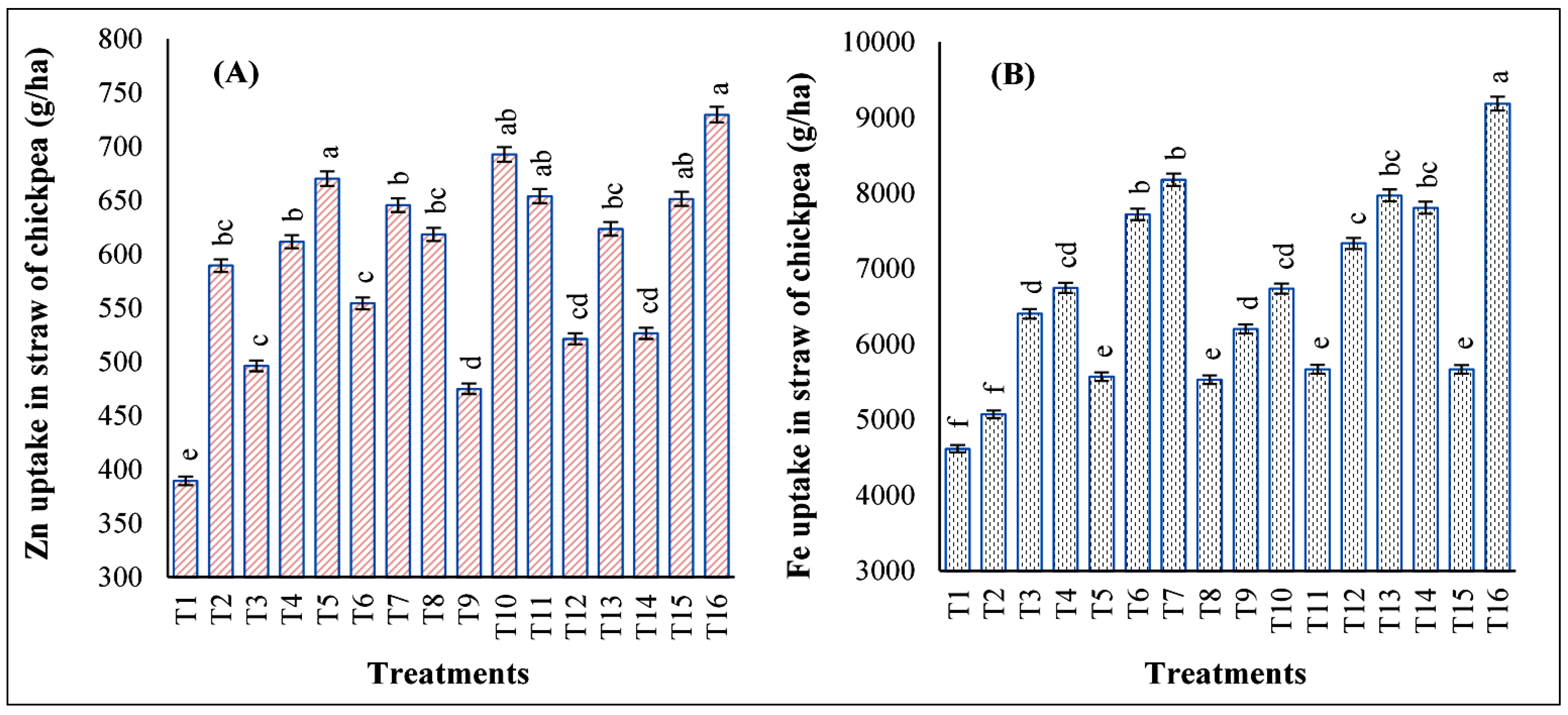
3.5. Impact of Foliar Application of Zn and Fe on Straw Zn and Fe Uptake of Chickpeas
3.6. Economic Analysis
4. Discussion
4.1. Impact of Zn and Fe on Grain and Straw Yield of Chickpeas
4.2. Impact of Zn and Fe on their Grain and Straw Concentrations in Chickpeas
4.3. Impact of Zn and Fe on their Grain and Straw Uptake in Chickpeas and Economic Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yegrem, L. Nutritional composition, antinutritional factors, and utilization trends of Ethiopian Chickpea Cicer Arietinum L. Int. J. Food Sci. 2021, 2021, 5570753. [Google Scholar] [CrossRef] [PubMed]
- Merga, B.; Haji, J. Economic importance of chickpea: Production, value, and world trade. Cogent Food Agric. 2019, 5, 1615718. [Google Scholar] [CrossRef]
- Pegoraro, R.F.; AlmeidaNeta, M.; Costa, C.; Sampaio, R.A.; Fernandes, L.A.; Neves Rodrigues, M. Chickpea production and soil chemical attributes after phosphorus and molybdenum fertilization. Ciênciae Agrotecnol. 2018, 42, 474–483. [Google Scholar] [CrossRef]
- Nadi, E.; Aynehband, A.; Mojaddam, M. Effect of nano-iron chelate fertilizer on grain yield, protein percent and chlorophyll content of faba bean Vicia faba L. Int. J. Biosci. 2013, 3, 267–272. [Google Scholar]
- Bozorgi, H.R. Study effects of nitrogen fertilizer management under nano iron chelate foliar spraying on yield and yield components of Eggplant. J. Agric. Biol. Sci. 2012, 7, 233–237. [Google Scholar]
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Hacisalihoglu, G. Zinc: The last nutrient in the alphabet and shedding light on Zn efficiency for the future of crop production under suboptimal Zn. Plants 2020, 9, 1471. [Google Scholar] [CrossRef]
- Tsonev, T.; Cebola Lidon, F.J. Zinc in plants-an overview. Emirates J. Food Agric. 2012, 24, 322–333. [Google Scholar]
- Umair Hassan, M.; Aamer, M.; Umer Chattha, M.; Haiying, T.; Shahzad, B.; Barbanti, L.; Nawaz, M.; Rasheed, A.; Afzal, A.; Liu, Y.; et al. The critical role of zinc in plants facing the drought stress. Agriculture 2020, 10, 396. [Google Scholar] [CrossRef]
- Karim, M.; Zhang, Y.Q.; Zhao, R.R.; Chen, X.; Zhang, F.; Zou, C. Alleviation of drought stress in winter wheat by late foliar application of zinc, boron, and manganese. J. Plant Nutr. Soil Sci. 2012, 175, 142–151. [Google Scholar] [CrossRef]
