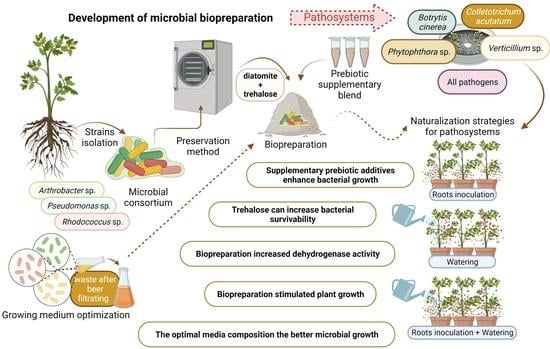
Figure 1.
The effect of different medium pH values—(a) and temperatures—(b) on the growth of bacteria in a liquid culture. Rhodococcus sp. B12/18, Pseudomonas sp. B37/18, Arthrobacter sp. B58/18, Rhodococcus sp. B75/18.
Figure 1.
The effect of different medium pH values—(a) and temperatures—(b) on the growth of bacteria in a liquid culture. Rhodococcus sp. B12/18, Pseudomonas sp. B37/18, Arthrobacter sp. B58/18, Rhodococcus sp. B75/18.
Figure 2.
Effect of the 1% supplementary blend addition (D-malic acid, N-acetyl-D-glucosamine, α-keto-glutaric acid, γ-aminobutyric acid) on the growth of bacteria during 144 h of culturing in the M9 medium. Figures represent OD600nm measured during cultivation every 24 h for particular strains of bacteria accordingly: (a) Rhodococcus sp. B12/18, (b) Pseudomonas sp. B37/18, (c) Arthrobacter sp. B58/18, (d) Rhodococcus sp B75/18; n = 3, error bars represent standard sample deviation. Stars represent the level of significance (p-value) of the differences within the particular hour. Three stars—p-value < 0.001, two stars—p-value < 0.01, one star—p-value < 0.05.
Figure 2.
Effect of the 1% supplementary blend addition (D-malic acid, N-acetyl-D-glucosamine, α-keto-glutaric acid, γ-aminobutyric acid) on the growth of bacteria during 144 h of culturing in the M9 medium. Figures represent OD600nm measured during cultivation every 24 h for particular strains of bacteria accordingly: (a) Rhodococcus sp. B12/18, (b) Pseudomonas sp. B37/18, (c) Arthrobacter sp. B58/18, (d) Rhodococcus sp B75/18; n = 3, error bars represent standard sample deviation. Stars represent the level of significance (p-value) of the differences within the particular hour. Three stars—p-value < 0.001, two stars—p-value < 0.01, one star—p-value < 0.05.
Figure 3.
The early effect of the bacterial consortium on Polana raspberry plant growth in the pot experiment depends on the pathosystem and naturalization strategy applied. The dry biomass of shoots varies, according to pathosystems Botrytis cinerea, Verticillium sp., Colletotrichum acutatum, Phytophthora sp., and all pathogens (Botrytis, Verticillium, Colletotrichum, Phytophthora) used and also without pathogens, within different biopreparation application strategies: no naturalization (NN), root inoculations (R), root inoculations and watering (RW), and watering (W). The error bars indicate a standard deviation, n = 3, different lowercase letters above the bars indicate differences within the particular pathosystems, and different capital letters above the bars indicate differences within the particular naturalization strategies (according to ANOVA with p < 0.05).
Figure 3.
The early effect of the bacterial consortium on Polana raspberry plant growth in the pot experiment depends on the pathosystem and naturalization strategy applied. The dry biomass of shoots varies, according to pathosystems Botrytis cinerea, Verticillium sp., Colletotrichum acutatum, Phytophthora sp., and all pathogens (Botrytis, Verticillium, Colletotrichum, Phytophthora) used and also without pathogens, within different biopreparation application strategies: no naturalization (NN), root inoculations (R), root inoculations and watering (RW), and watering (W). The error bars indicate a standard deviation, n = 3, different lowercase letters above the bars indicate differences within the particular pathosystems, and different capital letters above the bars indicate differences within the particular naturalization strategies (according to ANOVA with p < 0.05).
Figure 4.
The early effect of the bacterial consortium on Polana raspberry plant growth in the pot experiment depended on the pathosystem and naturalization strategy applied. The wet biomass of the roots, according to pathosystems Botrytis cinerea, Verticillium sp., Colletotrichum acutatum, Phytophthora sp., and all pathogens (Botrytis, Verticillium, Colletotrichum, Phytophthora) and without pathogens, within different biopreparation application strategies: no naturalization (NN), root inoculations (R), root inoculations and watering (RW), and watering (W). The error bars indicate a standard deviation, n = 3, different lowercase letters above the bars indicate differences within the particular pathosystem, and different capital letters above the bars indicate differences within the particular naturalization strategies (according to ANOVA with p < 0.05).
