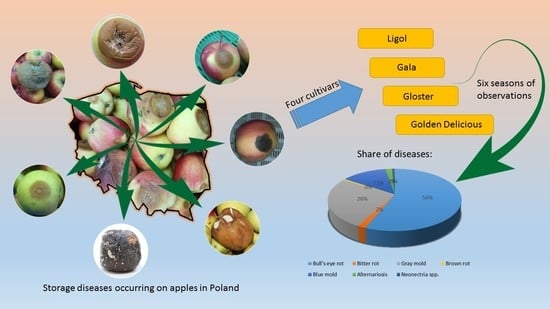
The Recent Occurrence of Biotic Postharvest Diseases of Apples in Poland
Abstract
1. Introduction
2. Materials and Methods
2.1. Monitoring and Sample Collection
2.2. Diagnostics of Apple Storage Diseases
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neri, F.; Gualanduzzi, S.; Brigati, S. Effect of harvest maturity on quality, physiological and pathological disorders during storage of ‘Gala’ apples. Acta Hort. 2005, 682, 2069–2076. [Google Scholar] [CrossRef]
- Ivić, D.; Sever, Z.; Miličević, T. Estimation of economic loss due to postharvest diseases of apple (cv. Idared) during four seasons. Pomol. Croat. 2012, 18, 51–62. [Google Scholar]
- Aguilar, C.G.; Mazzola, M.; Xiao, C.L. Timing of apple fruit infection by Neofabraea perennans and Neofabraea kienholzii in relation to bull’s-eye rot development in stored apple fruit. Plant Dis. 2017, 101, 800–806. [Google Scholar] [CrossRef][Green Version]
- Jones, A.L.; Aldwinckle, H.S. Compendium of Apple and Pear Diseases; APS Press the American Phytopathological Society: St. Paul, MN, USA, 1997; p. 100. [Google Scholar]
- Snowdon, A.L. A Colour Atlas of Post-Harvest Diseases and Disorders of Fruits and Vegetables. Vol. 1: General Introduction and Fruits; Wolf Scientific Ltd.: London, UK, 1990; pp. 170–218. [Google Scholar]
- Blažek, J.; Kloutvorová, J.; Křelinová, J. Incidence of storage diseases on apples of selected cultivars and advanced selections grown with and without fungicide treatments. Hort. Sci. 2006, 33, 87–94. [Google Scholar] [CrossRef]
- Rejman, A. Studia nad przechowywaniem 12 odmian jabłek. Roczniki Nauk Rolniczych 1954, 69, 349–383. [Google Scholar]
- Borecka, H. Cryptosporiopsis malicorticis (Zeller et Childs) Wollenw. syn. Gloeosporium perennans Zeller et Childs, jako czynnik chorobotwórczy na jabłkach w okresie przechowywania. Acta Agrobot. 1962, 12, 11–66. [Google Scholar]
- Bryk, H.; Bartosiewicz, B.; Rejnus, M. Nasilenie chorób grzybowych na przechowywanych jabłkach w latach 1993–1995. In Proceedings of the Materiały Ogólnopolskiej Konferencji Ochrony Roślin Sadowniczych, Skierniewice, Poland, 20–21 February 1996; pp. 134–135. [Google Scholar]