- Khan, H.R.; McDonald, G.K.; Rengel, Z. Zinc fertilization and water stress affects plant water relations, stomatal conductance and osmotic adjustment in chickpea Cicer arietinum L. Plant Soil 2004, 267, 271–284. [Google Scholar] [CrossRef]
- Vadlamudi, K.; Upadhyay, H.; Singh, A.; Reddy, M. Influence of zinc application in plant growth: An overview. Eur. J. Mol. Clin. Med. 2020, 7, 2321–2327. [Google Scholar]
- Pal, V.; Singh, G.; Dhaliwal, S. Yield enhancement and biofortification of chickpea Cicer arietinum L. grain with iron and zinc through foliar application of ferrous sulfate and urea. J. Plant Nutr. 2019, 42, 1789–1802. [Google Scholar] [CrossRef]
- Schmidt, W.; Thomine, S.; Buckhout, T.J. Iron nutrition and interactions in plants. Front. Plant Sci. 2020, 10, 1670. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Fernandez, A.; Garcıa-Lavina, P.; Fidalgo, C.; Abadıa, J.; Abadıa, A. Foliar fertilization to control iron chlorosis in pear Pyrus communis L. trees. Plant Soil 2004, 263, 271–284. [Google Scholar] [CrossRef]
- Cakmak, I. Enrichment of fertilizers with zinc: An excellent investment for humanity and crop production in India. J. Trace Elem. Med. Biol. 2009, 23, 281–289. [Google Scholar] [CrossRef]
- de Valenca, A.W.; Bake, A.; Brouwer, I.D.; Giller, K.E. Agronomic biofortification of crops to fight hidden hunger in sub-Saharan Africa. Glob. Food Sec. 2017, 12, 8–14. [Google Scholar] [CrossRef]
- Almendros, P.; Gonzalez, D.; Alvarez, J.M. Long-term bioavailability effects of synthesized zinc chelates fertilizers on the yield and quality of a flax (Linum usitatissimum L.) crop. Plant Soil 2013, 368, 251–265. [Google Scholar] [CrossRef]
- Slaton, N.A.; Norman, R.J.; Wilson, C.E. Effect of zinc source and application time on zinc uptake and grain yield of flood-irrigated rice. Agron. J. 2005, 97, 272–278. [Google Scholar] [CrossRef]
- Cakmak, I. Biofortification of cereals with zinc and iron through fertilization strategy. In Proceedings of the 19th World Congress of Soil Science, Brisbane, Australia, 1–6 August 2010. [Google Scholar]
- Rahman, A.M.; Hasegawa, H.; Kadohashi, K.; Maki, T.; Ueda, K. Hydroxyiminodisuccinic acid: A novel biodegradable chelating ligand for the increase of iron bioavailability and arsenic phytoextraction. Chemosphere 2009, 77, 207–213. [Google Scholar] [CrossRef] [Green Version]
