The Cumulative Effect of Various Tillage Systems and Stubble Management on the Biological and Chemical Properties of Soil in Winter Wheat Monoculture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Location and Layout
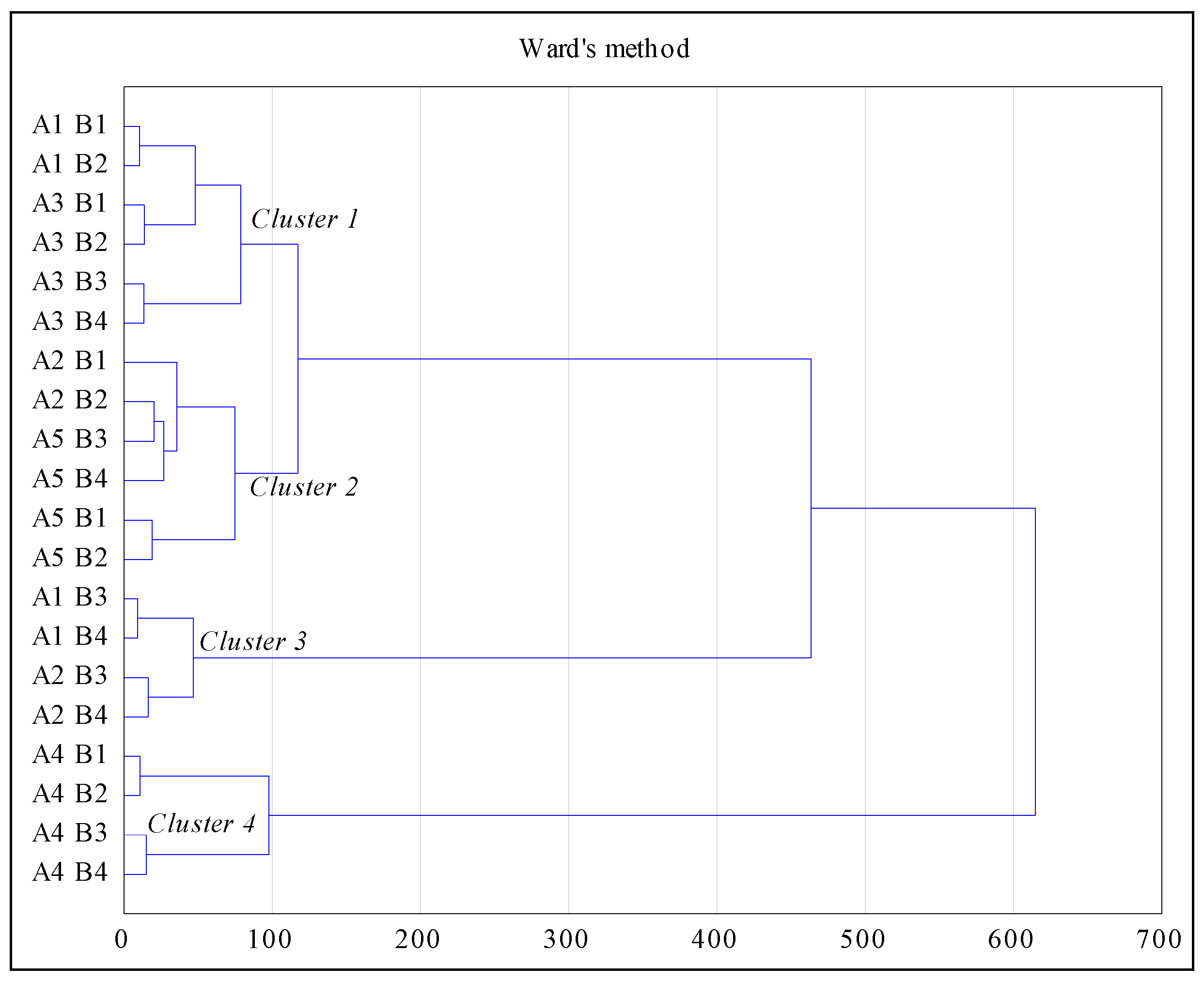
- A—tillage system (five variants):
- A1—post-harvest: Grubber with a roll; pre-sowing: grubber + seeder-cultivator unit
- A2—post-harvest: grubber with a roll; sow ploughing + seeder-cultivator unit
- A3—single ploughing + seeder-cultivator unit
- A4—post-harvest: manure + grubber with a roll; pre-sowing: sow ploughing + seeder-cultivator unit
- A5—direct sowing
- B—method of managing the post-harvest residues:
- B1—leaving shredded straw
- B2—leaving shredded straw + EM
- B3—removing straw + EM
- B4—removing straw
2.2. Soil Samples
2.3. Soil Microbiological Parameters
2.4. Soil Respiration Measurement
2.5. Soil Chemical Properties
2.6. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO Cereal Supply and Demand Brief. 2021. Available online: http://www.fao.org/worldfoodsituation/csdb/en/ (accessed on 23 June 2021).
- Bastos, L.M.; Carciochi, W.; Lollato, R.P.; Jaenisch, B.R.; Rezende, C.R.; Schwalbert, R.; Vara Prasad, P.V.; Zhang, G.; Fritz, A.K.; Foster, C.; et al. Winter Wheat Yield Response to Plant Density as a Function of Yield Environment and Tillering Potential: A Review and Field Studies. Front. Plant Sci. 2020, 11, 54. [Google Scholar] [CrossRef] [Green Version]
- Rodgers, H.R.; Norton, J.B.; van Diepen, L.T.A. Effects of Semiarid Wheat Agriculture Management Practices on Soil Microbial Properties: A Review. Agronomy 2021, 11, 852. [Google Scholar] [CrossRef]
- Willis, S.A.; Williams, C.; Duniway, M.; Veenstra, J.; Seybold, C.; Presley, D. Human Land-Use and Soil Change. In The Soils of the USA; West, L.T., Singer, M.J., Hartemink, A.E., Eds.; Springer: Cham, Switzerland, 2017; pp. 351–371. [Google Scholar]
- Pagliai, M.; Vignozzi, N.; Pellegrini, S. Soil structure and the effect of management practices. Soil Tillage Res. 2004, 79, 131–143. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Amundson, R. Managing for soil carbon sequestration: Let’s get realistic. Glob. Chang. Biol. 2019, 25, 386–389. [Google Scholar] [CrossRef] [Green Version]
- Jaskulska, I.; Romaneckas, K.; Jaskulski, D.; Gałęzewski, L.; Breza-Boruta, B.; Dębska, B.; Lemanowicz, J. Soil Properties after Eight Years of the Use of Strip-Till One-Pass Technology. Agronomy 2020, 10, 1596. [Google Scholar] [CrossRef]
- Panasiewicz, K.; Faligowska, A.; Szymańska, G.; Szukała, J.; Ratajczak, K.; Sulewska, H. The Effect of Various Tillage Systems on Productivity of Narrow-Leaved Lupin-Winter Wheat-Winter Triticale-Winter Barley Rotation. Agronomy 2020, 10, 304. [Google Scholar] [CrossRef] [Green Version]
- Stockfisch, N.; Forstreuter, T.; Ehlers, W. Ploughing effects on soil organic matter after twenty years of conservation tillage in Lower Saxony, