Polyphasic Analysis of Isolates from Kiwifruit Reveal New Genetic Lineages of Pseudomonas syringae pv. actinidifoliorum Look-Alike
Abstract
:1. Introduction
2. Materials and Methods
2.1. Surveys and Bacterial Isolations
2.2. Detection of Psa
2.3. Characterization of Pfm Look-Alike
2.3.1. Phenotypic Characterization
2.3.2. Pathogenicity Assays
2.3.3. DNA Fingerprinting Using Pulsed-Field Gel Electrophoresis (PFGE)
2.3.4. Housekeeping Genes Amplifications and Sequencing
2.3.5. Phylogenetic Analyses: 16S rRNA and MLSA
2.3.6. Repetitive-Sequence PCR and Detection of syrD, cfl, and tox-argK Genes
2.3.7. Detection of Type III Secretion System Effector Genes
3. Results
3.1. Surveys and Bacterial Isolations and Detection of Psa
3.2. Characterization of Pfm Look-Alike
3.2.1. Phenotypic Characterization
3.2.2. Pathogenicity Assays
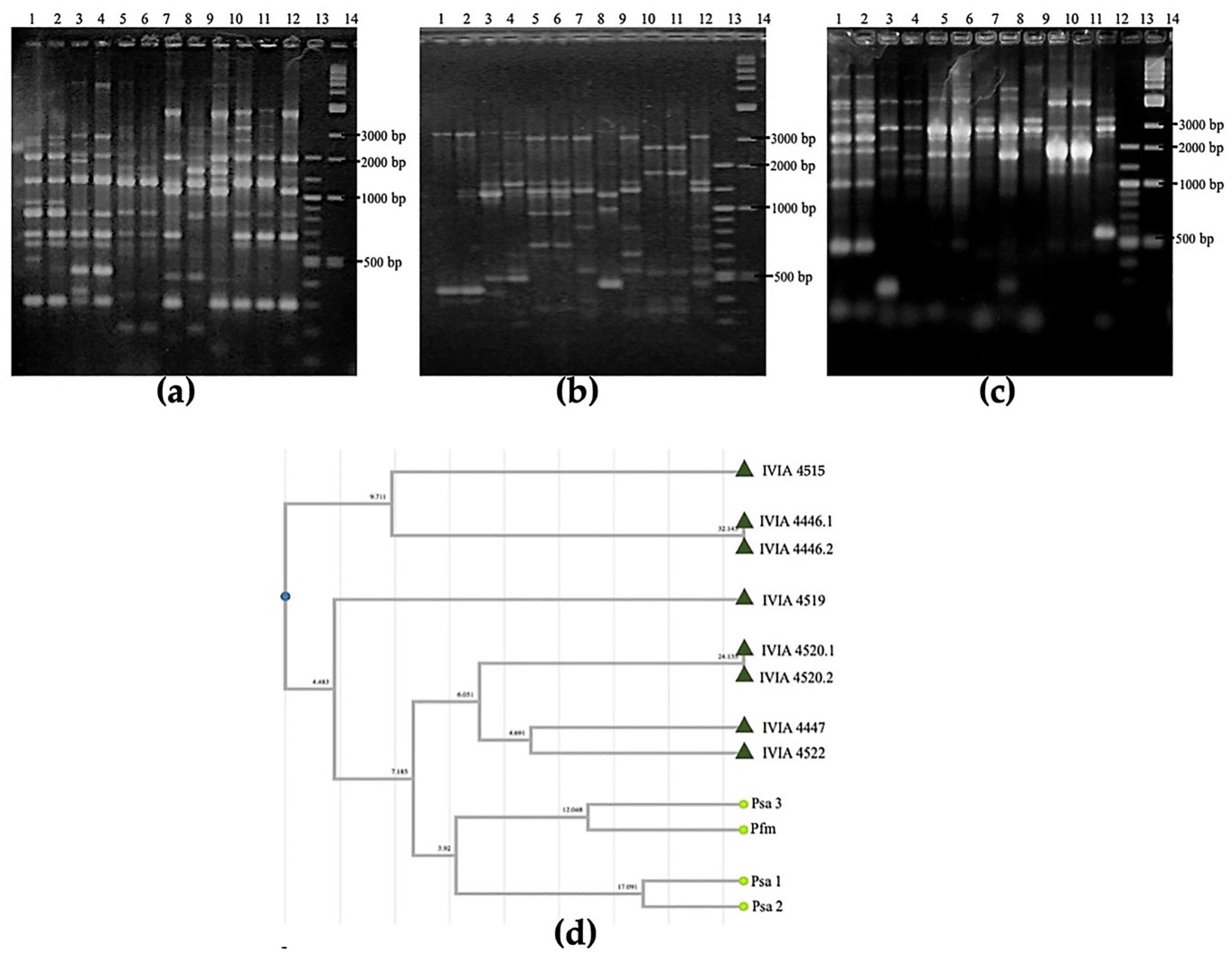
3.2.3. DNA Fingerprinting Using Pulsed-Field Gel Electrophoresis (PFGE)
3.2.4. Phylogenetic Analyses: 16S rRNA Gene and MLSA and Clonal Genealogy with gltA, gyrB, gapA and rpoD Genes
3.2.5. Repetitive-Sequence PCR and Detection of syrD, cfl and tox-argK Genes
3.2.6. Detection of Type III Secretion System Effector Genes
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vogelaar, M.; Schenk, M.; Delbianco, A.; Graziosi, I.; Vos, S. Pest survey card on Pseudomonas syringae pv. actinidiae. EFSA 2020, 17, 1986E. [Google Scholar] [CrossRef]
- Takikawa, Y.; Serizawa, S.; Ichikawa, T.; Tsuyumu, S.; Goto, M. Pseudomonas syringae pv. actinidiae pv. nov.: The causal bacterium of banker of biwifruit in Japan. Jpn. J. Phytopathol. 1989, 55, 437–444. [Google Scholar]