Figure 4.
The early effect of the bacterial consortium on Polana raspberry plant growth in the pot experiment depended on the pathosystem and naturalization strategy applied. The wet biomass of the roots, according to pathosystems Botrytis cinerea, Verticillium sp., Colletotrichum acutatum, Phytophthora sp., and all pathogens (Botrytis, Verticillium, Colletotrichum, Phytophthora) and without pathogens, within different biopreparation application strategies: no naturalization (NN), root inoculations (R), root inoculations and watering (RW), and watering (W). The error bars indicate a standard deviation, n = 3, different lowercase letters above the bars indicate differences within the particular pathosystem, and different capital letters above the bars indicate differences within the particular naturalization strategies (according to ANOVA with p < 0.05).
Figure 5.
The effect of the bacterial consortium on dehydrogenase activity in the soil samples depending on the pathosystem and naturalization strategy applied. Dehydrogenase activity varied according to the pathosystems used, Botrytis cinerea, Verticillium sp., Colletotrichum acutatum, Phytophthora sp., and all pathogens (Botrytis, Verticillium, Colletotrichum, Phytophthora) and without pathogens, within different biopreparation application strategies: no naturalization (NN), root inoculations (R), root inoculations and watering (RW), and watering (W). Error bars indicate the standard deviation, n = 3, different lowercase letters above the bars indicate differences within the particular pathosystem, and different capital letters above the bars indicate differences within the particular naturalization strategies (according to ANOVA with p < 0.05).
Figure 5.
The effect of the bacterial consortium on dehydrogenase activity in the soil samples depending on the pathosystem and naturalization strategy applied. Dehydrogenase activity varied according to the pathosystems used, Botrytis cinerea, Verticillium sp., Colletotrichum acutatum, Phytophthora sp., and all pathogens (Botrytis, Verticillium, Colletotrichum, Phytophthora) and without pathogens, within different biopreparation application strategies: no naturalization (NN), root inoculations (R), root inoculations and watering (RW), and watering (W). Error bars indicate the standard deviation, n = 3, different lowercase letters above the bars indicate differences within the particular pathosystem, and different capital letters above the bars indicate differences within the particular naturalization strategies (according to ANOVA with p < 0.05).
Table 1.
The effect of different carbon sources (glucose, lactose, sucrose), their concentrations 3%, 6%, 9% and the addition of peptone on the growth of bacteria in liquid culture. The control was prepared using the M9 minimal medium containing KH2PO4 15 g/L, NaCl, 2.5 g/L, Na2HPO4, 33.9 g/L, NH4Cl, 5 g/L. Rhodococcus sp. B12/18, Pseudomonas sp. B37/18, Arthrobacter sp. B58/18, and Rhodococcus sp. B75/18, pH = 7.2, culturing temperature = 30 °C.
Table 1.
The effect of different carbon sources (glucose, lactose, sucrose), their concentrations 3%, 6%, 9% and the addition of peptone on the growth of bacteria in liquid culture. The control was prepared using the M9 minimal medium containing KH2PO4 15 g/L, NaCl, 2.5 g/L, Na2HPO4, 33.9 g/L, NH4Cl, 5 g/L. Rhodococcus sp. B12/18, Pseudomonas sp. B37/18, Arthrobacter sp. B58/18, and Rhodococcus sp. B75/18, pH = 7.2, culturing temperature = 30 °C.
