A Greener HPTLC Approach for the Determination of β-Carotene in Traditional and Ultrasound-Based Extracts of Different Fractions of Daucus carota (L.), Ipomea batatas (L.), and Commercial Formulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Chemicals and Reagents
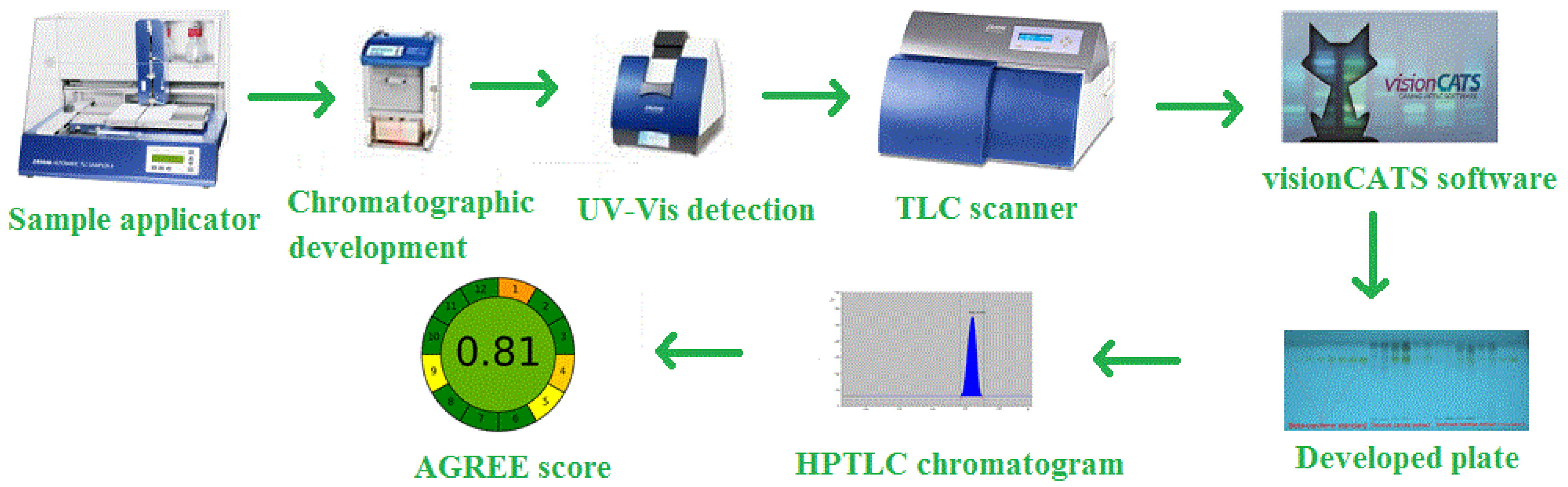
2.3. Chromatography and Instrumentation
2.4. β-Carotene Calibration Curve and Quality Control (QC) Samples
2.5. Sample Processing for the Determination of β-Carotene in TE of Carrots, Sweet Potato, and Commercial Formulation A
2.6. Sample Processing for the Determination of β-Carotene in UBE of Carrots, Sweet Potato, and Commercial Formulation A
2.7. Validation Studies
2.8. Determination of β-Carotene in TE and UBE of Carrots, Sweet Potato, and Marketed Formulation A
2.9. Greenness Assessment
3. Results and Discussion
3.1. Method Development
3.2. Validation Studies
3.3. Determination of β-Carotene in TE and UBE of Carrots, Sweet Potato, and Marketed Formulation A
3.4. Greenness Evaluation
3.5. Comparison with Literature Analytical Approaches
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haskell, M.J. The challenge to reach nutritional adequacy for vitamin A: β-carotene bioavailability and conversion—Evidence in humans. Am. J. Clin. Nutr. 2012, 96, 1193S–1203S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sommer, A.; Vyas, K.S. A global clinical view on vitamin A and carotenoids. Am. J. Clin. Nutr. 2012, 96, 1204S–1206S. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.K. An update on the potential health benefits of carotenes. EXCLI J. 2016, 15, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Prieto, J.M.; Chandrasekara, A.; Josheph, K.T. Roots and tuber crops as functional foods: A review on phytochemical constituents and their potential health benefits. Int. J. Food Sci. 2016, 2016, E3631647. [Google Scholar]
- Blando, F.; Marchello, S.; Maiorano, G.; Durante, M.; Signore, A.; Laus, M.; Soccio, M.; Mita, G. Bioactive Compounds and Antioxidant Capacity in Anthocyanin-Rich Carrots: A Comparison between the Black Carrot and the Apulian Landrace “Polignano” Carrot. Plants 2021, 10, 564. [Google Scholar] [CrossRef]
- Arscott, S.A.; Tanumihardjo, S.A. Carrots of Many Colors Provide Basic Nutrition and Bioavailable Phytochemicals Acting as a Functional Food. Compr. Rev. Food Sci. Food Saf. 2010, 9, 223–239. [Google Scholar] [CrossRef]
- Andersen, O.M.; Jordheim, M. Basic anthocyanin chemistry and dietary Sources. In Anthocyanins in Health and Disease; Wallace, T.C., Giusti, M.M., Eds.; Taylor & Francis Inc.: Abingdon, UK; CRC Press: New York, NY, USA, 2013; pp. 13–90. [Google Scholar]