- Bryk, H.; Wojtas-Kozieł, B.; Lewandowska, M.; Rejnus, M. Grzyby powodujące choroby podczas przechowywania oraz ocena skuteczności fungicydów w zwalczaniu tych chorób. Pr. Inst. Sad. Kwiac. Ser. A 1991, 30, 127–135. [Google Scholar]
- Weber, R. Apple storage rots: The situation in Northern Germany. In Proceedings of the COST 864 Expert Meeting, Bergen, Norway, 25–26 March 2009. [Google Scholar]
- Giraud, M. Fungal storage diseases of apple in France. In Proceedings of the COST 864 Expert Meeting, Bergen, Norway, 25–26 March 2009. [Google Scholar]
- Neri, F.; Mari, M.; Brigati, S.; Bertolini, P. Control of Neofabraea alba by plant volatile compounds and hot water. Postharvest Biol. Technol. 2009, 51, 425–430. [Google Scholar] [CrossRef]
- Henriquez, J.L.; Sugar, D.; Spotts, R.A. Etiology of bull’s eye rot of pear caused by Neofabraea spp. in Oregon, Washington, and California. Plant Dis. 2004, 88, 1134–1138. [Google Scholar] [CrossRef]
- De Jong, S.N.; Levesque, C.E.; Verkley, G.J.M.; Abeln, E.C.A.; Rahe, J.E.; Braun, P.G. Phylogenetic relationships among Neofabraea species causing tree cankers and bull’s-eye-rot of apple based on DNA sequencing of ITS nuclear rDNA, mitochondrial rDNA, and the ß-tubulin gene. Mycol. Res. 2001, 105, 658–669. [Google Scholar] [CrossRef]
- Soto-Alvear, S.; Lolas, M.; Rosales, I.M.; Chavez, E.R.; Latorre, B.A. Characterization of the bull’s eye rot of apple in Chile. Plant Dis. 2013, 97, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Cunnington, J.H. Three Neofabraea species on pome fruit in Australia. Australas. Plant Pathol. 2004, 33, 453–454. [Google Scholar] [CrossRef]
- Cameldi, I.; Neri, F.; Menghini, M.; Pirondi, A.; Nanni, I.M.; Collina, M.; Mari, M. Characterization of Neofabraea vagabunda isolates causing apple bull‘s eye rot in Italy (Emilia-Romagna region). Plant Pathol. 2017, 66, 1432–1444. [Google Scholar] [CrossRef]
- Michalecka, M.; Bryk, H.; Poniatowska, A.; Puławska, J. Identification of Neofabraea species causing bull‘s eye rot of apples in Poland and their direct detection in apple fruit using multiplex PCR. Plant Pathol. 2016, 65, 643–654. [Google Scholar] [CrossRef]
- Peres, N.A.; Timmer, L.w.; Adaskaveg, J.E.; Correl, J.C. Lifestyles of Colletotrichum acutatum. Plant Dis. 2005, 89, 784–796. [Google Scholar] [CrossRef] [PubMed]