- Wang, Z.T. Identification of the pathogenic bacterium for bacterial canker on actinidia in sichuan. J. Southwest Agric. Univ. 1992, 46, 6. [Google Scholar]
- Koh, Y.; Chung, H.; Cha, B.; Lee, D. Outbreak and spread of bacterial canker in kiwifruit. Korean. Korean J. Plant Pathol. 1994, 10, 69–72. [Google Scholar]
- Scortichini, M. Occurrence of Pseudomonas syringae pv. actinidiae on kiwifruit in Italy. Plant Pathol. 1994, 43, 1035–1038. [Google Scholar] [CrossRef]
- Everett, K.R.; Taylor, R.K.; Romberg, M.K.; Rees-George, J.; Fullerton, R.A.; Vanneste, J.L.; Manning, M.A. First report of Pseudomonas syringae pv. actinidiae causing kiwifruit bacterial canker in New Zealand. Australas. Plant Dis. Notes 2011, 6, 67–71. [Google Scholar] [CrossRef] [Green Version]
- Vanneste, J.L.; Poliakoff, F.; Audusseau, C.; Cornish, D.A.; Paillard, S.; Rivoal, C.; Yu, J. First report of Pseudomonas syringae pv. Actinidiae, the causal agent of bacterial canker of kiwifruit in France. Plant Dis. 2011, 95, 1311. [Google Scholar] [CrossRef]
- Abelleira, A.; López, M.M.; Peñalver, J.; Aguín, O.; Mansilla, J.P.; Picoaga, A.; García, M.J. First report of bacterial canker of kiwifruit caused by Pseudomonas syringae pv. actinidiae in Spain. Plant Dis. 2011, 95, 1583. [Google Scholar] [CrossRef]
- Balestra, G.M.; Renzi, M.; Mazzaglia, A. First report of bacterial canker of Actinidia deliciosa caused by Pseudomonas syringae pv. actinidiae in Portugal. New Dis. Rep. 2010, 22, 10. [Google Scholar] [CrossRef] [Green Version]
- EPPO Global Database. Pseudomonas syringae pv. actinidiae (PSDMAK), Distribution. Available online: https://gd.eppo.int/taxon/PSDMAK/distribution (accessed on 5 November 2021).