| Carbon Source | Peptone Concentration | Colony-Forming Units (CFU/mL) |
|---|
| Sugar | Concentration | Additive | Concentration | B12/18 | B37/18 | B58/18 | B75/18 |
|---|
| Glucose | 3% | Peptone | 0% | 2.45 × 1011 | 1.50 × 1012 | 6.15 × 1010 | 5.46 × 1011 |
| 3% | 0.20% | <108 | 1.37 × 1012 | 1.80 × 1011 | 1.50 × 1012 |
| 6% | Peptone | 0% | 1.00 × 109 | 1.50 × 1012 | 3.00 × 109 | 1.25 × 1010 |
| 6% | 0.20% | 6.80 × 109 | 4.45 × 1010 | 5.00 × 108 | 1.66 × 1011 |
| 9% | Peptone | 0% | <108 | <108 | <108 | <108 |
| 9% | 0.20% | <108 | 3.00 × 109 | <108 | <108 |
| Lactose | 3% | Peptone | 0% | 9.10 × 1011 | 7.50 × 1011 | <108 | 7.96 × 1011 |
| 3% | 0.20% | 1.50 × 1012 | 1.50 × 1012 | 1.00 × 1010 | 6.25 × 1010 |
| 6% | Peptone | 0% | 7.61 × 1011 | 7.50 × 1011 | <108 | <108 |
| 6% | 0.20% | 4.00 × 1010 | 5.20 × 1010 | <108 | 3.50 × 109 |
| 9% | Peptone | 0% | <108 | <108 | <108 | <108 |
| 9% | 0.20% | <108 | <108 | <108 | <108 |
| Sucrose | 3% | Peptone | 0% | 1.50 × 1012 | 1.22 × 1012 | 1.50 × 1012 | 1.28 × 1012 |
| 3% | 0.20% | 1.50 × 1012 | 1.50 × 1012 | 1.50 × 1012 | 1.50 × 1012 |
| 6% | Peptone | 0% | 1.50 × 1012 | 1.50 × 1012 | 8.66 × 1011 | 1.50 × 1012 |
| 6% | 0.20% | 8.50 × 109 | 5.20 × 1010 | 7.00 × 109 | 2.03 × 1011 |
| 9% | Peptone | 0% | 2.24 × 1011 | 1.00 × 1010 | 1.50 × 1010 | 3.29 × 1011 |
| 9% | 0.20% | 2.15 × 1011 | 6.20 × 1010 | 1.30 × 1010 | 1.80 × 1011 |
| Control using M9 medium | 1.11 × 1012 | 1.50 × 1012 | 1.42 × 1011 | 1.46 × 1012 |
Table 2.
The effect of different nitrogen sources (NaNO3, NH4NO3, (NH4)2SO4, NH4Cl) and their concentrations of 1%, 3%, 6%, on the growth of bacteria in a liquid culture. Rhodococcus sp. B12/18, Pseudomonas sp. B37/18, Arthrobacter sp. B58/18, and Rhodococcus sp. B75/18, pH = 7.2, culturing temperature = 30 °C.
Table 2.
The effect of different nitrogen sources (NaNO3, NH4NO3, (NH4)2SO4, NH4Cl) and their concentrations of 1%, 3%, 6%, on the growth of bacteria in a liquid culture. Rhodococcus sp. B12/18, Pseudomonas sp. B37/18, Arthrobacter sp. B58/18, and Rhodococcus sp. B75/18, pH = 7.2, culturing temperature = 30 °C.
| Nitrogen Source | Colony-Forming Units (CFU/mL) |
|---|
| Compound | Concentration | B12/18 | B37/18 | B58/18 | B75/18 |
|---|
| NaNO3 | 1% | 2.50 × 109 | <108 | 6.50 × 109 | 4.4 × 1011 |
| 3% | <108 | <108 | <108 | 3.1 × 1011 |
| 6% | <108 | <108 | <108 | <108 |
| NH4NO3 | 1% | <108 | <108 | 1.30 × 1010 | 2.05 × 1010 |
| 3% | <108 | 1.10 × 1011 | 2.00 × 1010 | 2.7 × 1010 |
| 6% | 5.20 × 109 | 2.23 × 1011 | 1.20 × 1010 | 2.6 × 1011 |
| (NH4)2SO4 | 1% | 3.00 × 109 | 1.00 × 109 | <108 | <108 |
| 3% | 1.00 × 109 | 1.50 × 1010 | <108 | <108 |
| 6% | 4.00 × 109 | 6.00 × 109 | 1.40 × 1010 | 3.5 × 1011 |
| NH4Cl | 1% | 4.26 × 108 | 4.50 × 109 | 1.50 × 109 | 6.47 × 1011 |
| 3% | <108 | <108 | 3.00 × 109 | 5.26 × 1011 |
| 6% | 6.00 × 109 | 4.00 × 109 | <108 | 4.67 × 1011 |
Table 3.
The effect of different drying methods and sample preparations on the survivability of bacteria used in naturalization biopreparation. Rhodococcus sp. B12/18, Pseudomonas sp. B37/18, Arthrobacter sp. B58/18, Rhodococcus sp. B75/18, D—drying, VD—vacuum drying, LF—lyophilization.
Table 3.