- Blando, F.; Calabriso, N.; Berland, H.; Maiorano, G.; Gerardi, C.; Carluccio, M.A.; Andersen, Ø.M. Radical scavenging and biological activities of representative anthocyanin groupings from pigment-rich fruits and vegetables. Int. J. Mol. Sci. 2018, 19, 169. [Google Scholar] [CrossRef] [Green Version]
- Esatbeyoglu, T.; Rodríguez-Werner, M.; Schlösser, A.; Liehr, M.; Ipharraguerre, I.; Winterhalter, P.; Rimbach, G. Fractionation of Plant Bioactives from Black Carrots (Daucus carota subspecies sativus varietas atrorubens Alef.) by Adsorptive Membrane Chromatography and Analysis of Their Potential Anti-Diabetic Activity. J. Agric. Food Chem. 2016, 64, 5901–5908. [Google Scholar] [CrossRef]
- Bendokas, V.; Stanys, V.; Mažeikienė, I.; Trumbeckaite, S.; Baniene, R.; Liobikas, J. Anthocyanins: From the Field to the Antioxidants in the Body. Antioxidants 2020, 9, 819. [Google Scholar] [CrossRef]
- Groppo, F.C.; Pochapski, M.T.; Fosquiera, E.C.; Esmerino, L.A.; Dos Santos, E.B.; Farago, P.V.; Santos, F.A. Phytochemical screening, antioxidant, and antimicrobial activities of the crude leaves′ extract from Ipomoea batatas (L.) Lam. Pharmacogn. Mag. 2011, 7, 165–170. [Google Scholar] [CrossRef]
- Dini, I.; Tenore, G.C.; Dini, A. Saponins in Ipomoea batatas tubers: Isolation, characterization, quantification and antioxidant properties. Food Chem. 2009, 113, 411–419. [Google Scholar] [CrossRef]
- Khan, M.Z.; Takemura, M.; Maoka, T.; Otani, M.; Misawa, N. Carotenoid analysis of sweetpotato Ipomoea batatas and functional identification of its lycopene β- and ε-cyclase genes. Zeitschrift für Naturforschung C 2016, 71, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Clifford, M.N. Profiling the chlorogenic acids of sweet potato (Ipomoea batatas) from China. Food Chem. 2008, 106, 147–152. [Google Scholar] [CrossRef]
- Rumbaoa, R.G.O.; Cornago, D.F.; Geronimo, I.M. Phenolic content and antioxidant capacity of Philippine sweet potato (Ipomoea batatas) varieties. Food Chem. 2009, 113, 1133–1138. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Kusano, S.; Doi, H.; Aki, O. Effects on immune response of antidiabetic ingredients from white-skinned sweet potato (Ipomoea batatas L.). Nutrition 2005, 21, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.C.; Jung, C.M.; Shin, C.G.; Lee, J.K.; Choi, S.U.; Kim, S.Y.; Lee, K.R. A New Caffeoyl Quinic Acid from Aster scaber and Its Inhibitory Activity against Human Immunodeficiency Virus-1(HIV-1) Integrase. Chem. Pharm. Bull. 2000, 48, 1796–1798. [Google Scholar] [CrossRef] [Green Version]
- Mustapha, Y.; Babura, S.R. Determination of carbohydrate and β-carotene content of some vegetables consumed in Kana metropolis, Nigeria. Bay. J. Pure Appl. Sci. 2009, 2, 119–121. [Google Scholar]