- López-Rayo, S.; Sanchis-Pérez, I.; Ferreira, C.M.H.; Lucena, J.J. [S,S]-EDDS/Fe: A new chelate for the environmentally sustainable correction of iron chlorosis in calcareous soil. Sci. Total Environ. 2019, 647, 1508–1517. [Google Scholar] [CrossRef]
- Mahender, A.; Swamy, B.P.M.; Anandan, A.; Ali, J. Tolerance of iron deficient and -toxic soil conditions in rice. Plants 2019, 8, 31. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Shohag, M.J.I.; Yang, X.; Yibin, Z. Effects of foliar iron application on iron concentration in polished rice grain and its bioavailability. J. Agric. Food Chem. 2012, 60, 11433–11439. [Google Scholar] [CrossRef] [PubMed]
- Brennan, R.F. Effectiveness of different sources of manganese foliar sprays in alleviating manganese deficiency of Lupinus angustifolius L. grown on manganese deficient soils in western Australia. J. Plant Nutr. 1996, 19, 293–304. [Google Scholar] [CrossRef]
- Kinaci, E.; Gulmezoglu, N. Grain yield and yield components of triticale upon application of different foliar fertilizers. Interciencia 2007, 32, 624–628. [Google Scholar]
- Kayan, N.; Gulmezoglu, N.; Kaya, M.D. The optimum foliar zinc source and level for improving Zn content in grain of chickpea. Legume Res. 2015, 38, 826–831. [Google Scholar]
- Fatima, M.; Hashim, A.; Anees, S. Efficacy of nanoparticles as nanofertilizer production: A review. Environ. Sci. Pollut. Res. 2020, 28, 1292–1303. [Google Scholar] [CrossRef]
- Zulfidar, F.; Navarro, M.; Ashraf, M.; Akram, N.A.; Munne-Bosch, S. Nanofertilizer use for sustainable agriculture: Advantages and limitations. Plant Sci. 2019, 289, 110270. [Google Scholar] [CrossRef]
- Sadak, M.; Bakry, B.A. Zinc-oxide and nano ZnO oxide effects on growth, some biochemical aspects, yield quantity, and quality of flax (Linum uitatissimum L.) in absence and presence of compost under sandy soil. Bull. Nat. Res. Cent. 2020, 44, 98. [Google Scholar] [CrossRef]
- Kaur, M.; Kaur, N.; Vibha, V. Ferrites: Synthesis and applications for environmental remediation. In Ferrites and Ferrates: Chemistry and Applications in Sustainable Energy and Environmental Remediation; Sharma, V.S., Doong, R., Kim, H., Varma, R.S., Dionysiou, D.D., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2016; pp. 113–136. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall of India Pvt Ltd.: New Delhi, India, 1973. [Google Scholar]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Verma, V.; Kaur, M.; Greneche, J.M. Tailored structural, optical and magnetic properties of ternary nanohybrid Mn0.4Co0.6-xCuxFe2O4 (x= 0, 0.2, 0.4, 0.6) spinel ferrites. Ceramics Int. 2019, 45, 10865–10875. [Google Scholar] [CrossRef]