The effect of different drying methods and sample preparations on the survivability of bacteria used in naturalization biopreparation. Rhodococcus sp. B12/18, Pseudomonas sp. B37/18, Arthrobacter sp. B58/18, Rhodococcus sp. B75/18, D—drying, VD—vacuum drying, LF—lyophilization.
| Drying Technique and Sample Type | Colony-Forming Units (CFU/g) |
|---|
| B12/18 | B37/18 | B58/18 | B75/18 |
|---|
| Control | Fresh culture | 7.50 × 1010 | 6.00 × 108 | 4.10 × 109 | 8.50 × 109 |
| Bacterial pellet | 1.00 × 108 | 2.00 × 107 | 2.00 × 108 | 1.50 × 109 |
| D | Bacterial pellet | <106 | <106 | 1.00 × 106 | 1.00 × 108 |
| Medium with bacteria | <106 | <106 | <106 | 1.00 × 108 |
| Medium with bacteria absorbed on diatomite | 4.00 × 1010 | 1.00 × 106 | 9.90 × 108 | <106 |
| VD | Bacterial pellet | <106 | <106 | 1.00 × 107 | 2.00 × 107 |
| Medium with bacteria | 2.00 × 108 | <106 | <106 | <106 |
| Medium with bacteria absorbed on diatomite | <106 | 5.30 × 109 | 7.00 × 108 | 4.70 × 109 |
| LF | Bacterial pellet | 7.00 × 106 | 1.00 × 107 | 2.00 × 106 | 2.00 × 108 |
| Medium with bacteria | 3.00 × 108 | <106 | 1.00 × 107 | 1.00 × 108 |
| Medium with bacteria absorbed on diatomite | 7.00 × 108 | <106 | 1.00 × 106 | 1.00 × 108 |
Table 4.
The effect of trehalose addition and the chosen drying methods on bacterial survivability in drying (D) and vacuum drying (VD) processes used in manufacturing and in the naturalization biopreparation. Rhodococcus sp. B12/18, Pseudomonas sp. B37/18, Arthrobacter sp. B58/18, Rhodococcus sp. B75/18, D—drying, VD—vacuum drying.
Table 4.
The effect of trehalose addition and the chosen drying methods on bacterial survivability in drying (D) and vacuum drying (VD) processes used in manufacturing and in the naturalization biopreparation. Rhodococcus sp. B12/18, Pseudomonas sp. B37/18, Arthrobacter sp. B58/18, Rhodococcus sp. B75/18, D—drying, VD—vacuum drying.
| Bacterial Isolate | Trehalose Addition | Drying Technique | Colony-Forming Units (CFU/g) |
|---|
| To the Culture | To Drying |
|---|
| B12/18 | 0.1 M | - | D | <107 |
| B58/18 | 0.1 M | - | D | 1.20 × 109 |
| B37/18 | 0.1 M | - | VD | 2.00 × 108 |
| B75/18 | 0.1 M | - | VD | 1.96 × 1010 |
| B12/18 | 0.1 M | 0.1 M | D | <107 |
| B58/18 | 0.1 M | 0.1 M | D | 1.00 × 108 |
| B37/18 | 0.1 M | 0.1 M | VD | 1.00 × 108 |
| B75/18 | 0.1 M | 0.1 M | VD | 5.50 × 109 |
| B12/18 + B58/18 | 0.1 M | - | D | 1.50 × 109 |
| B37/18 + B75/18 | 0.1 M | - | VD | 3.97 × 109 |
| B12/18 + B58/18 | 0.1 M | 0.1 M | D | 1.67 × 108 |
| B37/18 + B75/18 | 0.1 M | 0.1 M | VD | 8.87 × 109 |
Table 5.
The effect of the bacterial consortium on the macronutrient amount in the plant, root and in the soil samples depends on the pathosystem and naturalization strategy applied. The amount of macronutrients varied according to the pathosystem used, Botrytis cinerea, Verticillium sp., Colletotrichum acutatum, Phytophthora sp. and all pathogens (Botrytis, Verticillium, Colletotrichum, Phytophthora) and without pathogens, within different biopreparation application strategies: no naturalization (NN), root inoculations (R), root inoculations and watering (RW), and watering (W).
Table 5.