- Nagata, M. A simple spectrophotometric method for the estimation of beta-carotene content in spinach acetone extracts. Bull. Natl. Inst. Veg. Tea Sci. 2009, 8, 1–5. [Google Scholar]
- Speek, A.; Temalilwa, C.; Schrijver, J. Determination of β-carotene content and vitamin A activity of vegetables by high-performance liquid chromatography and spectrophotometry. Food Chem. 1986, 19, 65–74. [Google Scholar] [CrossRef]
- Takahata, Y.; Noda, T.; Nagata, T. HPLC determination of β-carotene content of sweet potato cultivars and its relationship with color values. Jpn. J. Breed. 1993, 43, 421–427. [Google Scholar] [CrossRef] [Green Version]
- Schuep, W.; Schierle, J. Determination of β-Carotene in Commercial Foods: Interlaboratory Study. J. AOAC Int. 1997, 80, 1057–1064. [Google Scholar] [CrossRef] [Green Version]
- Szpylka, J.; DeVries, J.W.; Bhandari, S.; Bui, M.H.; Ji, D.; Konings, E.; Lewis, R.; Mass, P.; Parish, H.; Post, B.; et al. Determination of β-carotene in supplements and raw materials by reversed-phase high pressure liquid chromatography. J. AOAC Int. 2005, 88, 1279–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahamad, M.N.; Saleemullah, M.; Shah, H.U.; Khalil, I.A.; Saljoqi, A.U.R. Determination of beta carotene content in fresh vegetables using high performance liquid chromatography. Sarhad J. Agric. 2007, 23, 767–770. [Google Scholar]
- Gupta, P.; Sreelakshmi, Y.; Sharma, R. A rapid and sensitive method for determination of carotenoids in plant tissues by high performance liquid chromatography. Plant Methods 2015, 11, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varzakas, T.; Kiokias, S. HPLC Analysis and Determination of Carotenoid Pigments in Commercially Available Plant Extracts. Curr. Res. Nutr. Food Sci. J. 2015, 4, 1–14. [Google Scholar] [CrossRef]
- Dhankhar, J.; Sharma, R.; Mann, B. Optimization of various steps for RP-HPLC determination of β-carotene in milk fat. Int. Food Res. J. 2017, 24, 1393–1398. [Google Scholar]
- Dai, Y.; Row, K.H. Isolation and Determination of Beta-Carotene in Carrots by Magnetic Chitosan Beta-Cyclodextrin Extraction and High-Performance Liquid Chromatography (HPLC). Anal. Lett. 2019, 52, 1828–1843. [Google Scholar] [CrossRef]
- Shi, L.; Zhu, X.L.; Liu, B.Z.; Gao, Y. Determination of beta-carotene in tobacco by HPTLC. Chin. J. Chromatogr. 1999, 17, 606–607. [Google Scholar]
- Marsit, C.J.; Fried, B.; Sherma, J. High-performance thin-layer chromatographic analysis of lutein and beta-carotene in Cerithidia californica (Gastropoda) infected with two species of larval trematodes. J. Parasitol. 2000, 86, 635–636. [Google Scholar] [CrossRef]