- Wenneker, M.; Pham, K.; Kohl, J. Postharvest diseases of pome fruit and sources of inokulum in orchard in The Netherlands. Agropecu. Catarin. 2018, 31, 57–63. [Google Scholar]
- Xiao, C.L.; Kim, Y.K. Postharvest fruit rots in apples caused by Botrytis cinerea, Phacidiopycnis washingtonensis, and Sphaeropsis pyriputrescens. Plant Health Prog. 2008, 9, 34. [Google Scholar] [CrossRef]
- Marcinkowska, J. Oznaczanie Rodzajów Grzybów Ważnych w Patologii Roślin; Fundacja “Rozwój SGGW”: Warszawa, Poland, 2003; p. 328. ISBN 8372740569. [Google Scholar]
- Sreenivasaprasad, S.; Sharada, K.; Brown, A.É.; Mills, P.R. PCR-based detection of Colletotrichum acutatum on strawberry. Plant Pathol. 1996, 45, 650–655. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In Book: PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Romanazzi, G.; Smilanick, J.L.; Feliziani, E.; Droby, S. Integrated management of postharvest gray mold on fruit crops. Postharvest Biol. Technol. 2016, 113, 69–76. [Google Scholar] [CrossRef]
- Munoz, C.; Sanz, M.I. Detection transposable elements in Botrytis cinerea in latent infection stage from symptomless apples. J. Coast. Life Med. 2014, 2, 125–131. [Google Scholar]
- Wenneker, M.; Kohl, J. Postharvest decay of apples and pears in the Netherlands. Acta Hort. 2014, 1053, 107–111. [Google Scholar] [CrossRef]
- Florian, V.C.; Puia, C.; Groza, R.; Suciu, L.A.; Florian, T. Study of the major pathogens that lead to apple fruit decay during storage. Not. Bot. Horti Agrobot. 2018, 46, 538–545. [Google Scholar] [CrossRef]
- Konstantinou, S.; Karaoglanidis, G.S.; Bardas, G.A.; Minas, I.S.; Doukas, E.; Markoglou, A.N. Postharvest fruit rots of apple in Greece: Pathogen incidence and relationships between fruit quality parameters, cultivar susceptibility, and patulin production. Plant Dis. 2011, 95, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Bryk, H. Choroby przechowalnicze jabłek i gruszek. In Proceedings of the Materiały VI Międzynarodowych Targów Agrotechniki Sadowniczej, Warszawa, Poland, 15–16 January 2010; pp. 17–26. [Google Scholar]
- Jurick, W.M., II; Gaskins, V.L.; Luo, Y.G. First report of Alternaria alternata causing postharvest decay on apple fruit during cold storage in Pensylvannia. Plant Dis. 2014, 98, 690. [Google Scholar] [CrossRef]