- Dreo, T.; Pirc, M.; Ravnikar, M.; Žežlina, I.; Poliakoff, F.; Rivoal, C.; Nice, F.; Cunty, A.; Ouest, A. First report of Pseudomonas syringae pv. actinidiae, the causal agent of bacterial canker of kiwifruit in Slovenia. Plant Dis. 2014, 98, 1578. [Google Scholar] [PubMed]
- Holeva, M.C.; Glynos, P.E.; Karafla, C.D. First report of bacterial canker of kiwifruit caused by Pseudomonas syringae pv. Actinidiae in Greece. Plant Dis. 2015, 99, 723. [Google Scholar] [CrossRef]
- Donati, I.; Cellini, A.; Sangiorgio, D.; Vanneste, J.L.; Scortichini, M.; Balestra, G.M.; Spinelli, F. Pseudomonas syringae pv. actinidiae: Ecology, infection dynamics and disease epidemiology. Microb. Ecol. 2020, 80, 81–102. [Google Scholar] [CrossRef] [PubMed]
- Morán, F.; Marco-Noales, E.; Escrich, A.; Barbé, S.; López, M.M. Biodiversity and biogeography of three Pseudomonas syringae pathovars which affect kiwi fruit cultivation. Biodivers. Online J. 2018, 1, 1–3. [Google Scholar]
- Vanneste, J.L.; Yu, J.; Cornish, D.A.; Tanner, D.J.; Windner, R.; Chapman, J.R.; Taylor, R.K.; Mackay, J.F.; Dowlut, S. Identification, virulence, and distribution of two biovars of Pseudomonas syringae pv. actinidiae in New Zealand. Plant Dis. 2013, 97, 708–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunty, A.; Poliakoff, F.; Rivoal, C.; Cesbron, S.; Fischer-Le Saux, M.; Lemaire, C.; Jacques, M.A.; Manceau, C.; Vanneste, J.L. Characterization of Pseudomonas syringae pv. actinidiae (Psa) isolated from France and assignment of Psa biovar 4 to a de novo pathovar: Pseudomonas syringae pv. actinidifoliorum pv. nov. Plant Pathol. 2015, 64, 582–596. [Google Scholar] [CrossRef]
- Sawada, H.; Miyoshi, T.; Ide, Y. Novel MLSA group (Psa5) of Pseudomonas syringae pv. actinidiae causing bacterial canker of kiwifruit (Actinidia chinensis) in Japan. Jpn. J. Phytopathol. 2014, 80, 171–184. [Google Scholar] [CrossRef] [Green Version]
- Sawada, H.; Kondo, K.; Nakaune, R. Novel biovar (biovar 6) of Pseudomonas syringae pv. actinidiae causing bacterial canker of kiwifruit (Actinidia deliciosa) in Japan. Jpn. J. Phytopathol. 2016, 82, 101–115. [Google Scholar] [CrossRef] [Green Version]
- Fujikawa, T.; Sawada, H. Genome analysis of Pseudomonas syringae pv. actinidiae biovar 6, which produces the phytotoxins, phaseolotoxin and coronatine. Sci. Rep. 2019, 9, 3836. [Google Scholar] [CrossRef] [PubMed]
- Abelleira, A.; Ares, A.; Aguín, O.; Picoaga, A.; López, M.M.; Mansilla, P. Current situation and characterization of Pseudomonas syringae pv. actinidiae on kiwifruit in Galicia (northwest Spain). Plant Pathol. 2014, 63, 691–699. [Google Scholar] [CrossRef]
- Abelleira, A.; Ares, A.; Aguin, O.; Peñalver, J.; Morente, M.C.; López, M.M.; Sainz, M.J.; Mansilla, J.P. Detection and characterization of Pseudomonas syringae pv. actinidifoliorum in kiwifruit in Spain. J. Appl. Microbiol. 2015, 119, 1659–1671. [Google Scholar] [CrossRef] [PubMed]
- EPPO. PM 7/120 (1) Pseudomonas syringae pv. actinidiae. EPPO Bull. 2014, 44, 360–375. [Google Scholar] [CrossRef] [Green Version]
- Crosse, J.E. Bacterial canker of stone-fruits: Iv. investigation of a method for measuring the inoculum potential of cherry trees. Ann. Appl. Biol. 1959, 47, 306–317. [Google Scholar] [CrossRef]
- King, E.O.; Ward, M.K.; Raney, D.E. Two simple media for the demonstration of pyocyanin and fluorescin. J. Lab. Clin. Med. 1954, 44, 301–307. [Google Scholar]
- Mohan, S.K. An Improved Agar Plating Assay for Detecting Pseudomonas syringae pv. syringae and P. s. pv. phaseolicola in contaminated bean seed. Phytopathology 1987, 77, 1390. [Google Scholar] [CrossRef]
- Rees-George, J.; Vanneste, J.L.; Cornish, D.A.; Pushparajah, I.P.S.; Yu, J.; Templeton, M.D.; Everett, K.R. Detection of Pseudomonas syringae pv. actinidiae using polymerase chain reaction (PCR) primers based on the 16S-23S rDNA intertranscribed spacer region and comparison with PCR primers based on other gene regions. Plant Pathol. 2010, 59, 453–464. [Google Scholar] [CrossRef]
- Gallelli, A.; L’Aurora, A.; Loreti, S. Gene sequence analysis for the molecular detection of Pseudomonas syringae pv. actinidiae: Developing diagnostic protocols. J. Plant Pathol. 2011, 93, 425–435. [Google Scholar]
- Gallelli, A.; Talocci, S.; Pilotti, M.; Loreti, S. Real-time and qualitative PCR for detecting Pseudomonas syringae pv. actinidiae isolates causing recent outbreaks of kiwifruit bacterial canker. Plant Pathol. 2014, 63, 264–276. [Google Scholar] [CrossRef]
- Lelliott, R.; Stead, D. Methods for the Diagnosis of Bacterial Diseases of Plants; Blackwell Scientific Publications: Oxford, UK, 1987; p. 216. [Google Scholar]
- Lindow, S.E. The role of bacterial ice nucleation in frost injury to plants. Ann. Rev. Phytopathol. 1983, 21, 363–384. [Google Scholar] [CrossRef]
- Latorre, B.A. Pseudomonas morsprunorum, the cause of bacterial canker of sour cherry in Michigan, and its epiphytic association with P. syringae. Phytopathology 1979, 69, 335. [Google Scholar] [CrossRef]
- Pethybridge, S.J.; Nelson, S.C. Leaf Doctor: A new portable application for quantifying plant disease severity. Plant Dis. 2015, 99, 1310–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leaf Doctor on the App Store. Available online: https://apps.apple.com/us/app/leaf-doctor/id874509900 (accessed on 5 November 2021).