- Starek, M.; Guja, A.; Dąbrowska, M.; Krzek, J. Assay of β-Carotene in Dietary Supplements and Fruit Juices by TLC-Densitometry. Food Anal. Methods 2015, 8, 1347–1355. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Gupta, P.; De, B. Thin Layer Chromatographic Characterization of Carotenoid Isolates in Sugar Date Palm (Phoenix sylvestris) Fruit Epicarp and Inflorescence Axis. Int. J. Pharmacogn. Phytochem. Res. 2017, 9, 680–684. [Google Scholar] [CrossRef]
- Hynstova, V.; Štěrbová, D.; Klejdus, B.; Hedbavny, J.; Huska, D.; Adam, V. Separation, identification and quantification of carotenoids and chlorophylls in dietary supplements containing Chlorella vulgaris and Spirulina platensis using High Performance Thin Layer Chromatography. J. Pharm. Biomed. Anal. 2018, 148, 108–118. [Google Scholar] [CrossRef]
- Ghosh, S.; Chatterjee, J.K.; Chalkroborty, B.; Kundu, P. Estimation of beta carotene from fruit peel waste by high performance thin layer chromatography. J. Pharmacog. Phytochem. 2019, 8, 2598–2600. [Google Scholar]
- Condurso, C.; Cincotta, F.; Tripodi, G.; Merlino, M.; Giarratana, F.; Verzera, A. A new approach for the shelf-life definition of minimally processed carrots. Postharvest Biol. Technol. 2020, 163, 111138. [Google Scholar] [CrossRef]
- Cioates, C.N.; van Staden, J.F. Fluorimetric determination of β-carotene in food samples using a fluorescent dye. Anal. Lett. 2020, 53, 152–163. [Google Scholar] [CrossRef]
- Baranska, M.; Schütze, A.W.; Schulz, H. Determination of Lycopene and β-Carotene Content in Tomato Fruits and Related Products: Comparison of FT-Raman, ATR-IR, and NIR Spectroscopy. Anal. Chem. 2006, 78, 8456–8461. [Google Scholar] [CrossRef]
- Alam, P.; Ezzeldin, E.; Iqbal, M.; Anwer, K.; Mostafa, G.A.E.; Alqarni, M.H.; Foudah, A.I.; Shakeel, F. Ecofriendly densitometric RP-HPTLC method for determination of rivaroxaban in nanoparticle formulations using green solvents. RSC Adv. 2020, 10, 2133–2140. [Google Scholar] [CrossRef] [Green Version]
- Alam, P.; Iqbal, M.; Ezzeldin, E.; Khalil, N.Y.; Foudah, A.I.; Alqarni, M.H.; Shakeel, F. Simple and accurate HPTLC-densitometry method for quantification of delafloxacin (a novel fluoroquinolone antibiotic) in plasma samples: Application to pharmacokinetic study in rats. Antibiotics 2020, 9, 134. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, F.A.; Elmansi, H.; Fathy, M.E. Green RP-HPLC method for simultaneous determination of moxifloxacin combinations: Investigation of the greenness for the proposed method. Microchem. J. 2019, 148, 151–161. [Google Scholar] [CrossRef]
- Abou-Taleb, N.H.; El-Enany, N.M.; El-Sherbiny, D.T.; El-Subbagh, H.I. Digitally enhanced thin layer chromatography for simultaneous determination of norfloxacin and tinidazole with the aid of Taguchi orthogonal array and desirability function approach: Greenness assessment by analytical Eco-Scale. J. Sep. Sci. 2019, 43, 1195–1202. [Google Scholar] [CrossRef]