The effect of the bacterial consortium on the macronutrient amount in the plant, root and in the soil samples depends on the pathosystem and naturalization strategy applied. The amount of macronutrients varied according to the pathosystem used, Botrytis cinerea, Verticillium sp., Colletotrichum acutatum, Phytophthora sp. and all pathogens (Botrytis, Verticillium, Colletotrichum, Phytophthora) and without pathogens, within different biopreparation application strategies: no naturalization (NN), root inoculations (R), root inoculations and watering (RW), and watering (W).
| Pathogen Contamination | Naturalization | Macronutrients in Shoots | Absorbable Forms of Minerals in the Soil | Nitrogen in Soil |
|---|
| N | P | K | Ca | Mg | P2O5 | K2O | Mg | N-NO3 | N-NH4 | Nmin |
|---|
| % d.m. | mg/100 g Soil | mg/kg d.m. | kg/ha |
|---|
| without pathogen | NN | 1.44 | 0.29 | 2.47 | 1.22 | 0.44 | 18.3 | 32.5 | 14.3 | 4.41 | 6.26 | 45.90 |
| R | 1.44 | 0.3 | 2.63 | 1.38 | 0.48 | 17.6 | 31.8 | 12.9 | 4.18 | 6.34 | 45.20 |
| RW | 1.4 | 0.27 | 2.15 | 1.2 | 0.48 | 20.3 | 33.9 | 14.5 | 1.41 | 7.87 | 39.90 |
| W | 1.72 | 0.29 | 2.46 | 1.12 | 0.48 | 20.5 | 34.3 | 15 | 3.33 | 7.10 | 44.80 |
| Botrytis cinerea | NN | 1.41 | 0.28 | 2.12 | 1.13 | 0.46 | 19.6 | 35.6 | 12.6 | 3.65 | 7.71 | 48.80 |
| R | 1.52 | 0.29 | 2.65 | 1.28 | 0.48 | 20.2 | 35.3 | 12.7 | 10.7 | 3.35 | 60.40 |
| RW | 1.48 | 0.3 | 2.29 | 1.21 | 0.44 | 21.1 | 36.1 | 13.9 | 3.11 | 7.40 | 45.20 |
| W | 1.34 | 0.29 | 2.21 | 1.22 | 0.42 | 20.5 | 35.4 | 14.9 | 2.83 | 6.61 | 40.60 |
| Verticilium sp. | NN | 1.51 | 0.29 | 2.04 | 1.2 | 0.42 | 16.1 | 31.8 | 14 | 2.23 | 9.37 | 49.90 |
| R | 1.66 | 0.3 | 2.31 | 1.16 | 0.4 | 15.5 | 29.8 | 13.5 | 2.75 | 8.02 | 46.30 |
| RW | 1.65 | 0.29 | 2.35 | 1.15 | 0.32 | 16.1 | 31.7 | 14.6 | 2.45 | 8.94 | 49.00 |
| W | 1.34 | 0.27 | 2.05 | 1.33 | 0.4 | 18.1 | 33.9 | 14.8 | 1.34 | 8.74 | 37.60 |
| Colletotrichum acutatum | NN | 1.18 | 0.26 | 2.01 | 1.33 | 0.38 | 17.5 | 31.8 | 12.8 | 2.78 | 7.45 | 44.00 |
| R | 1.6 | 0.27 | 2.03 | 1.43 | 0.44 | 18 | 33.1 | 12 | 1.36 | 8.06 | 40.50 |
| RW | 1.62 | 0.29 | 2.17 | 1.34 | 0.4 | 18 | 33.6 | 13.7 | 9.36 | 3.72 | 56.20 |
| W | 1.67 | 0.29 | 2.63 | 1.36 | 0.42 | 18.4 | 33.2 | 13.4 | 12.1 | 1.96 | 60.70 |
| Phytophthora sp. | NN | 1.6 | 0.28 | 2.56 | 1.36 | 0.4 | 18.5 | 32.3 | 14.4 | 4.40 | 6.61 | 47.30 |
| R | 1.47 | 0.24 | 2.14 | 1.3 | 0.38 | 19.7 | 36.7 | 15 | 4.33 | 5.36 | 41.70 |
| RW | 1.16 | 0.24 | 2.04 | 1.17 | 0.32 | 15.5 | 32.9 | 13.9 | 2.84 | 7.85 | 46.00 |
| W | 1.61 | 0.28 | 2.81 | 1.39 | 0.42 | 17.4 | 29.2 | 13.1 | 3.38 | 9.83 | 56.80 |
| all pathogens | NN | 1.54 | 0.27 | 2.23 | 1.24 | 0.39 | 16.7 | 30.4 | 12.9 | 4.32 | 9.15 | 57.90 |
| R | 1.57 | 0.27 | 2.21 | 1.22 | 0.38 | 14.9 | 28.8 | 13.1 | 4.11 | 9.55 | 58.70 |
| RW | 1.44 | 0.3 | 2.19 | 1.33 | 0.44 | 16.3 | 32.4 | 13.4 | 4.59 | 7.8 | 53.30 |
| W | 1.34 | 0.3 | 2.26 | 1.2 | 0.38 | 18.4 | 34.7 | 13.9 | 2.65 | 9.39 | 51.80 |