- Rainey, P.B.; Bailey, M.J.; Thompson, I.P. Phenotypic and genotypic diversity of fluorescent pseudomonads isolated from field-grown sugar beet. Microbiology 1994, 140, 2315–2331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guven, K.; Jones, J.B.; Momol, M.T.; Dickstein, E.R. Phenotypic and genetic diversity among Pseudomonas syringae pv. phaseolicola. J. Phytopathol. 2004, 152, 658–666. [Google Scholar] [CrossRef]
- Hunter, S.B.; Vauterin, P.; Lambert-Fair, M.A.; Van Duyne, M.S.; Kubota, K.; Graves, L.; Wrigley, D.; Barrett, T.; Ribot, E. Establishment of a universal size standard strain for use with the pulsenet standardized pulsed-field gel electrophoresis protocols: Converting the national databases to the new size standard. J. Clin. Microbiol. 2005, 43, 1045–1050. [Google Scholar] [CrossRef] [Green Version]
- Genetic Analysis Strategies. Available online: https://geneticpcr.com/en/ (accessed on 5 November 2021).
- Chapman, J.R.; Taylor, R.K.; Weir, B.S.; Romberg, M.K.; Vanneste, J.L.; Luck, J.; Alexander, B.J.R. Phylogenetic relationships among global populations of Pseudomonas syringae pv. actinidiae. Phytopathology 2012, 102, 1034–1044. [Google Scholar] [CrossRef] [Green Version]
- Ferrante, P.; Scortichini, M. Redefining the global populations of Pseudomonas syringae pv. actinidiae based on pathogenic, molecular and phenotypic characteristics. Plant Pathol. 2015, 64, 51–62. [Google Scholar] [CrossRef]
- Sarkar, S.F.; Guttman, D.S. Evolution of the core genome of Pseudomonas syringae, a highly clonal, endemic plant pathogen. Appl. Environ. Microbiol. 2004, 70, 1999–2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, M.S.H.; Morgan, R.L.; Sarkar, S.F.; Wang, P.W.; Guttman, D.S. Phylogenetic characterization of virulence and resistance phenotypes of Pseudomonas syringae. Appl. Environ. Microbiol. 2005, 71, 5182–5191. [Google Scholar] [CrossRef] [Green Version]
- Okonechnikov, K.; Golosova, O.; Fursov, M. Genome analysis Unipro UGENE: A unified bioinformatics toolkit. Bioinform. Appl. 2012, 28, 1166–1167. [Google Scholar] [CrossRef] [Green Version]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; Mcgettigan, P.A.; Mcwilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef] [PubMed]
- Hillis, D.M.; Bull, J.J. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst. Biol. 1993, 42, 182–192. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 15 January 2018).