- Piper, C.S. Soil and Plant Analysis; Hans Publishers: Bombay, India, 1966. [Google Scholar]
- Dhaliwal, S.S.; Sharma, V.; Taneja, P.K.; Shukla, A.K.; Kaur, L.; Verma, G.; Verma, V.; Singh, J. Effect of cadmium and ethylenediamine tetraacetic acid supplementation on cadmium accumulation by roots of Brassica species in Cd spiked soil. Environ. Sci Pollut. Res. 2021. [Google Scholar] [CrossRef]
- Singh, V.K.; Gautam, P.; Nanda, G.; Dhaliwal, S.S.; Pramanick, B.; Meena, S.S.; Alsanie, W.F.; Gaber, A.; Sayed, S.; Hossain, A. Soil test based fertilizer application imrpoves productivity, profitability and nutrient use efficiency of rice Oryza sativa L. under direct seeded condition. Agronomy 2021, 11, 1756. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Sadana, U.S.; Manchanda, J.S.; Dhadli, H.S. Biofortification of wheat grains with zinc and iron in typic Ustochrept soils of Punjab. Indian J. Fert. 2009, 5, 13–16. [Google Scholar]
- Dhaliwal, S.S.; Sadana, U.S.; Manchanda, J.S.; Kumar, D. Differential response of maize cultivars to zinc in relation to food security and alleviation of Zn malnutrition. Indian J. Fert. 2013, 9, 24–30. [Google Scholar]
- Poornima, R.; Koti, R.V. Effect of nano zinc oxide on growth, yield and grain zinc content of sorghum Sorghum Bicolor. J Pharmacogn. Phytochem. 2019, 8, 727–731. [Google Scholar]
- Dhaliwal, S.S.; Sadana, U.S.; Khurana, M.P.; Sidhu, S.S. Enrichment of rice grains with zinc and iron through ferti-fortification. Indian J. Fert. 2010, 6, 28–35. [Google Scholar]
- Dhaliwal, S.S.; Sadana, U.S.; Khurana, M.P.; Sidhu, S.S. Enrichment of wheat grains with Zn through ferti-fortification. Indian J. Fert. 2012, 8, 48–55. [Google Scholar]
- Kawakami, Y.; Bhullar, N.K. Molecular processes in iron and zinc homeostasis and their modulation for biofortification in rice. J. Integr. Plant Biol. 2018, 60, 1181–1198. [Google Scholar] [CrossRef]
- Faraz, A.; Faizan, M.; Sami, F.; Siddiqui, H.; Pichtel, J.; Hayat, S. Nanoparticles: Biosynthesis, translocation and role in plant metabolism. IET Nanobiotechnol. 2019, 1, 345–352. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Sandhu, A.S.; Shukla, A.K.; Sharma, V.; Kumar, B.; Singh, R. Bio-fortification of oats fodder through zinc enrichment to reduce animal malnutrition. J. Agric. Sci. Technol. A 2020, 10, 98–108. [Google Scholar]