- Abdelrahman, M.M.; Abdelwahab, N.S.; Hegazy, M.A.; Fares, M.Y.; El-Sayed, G.M. Determination of the abused intravenously self-administered madness drops (Tropicamide) by liquid chromatography in rat plasma; an application to pharmacokinetic study and greenness profile assessment. Microchem. J. 2020, 159, 105582. [Google Scholar] [CrossRef]
- Duan, X.; Liu, X.; Dong, Y.; Yang, J.; Zhang, J.; He, S.; Yang, F.; Wang, Z.; Dong, Y. A Green HPLC Method for Determination of Nine Sulfonamides in Milk and Beef, and Its Greenness Assessment with Analytical Eco-Scale and Greenness Profile. J. AOAC Int. 2020, 103, 1181–1189. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef] [PubMed]
- Nowak, P.M.; Koscielniak, P. What Color Is Your Method? Adaptation of the RGB Additive Color Model to Analytical Method Evaluation. Anal. Chem. 2019, 91, 10343–10352. [Google Scholar] [CrossRef] [PubMed]
- Guideline, I.H.T. Validation of analytical procedures: Text and methodology. Q2(R1) 2005, 1, 5. [Google Scholar]
- Foudah, A.I.; Shakeel, F.; Alqarni, M.H.; Alam, P. A rapid and sensitive stability-indicating green RP-HPTLC method for the quantitation of flibanserin compared to green NP-HPTLC method: Validation studies and greenness assessment. Microchem. J. 2021, 164, 105960. [Google Scholar] [CrossRef]
- Fish, W.W.; Perkins-Veazie, P.; Collins, J.K. A Quantitative Assay for Lycopene That Utilizes Reduced Volumes of Organic Solvents. J. Food Compos. Anal. 2002, 15, 309–317. [Google Scholar] [CrossRef] [Green Version]





| Parameters | Values |
|---|---|
| Linearity range (ng Band−1) | 25–1000 |
| Regression equation | y = 47.696x + 297.73 |
| R2 | 0.9985 |
| R | 0.9992 |
| Slope ± SD | 47.696 ± 1.9400 |
| Intercept ± SD | 297.73 ± 3.4100 |
| Standard error of slope | 0.79216 |
| Standard error of intercept | 1.3924 |
| 95% confidence interval of slope | 44.287–51.104 |
| 95% confidence interval of intercept | 291.73–303.72 |
| LOD ± SD (ng Band−1) | 8.84 ± 0.12 |
| LOQ ± SD (ng Band−1) | 26.52 ± 0.36 |
| Parameters | Value |
|---|---|
| Rf | 0.64 ± 0.02 |
| As | 1.03 ± 0.03 |
| N m−1 | 5741 ± 3.52 |
| Conc. (ng Band−1) | Conc. Found (ng Band−1) ± SD | Recovery (%) | CV (%) |
|---|---|---|---|
| 100 | 101.23 ± 0.61 | 101.23 | 0.60 |
| 400 | 397.64 ± 2.17 | 99.41 | 0.54 |
| 1000 | 1010.24 ± 4.64 | 101.02 | 0.45 |
| Conc. (ng Band−1) | Intraday Precision | Interday Precision | ||||
| Conc. (ng Band−1)± SD | Standard Error | CV (%) | Conc. (ng Band−1)± SD | Standard Error | CV (%) | |
| 100 | 98.36 ± 0.50 | 0.20 | 0.50 | 98.74 ± 0.64 | 0.26 | 0.64 |
| 400 | 406.31 ± 1.94 | 0.79 | 0.47 | 396.21 ± 2.19 | 0.89 | 0.55 |
| 1000 | 988.23 ± 4.58 | 1.87 | 0.46 | 1008.54 ± 4.78 | 1.95 | 0.47 |
| Conc. (ng Band−1) | Mobile Phase Composition (EtOH-CY-A, v v v−1) | Results | ||||