- Borecki, Z. Badania nad gorzką zgnilizną jabłek powodowaną przez grzyby Gloeosporium perennans Zeller et Childs, Gloeosporium album Osterw. I Gloeosporium fructigenum Berk. Acta Agrobot. 1961, 10, 53–97. [Google Scholar] [CrossRef]
- Sutton, T.B.; Shane, W.W. Epidemiology of the perfect stage of Glomerella cingulata on apples. Phytopathology 1983, 73, 1179–1183. [Google Scholar] [CrossRef]
- Bryk, H.; Michalecka, M.; Jakubowska, A. Grzyby z rodzaju Colletotrichum—Sprawcy gorzkiej zgnilizny jabłek. In Proceedings of the Materiały 54 Ogólnopolskiej. Konferencji Ochrony Roślin Sadowniczych, Ossa k. Białej Rawskiej, Poland, 23–24 February 2011; pp. 108–110. [Google Scholar]
- Damm, U.; Cannon, P.F.; Woudenberg, J.H.C.; Crous, P.W. The Colletotrichum acutatum species complex. Stud. Mycol. 2012, 73, 37–113. [Google Scholar] [CrossRef]
- Cattiaux, J.; Vautard, R.; Cassou, C.; Yiou, P.; Masson-Delmotte, V.; Codron, F. Winter 2010 in Europe: A cold extreme in a warming climate. Geophys. Res. Lett. 2010, 37, L20704. [Google Scholar] [CrossRef]
- West, J.S.; Townsend, J.A.; Stevens, M.; Fitt, B.D.L. Comparative biology of different plant pathogens to estimate effects of climate change on crop diseases in Europe. Eur. J. Plant Pathol. 2012, 133, 315–331. [Google Scholar] [CrossRef]
- Berrie, A.M. Storage rots of apple and pear in South East England 1980–1988: Incidence and fungicide resistance. IOBC Bull. 1989, 12, 229–239. [Google Scholar]
- Weber, R.W.S. Biology and control of the apple canker fungus Neonectria ditissima (syn. N. galligena) from a Northwestern European perspective. Erwerbs-Obstbau 2014, 56, 95–107. [Google Scholar] [CrossRef]
- Głos, H.; Bryk, H.; Michalecka, M.; Poniatowska, A.; Puławska, J. First report of Diaporthe eres, a new pathogen causing rot of apples during storage period in Poland. J. Plant Pathol. 2021, 103, 393–394. [Google Scholar] [CrossRef]
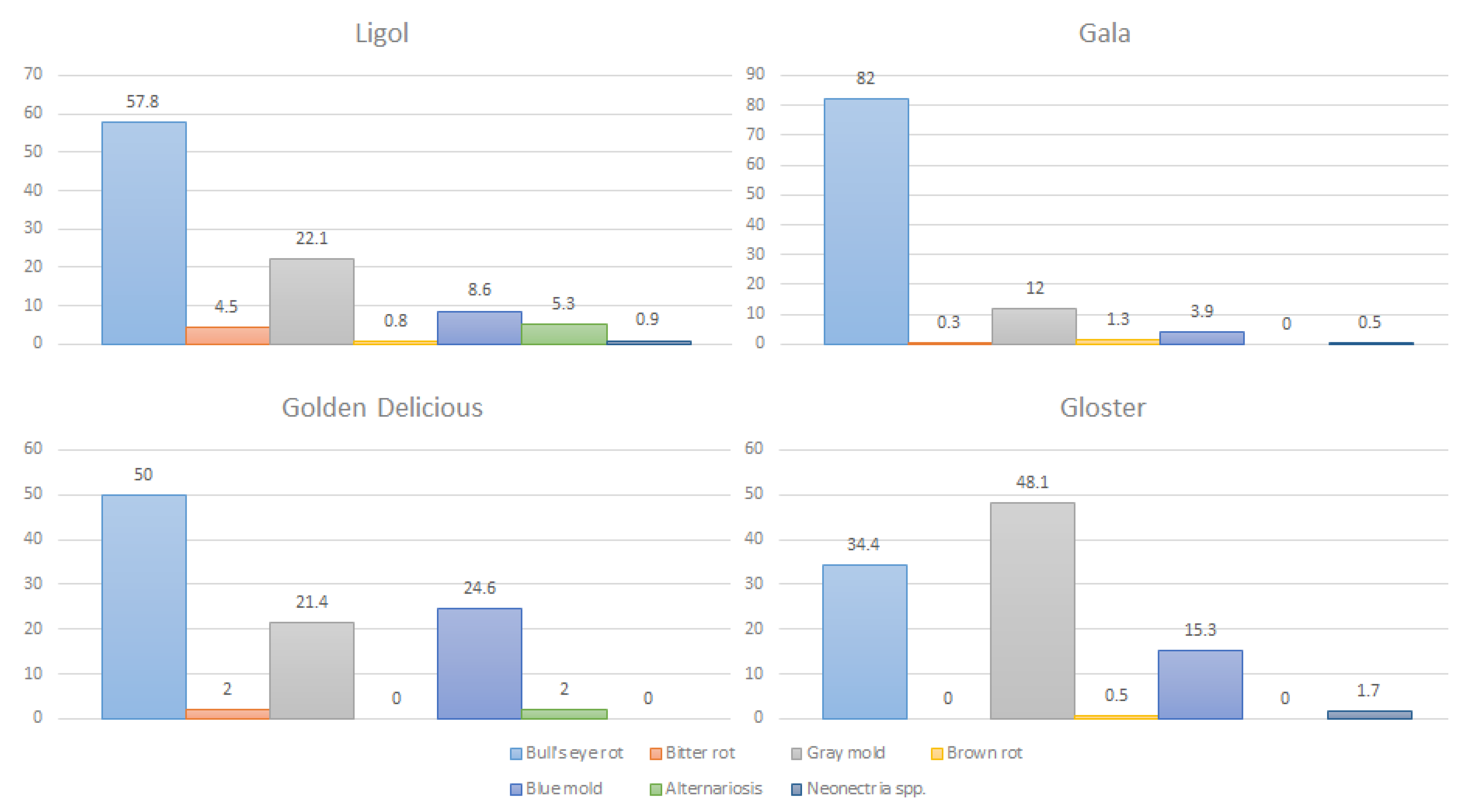
Storage Season Orchard | % of Apples with Disease Syptoms after Storage | |||||||
|---|---|---|---|---|---|---|---|---|
Total | Bull’s Eye Rot Neofabraea spp. | Bitter Rot (anthracnose) Colletotrichum spp. | Gray Mold Botrytis cinerea | Brown Rot Monilinia fructigena | Blue Mold Penicillium expansum | Alternariosis Alternaria spp. | Rot Caused by Neonectria ditissima | |