- Elemike, E.E.; Uzoh, I.M.; Onwudiwe, D.C.; Babalola, O.O. The role of nanotechnology in the fortification of plant nutrients and improvement of crop production. Appl. Sci. 2019, 9, 499. [Google Scholar] [CrossRef] [Green Version]
- Rossi, L.; Fedenia, L.N.; Sharifan, H.; Ma, X.; Lombardini, L. Effects of foliar application of zinc sulfate and zinc nanoparticles in coffee Coffea arabica L. plants. Plant Physiol. Biochem. 2019, 135, 160–166. [Google Scholar] [CrossRef] [PubMed]

| Treatments | Treatments |
|---|---|
| T1 | RDF control |
| T2 | RDF + 0.5% ZnSO4.7H2O spray pre-flowering stage |
| T3 | RDF + 0.5% FeSO4.7H2O spray pre-flowering stage |
| T4 | RDF + 0.5% ZnSO4.7H2O+ 0.5% FeSO4.7H2O spray pre-flowering stage |
| T5 | RDF + 0.5% ZnSO4.7H2O spray pre-flowering stage + pod formation |
| T6 | RDF + 0.5% FeSO4.7H2O spray pre-flowering stage + pod formation |
| T7 | RDF + 0.5% ZnSO4.7H2O + 0.5% FeSO4.7H2O spray pre-flowering stage + pod formation |
| T8 | RDF + 0.3% Zn-EDTA spray pre-flowering stage |
| T9 | RDF + 0.3% Fe-EDTA spray pre-flowering stage |
| T10 | RDF + 0.3% Zn-EDTA+ 0.3% Fe-EDTA spray pre-flowering stage |
| T11 | RDF + 0.3% Zn-EDTA spray pre-flowering stage + pod formation |
| T12 | RDF + 0.3% Fe-EDTA spray pre-flowering stage + pod formation |
| T13 | RDF + 0.3% Zn-EDTA+ 0.3% Fe-EDTA spray pre-flowering stage + pod formation |
| T14 | RDF + 0.5% Fe2O3 NPs spray pre-flowering stage |
| T15 | RDF + 0.5% ZnO NPs spray pre-flowering stage |
| T16 | RDF + 0.5% ZnO NPs + 0.5% Fe2O3 NPs spray pre-flowering stage |
| Treatments | Grain Yield (q ha−1) | Straw Yield (q ha−1) | ||||
|---|---|---|---|---|---|---|
| 1st Year | 2nd Year | Mean | 1st Year | 2nd Year | Mean | |
| T1 | 0.17 | 0.41 | 0.11 | 1.32 | 2.77 | 0.99 |
| T2 | 0.14 | 0.91 | 0.16 | 0.55 | 2.16 | 1.30 |
| T3 | 0.19 | 0.47 | 0.18 | 0.28 | 0.35 | 1.45 |
| T4 | 0.40 | 1.16 | 0.63 | 0.65 | 3.50 | 0.48 |
| T5 | 0.09 | 0.42 | 1.12 | 0.27 | 0.96 | 0.74 |
| T6 | 0.33 | 1.54 | 0.31 | 1.60 | 2.66 | 0.83 |
| T7 | 0.24 | 1.42 | 0.36 | 1.24 | 3.97 | 1.07 |
| T8 | 0.14 | 0.06 | 0.16 | 0.84 | 2.05 | 0.65 |
| T9 | 0.08 | 0.67 | 0.46 | 0.24 | 2.37 | 0.38 |
| T10 | 1.29 | 1.03 | 0.34 | 0.24 | 3.94 | 1.22 |