- Ferrante, P.; Scortichini, M. Identification of Pseudomonas syringae pv. actinidiae as causal agent of bacterial canker of yellow kiwifruit (Actinidia chinensis Planchon) in central Italy. J. Phytopathol. 2009, 157, 768–770. [Google Scholar] [CrossRef]
- Ferrante, P.; Scortichini, M. Molecular and phenotypic features of Pseudomonas syringae pv. actinidiae isolated during recent epidemics of bacterial canker on yellow kiwifruit (Actinidia chinensis) in central Italy. Plant Pathol. 2010, 59, 954–962. [Google Scholar] [CrossRef]
- Sorensen, K.N.; Kim, K.H.; Takemoto, J.Y. PCR detection of cyclic lipodepsinonapeptide-producing Pseudomonas syringae pv. syringae and similarity of strains. Appl. Environ. Microbiol. 1998, 64, 226–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bereswill, S.; Bugert, P.; Volksch, B.; Ullrich, M.; Bender, C.L.; Geider, K. Identification and relatedness of coronatine-producing Pseudomonas syringae pathovars by PCR analysis and sequence determination of the amplification products. Appl. Environ. Microbiol. 1994, 60, 2924–2930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Templeton, M.D.; Reinhardt, L.A.; Collyer, C.A.; Mitchell, R.E.; Cleland, W.W. Kinetic analysis of the L-ornithine transcarbamoylase from Pseudomonas savastanoi pv. phaseolicola that is resistant to the transition state analogue (R)-Nδ-(N′-sulfodiaminophosphinyl)-L-ornithine. Biochemistry 2005, 44, 4408–4415. [Google Scholar] [CrossRef]
- GenBank Database. Available online: https://www.ncbi.nlm.nih.gov/genbank/ (accessed on 15 January 2019).
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef] [PubMed]
- Parte, A.C. List of Prokaryotic names with standing in nomenclature (bacterio.net), 20 years on. Int. J. Syst. Evol. Microbiol. 2018, 68, 1825–1829. [Google Scholar] [CrossRef]
- Lalucat, J.; Mulet, M.; Gomila, M.; García-Valdés, E. Genomics in bacterial taxonomy: Impact on the genus Pseudomonas. Genes 2020, 11, 139. [Google Scholar] [CrossRef] [Green Version]
- Arnold, D.L.; Preston, G.M. Pseudomonas syringae: Enterprising epiphyte and stealthy parasite. Microbiology 2019, 165, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.E.; Monteil, C.L.; Berge, O. The life history of Pseudomonas syringae: Linking agriculture to earth system processes. Annu. Rev. Phytopathol. 2013, 51, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.E.; Sands, D.C.; Vanneste, J.L.; Montarry, J.; Oakley, B.; Guilbaud, C.; Glaux, C. Inferring the evolutionary history of the plant pathogen Pseudomonas syringae from its biogeography in headwaters of rivers in North America, Europe, and New Zealand. MBio 2010, 1, e00107-10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, J.M. Minireview taxonomy of Pseudomonas syringae. J. Plant Pathol. 2010, 92, S1.5–S1.14. [Google Scholar]
- Baltrus, D.A.; McCann, H.C.; Guttman, D.S. Evolution, genomics and epidemiology of Pseudomonas syringae: Challenges in Bacterial Molecular Plant Pathology. Mol. Plant Pathol. 2017, 18, 152–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, W.E.C.; Stackebrandt, E.; Kandler, O.; Colwell, R.R.; Krichevsky, M.I.; Truper, H.G.; Murray, R.G.E.; Wayne, L.G.; Grimont, P.A.D.; Brenner, D.J.; et al. Report of the Ad Hoc committee on reconciliation of approaches to bacterial systematics. Int. J. Syst. Evol. Microbiol. 1987, 37, 463–464. [Google Scholar] [CrossRef] [Green Version]
- Quigley, N.B. Syringomycin production among strains of Pseudomonas syringae pv. syringae: Conservation of the syrB and syrD genes and activation of phytotoxin production by plant signal molecules. Mol. Plant Microbe Interact. 1994, 7, 78. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morán, F.; Marco-Noales, E.; Landeras, E.; Roselló, M.; Abelleira, A.; Gonzalez, A.J.; López, M.M. Polyphasic Analysis of Isolates from Kiwifruit Reveal New Genetic Lineages of Pseudomonas syringae pv. actinidifoliorum Look-Alike. Agronomy 2021, 11, 2464. https://doi.org/10.3390/agronomy11122464
Morán F, Marco-Noales E, Landeras E, Roselló M, Abelleira A, Gonzalez AJ, López MM. Polyphasic Analysis of Isolates from Kiwifruit Reveal New Genetic Lineages of Pseudomonas syringae pv. actinidifoliorum Look-Alike. Agronomy. 2021; 11(12):2464. https://doi.org/10.3390/agronomy11122464
Chicago/Turabian StyleMorán, Félix, Ester Marco-Noales, Elena Landeras, Montserrat Roselló, Adela Abelleira, Ana J. Gonzalez, and María M. López. 2021. "Polyphasic Analysis of Isolates from Kiwifruit Reveal New Genetic Lineages of Pseudomonas syringae pv. actinidifoliorum Look-Alike" Agronomy 11, no. 12: 2464. https://doi.org/10.3390/agronomy11122464