| “Ligol” | ||||||||
| 2012/2013 Orchard A Orchard B | 5.5 16.9 | 2.7 5.7 | 0 0 | 0.1 6.9 | 0.2 0.2 | 1.8 3.1 | 0.7 1.0 | 0 0 |
| 2013/2014 Orchard A | 41.3 20.6 | 33.3 17.2 | 0.4 0.2 | 5.4 2.3 | 0 0 | 1.0 0.5 | 0.9 0.2 | 0.3 0.2 |
| Orchard B | ||||||||
| 2014/2015 Orchard A Orchard B Orchard D | 22.2 3.0 7.0 | 10.0 0.6 3.4 | 4.2 0.1 0 | 5.0 1.2 2.8 | 0 0.1 0 | 1.7 0.2 0.3 | 1.1 0.6 0 | 0.2 0.2 0.5 |
| 2015/2016 Orchard A Orchard D | 12.8 5.9 | 5.6 2.7 | 0.1 0 | 2.8 2.0 | 0.2 0.1 | 1.9 0.5 | 2.2 0.6 | 0 0 |
| 2016/2017 Orchard A | 9.7 | 2.5 | 1.5 | 3.5 | 0.3 | 1.5 | 0.4 | 0 |
| “Golden Delicious” | ||||||||
| 2012/2013 Orchard A | 4.7 | 1.2 | 0 | 0.1 | 0 | 3.3 | 0.1 | 0 |
| 2013/2014 Orchard A | 13.2 | 10.6 | 0.1 | 1.3 | 0 | 1.1 | 0.1 | 0 |
| 2016/2017 Orchard A | 6.9 | 0.6 | 0.4 | 3.9 | 0 | 1.7 | 0.3 | 0 |
| “Gala” | ||||||||
| 2013/2014 Orchard A Orchard C | 12.8 59.1 | 11.7 55.8 | 0.1 0 | 0.5 2.2 | 0 0.5 | 0.3 0.5 | 0 0 | 0.2 0.1 |
| 2015/2016 Orchard C | 25.3 | 21.8 | 0 | 2.8 | 0.4 | 0.2 | 0 | 0.1 |
| 2016/2017 Orchard C Orchard C | 19.8 13.8 | 13.2 4.7 | 0.3 0 | 5.1 5.1 | 0.4 0.4 | 0.7 3.4 | 0 0 | 0.1 0.2 |
| 2017/2018 Orchard A Orchard B | 42.0 25.2 | 38.8 23.6 | 0 0 | 1.3 0.03 | 0 0 | 1.9 1.6 | 0 0 | 0 0 |
| “Gloster” | ||||||||
| 2012/2013 Orchard A | 8.7 | 0.4 | 0 | 6.8 | 0.1 | 1.4 | 0 | 0 |
| 2013/2014 Orchard A | 9.6 | 5.9 | 0 | 2.0 | 0 | 1.4 | 0 | 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Głos, H.; Bryk, H.; Michalecka, M.; Puławska, J. The Recent Occurrence of Biotic Postharvest Diseases of Apples in Poland. Agronomy 2022, 12, 399. https://doi.org/10.3390/agronomy12020399
Głos H, Bryk H, Michalecka M, Puławska J. The Recent Occurrence of Biotic Postharvest Diseases of Apples in Poland. Agronomy. 2022; 12(2):399. https://doi.org/10.3390/agronomy12020399
Chicago/Turabian StyleGłos, Hubert, Hanna Bryk, Monika Michalecka, and Joanna Puławska. 2022. "The Recent Occurrence of Biotic Postharvest Diseases of Apples in Poland" Agronomy 12, no. 2: 399. https://doi.org/10.3390/agronomy12020399
APA StyleGłos, H., Bryk, H., Michalecka, M., & Puławska, J. (2022). The Recent Occurrence of Biotic Postharvest Diseases of Apples in Poland. Agronomy, 12(2), 399. https://doi.org/10.3390/agronomy12020399