| T11 | 0.34 | 1.88 | 0.24 | 0.58 | 4.26 | 0.04 |
| T12 | 1.06 | . 1.05 | 0.29 | 1.03 | 1.79 | 0.66 |
| T13 | 0.46 | 1.09 | 0.74 | 0.96 | 2.48 | 0.51 |
| T14 | 0.51 | 0.57 | 0.24 | 0.63 | . 0.67 | 1.25 |
| T15 | 0.12 | 0.50 | 0.12 | 0.52 | 1.67 | 1.21 |
| T16 | 0.11 | 0.41 | 0.12 | 0.38 | 2.10 | 1.29 |
| LSD (p ≤ 0.05) | 0.84 | 1.63 | 0.85 | 1.29 | NS | NS |
| Treatments | Zn Concentration in Grains (mg kg−1) | Fe Concentration in Grains (mg kg−1) | ||||
|---|---|---|---|---|---|---|
| 1st Year | 2nd Year | Mean | 1st Year | 2nd Year | Mean | |
| T1 | 3.62 | 3.12 | 0.37 | 0.36 | 1.57 | 2.21 |
| T2 | 1.12 | 0.11 | 0.87 | 0.19 | 3.62 | 0.21 |
| T3 | 2.21 | 0.19 | 1.45 | 2.73 | 3.19 | 2.29 |
| T4 | 1.38 | 2.01 | 1.38 | 1.90 | 3.92 | 0.52 |
| T5 | 0.88 | 0.12 | 2.06 | . 0.38 | 3.31 | 4.58 |
| 0.13 | 1.16 | 0.21 | 0.70 | 4.55 | 0.13 | |
| T7 | 2.38 | 0.17 | 0.04 | 1.51 | 2.59 | 0.60 |
| T8 | 1.00 | 0.15 | 1.04 | 1.95 | 3.23 | 0.20 |
| T9 | 3.37 | 3.22 | 0.59 | 2.18 | 4.43 | 1.21 |
| T10 | 0.13 | 0.15 | 2.41 | 1.66 | 2.17 | 1.29 |
| T11 | 2.50 | 1.10 | 2.52 | 2.01 | 3.80 | . 1.55 |
| T12 | 0.25 | 0.22 | 0.45 | 1.00 | 1.41 | 8.41 |
| T13 | 1.50 | 0.21 | 1.20 | 0.54 | 2.90 | 7.63 |
| T14 | 1.34 | 1.56 | 1.01 | 4.06 | 8.16 | 4.71 |
| T15 | 0.42 | 1.34 | 1.70 | 0.46 | 2.85 | 4.88 |
| T16 | 0.74 | 0.19 | 2.40 | 1.83 | 9.36 | 0.78 |
| LSD (p ≤ 0.05) | 3.08 | 3.75 | 2.01 | 2.48 | 9.09 | 2.77 |
| Treatments | Zn Concentration in Straw (mg kg−1) | Fe Concentration in Straw (mg kg−1) | ||||
|---|---|---|---|---|---|---|
| 1st Year | 2nd Year | Mean | 1st Year | 2nd Year | Mean | |
| T1 | 0.91 | 0.54 | 3.33 | 7.38 | 5.40 | 8.35 |
| T2 | 1.05 | 2.24 | 4.61 | 11.73 | 10.28 | 5.79 |
| T3 | 1.54 | 2.45 | 1.91 | 14.87 | 9.18 | 9.90 |
| T4 | 1.53 | 3.50 | 4.78 | 15.50 | 9.97 | 7.81 |
| T5 | 0.72 | 2.60 | 4.21 | 9.83 | 6.31 | 4.86 |
| T6 | 0.37 | 1.53 | 4.23 | 9.38 | 3.13 | 14.90 |
| T7 | 1.74 | 0.79 | 3.77 | 7.64 | 8.88 | 54.85 |
| T8 | 0.81 | 5.05 | 3.16 | 4.95 | 17.04 | 13.94 |
| T9 | 0.32 | 2.23 | 4.11 | 17.32 | 2.48 | 11.47 |
| T10 | 1.03 | 1.05 | 3.55 | 16.40 | 13.93 | 11.52 |
| T11 | 1.12 | 0.54 | 3.21 | 10.92 | 10.46 | 10.59 |
| T12 | 0.69 | 0.97 | 3.63 | 21.55 | 17.23 | 2.84 |
| T13 | 0.05 | 0.52 | 4.46 | 8.49 | 15.02 | 19.13 |
| T14 | 0.25 | 1.76 | 4.60 | 13.87 | 11.34 | 10.84 |
| T15 | 0.55 | 2.55 | 1.99 | 7.03 | 15.80 | 10.53 |
| T16 | 0.30 | 2.56 | 0.58 | 15.33 | 15.79 | 21.37 |