|---|---|---|---|---|---|---|
| Original | Used | Level | Conc. (ng Band−1) ± SD | % CV | Rf | |
| 96:2:2 | +1.0 | 387.42 ± 2.87 | 0.74 | 0.63 | ||
| 400 | 95:2.5:2.5 | 95:2.5:2.5 | 0.0 | 398.21 ± 3.14 | 0.78 | 0.64 |
| 94:3:3 | −1.0 | 406.21 ± 3.45 | 0.84 | 0.65 | ||
| Samples | TE | UBE |
|---|---|---|
| Amount of β-Carotene (% w w−1) | ||
| D. carota (Hexane 100%) | 0.00 ± 0.00 | 0.00 ± 0.00 |
| D. carota (Acetone 100%) | 3.22 ± 0.08 | 4.31 ± 0.11 |
| D. carota (Hexane: acetone 50:50%) | 10.32 ± 0.14 | 12.35 ± 0.20 |
| I. batatas (Hexane 100%) | 0.85 ± 0.02 | 1.06 ± 0.03 |
| I. batatas (Acetone 100%) | 2.29 ± 0.04 | 3.11 ± 0.05 |
| I. batatas (Hexane: acetone 50:50%) | 3.73 ± 0.09 | 4.86 ± 0.10 |
| Formulation A | 6.73 ± 0.13 | 8.52 ± 0.16 |
| Analytical Method | Linearity Range | Accuracy (% Recovery) | Precision (% CV) | Ref. |
|---|---|---|---|---|
| HPLC | 0.20–35 (µg g−1) | 97.94–101.02 | - | [18] |
| HPLC | 0.10–50 (µg mL−1) | 97.50–102.10 | 1.20–4.40 | [23] |
| HPTLC | 0.76–9.14 (µg Band−1) | 99.59–101.04 | 0.68–0.87 | [31] |
| HPTLC | 25–1000 (ng Band−1) | 99.41–101.23 | 0.46–0.64 | Present work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqarni, M.H.; Alam, P.; Alam, A.; Ali, A.; Foudah, A.I.; Alshehri, S.; Ghoneim, M.M.; Shakeel, F. A Greener HPTLC Approach for the Determination of β-Carotene in Traditional and Ultrasound-Based Extracts of Different Fractions of Daucus carota (L.), Ipomea batatas (L.), and Commercial Formulation. Agronomy 2021, 11, 2443. https://doi.org/10.3390/agronomy11122443
Alqarni MH, Alam P, Alam A, Ali A, Foudah AI, Alshehri S, Ghoneim MM, Shakeel F. A Greener HPTLC Approach for the Determination of β-Carotene in Traditional and Ultrasound-Based Extracts of Different Fractions of Daucus carota (L.), Ipomea batatas (L.), and Commercial Formulation. Agronomy. 2021; 11(12):2443. https://doi.org/10.3390/agronomy11122443
Chicago/Turabian StyleAlqarni, Mohammed H., Prawez Alam, Aftab Alam, Abuzer Ali, Ahmed I. Foudah, Sultan Alshehri, Mohammed M. Ghoneim, and Faiyaz Shakeel. 2021. "A Greener HPTLC Approach for the Determination of β-Carotene in Traditional and Ultrasound-Based Extracts of Different Fractions of Daucus carota (L.), Ipomea batatas (L.), and Commercial Formulation" Agronomy 11, no. 12: 2443. https://doi.org/10.3390/agronomy11122443
APA StyleAlqarni, M. H., Alam, P., Alam, A., Ali, A., Foudah, A. I., Alshehri, S., Ghoneim, M. M., & Shakeel, F. (2021). A Greener HPTLC Approach for the Determination of β-Carotene in Traditional and Ultrasound-Based Extracts of Different Fractions of Daucus carota (L.), Ipomea batatas (L.), and Commercial Formulation. Agronomy, 11(12), 2443. https://doi.org/10.3390/agronomy11122443