| LSD (p ≤ 0.05) | 1.62 | 3.82 | 2.10 | 21.65 | 29.45 | 18.58 |
| Treatments | Cost of Cultivation (US$) | Net Return (US$) | B:C Ratio |
|---|---|---|---|
| T1 | US$401.2 d | US$459.0 j | 2.14 |
| T2 | US$409.0 cd | US$499.0 i | 2.22 |
| T3 | US$409.8 cd | US$511.2 i | 2.25 |
| T4 | US$417.6 cd | US$596.1 h | 2.43 |
| T5 | US$414.1 cd | US$804.3 bc | 2.94 |
| T6 | US$415.0 cd | US$813.7 b | 2.96 |
| T7 | US$417.6 cd | US$882.2 a | 3.11 |
| T8 | US$416.8 cd | US$584.7 h | 2.40 |
| T9 | US$416.8 cd | US$566.9 h | 2.36 |
| T10 | US$432.3 c | US$653.3 g | 2.51 |
| T11 | US$421.9 c | US$677.8 g | 2.61 |
| T12 | US$421.9 c | US$722.6 f | 2.71 |
| T13 | US$432.3 c | US$886.2 a | 3.05 |
| T14 | US$458.3 b | US$771.3 cde | 2.68 |
| T15 | US$475.7 b | US$754.9 ef | 2.59 |
| T16 | US$532.8 a | US$782.9 bcd | 2.47 |
| LSD (p ≤ 0.05) | 25.1 | 36.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhaliwal, S.S.; Sharma, V.; Shukla, A.K.; Verma, V.; Behera, S.K.; Singh, P.; Alotaibi, S.S.; Gaber, A.; Hossain, A. Comparative Efficiency of Mineral, Chelated and Nano Forms of Zinc and Iron for Improvement of Zinc and Iron in Chickpea (Cicer arietinum L.) through Biofortification. Agronomy 2021, 11, 2436. https://doi.org/10.3390/agronomy11122436
Dhaliwal SS, Sharma V, Shukla AK, Verma V, Behera SK, Singh P, Alotaibi SS, Gaber A, Hossain A. Comparative Efficiency of Mineral, Chelated and Nano Forms of Zinc and Iron for Improvement of Zinc and Iron in Chickpea (Cicer arietinum L.) through Biofortification. Agronomy. 2021; 11(12):2436. https://doi.org/10.3390/agronomy11122436
Chicago/Turabian StyleDhaliwal, Salwinder Singh, Vivek Sharma, Arvind Kumar Shukla, Vibha Verma, Sanjib Kumar Behera, Prabhjot Singh, Saqer S. Alotaibi, Ahmed Gaber, and Akbar Hossain. 2021. "Comparative Efficiency of Mineral, Chelated and Nano Forms of Zinc and Iron for Improvement of Zinc and Iron in Chickpea (Cicer arietinum L.) through Biofortification" Agronomy 11, no. 12: 2436. https://doi.org/10.3390/agronomy11122436
APA StyleDhaliwal, S. S., Sharma, V., Shukla, A. K., Verma, V., Behera, S. K., Singh, P., Alotaibi, S. S., Gaber, A., & Hossain, A. (2021). Comparative Efficiency of Mineral, Chelated and Nano Forms of Zinc and Iron for Improvement of Zinc and Iron in Chickpea (Cicer arietinum L.) through Biofortification. Agronomy, 11(12), 2436. https://doi.org/10.3390/agronomy11122436











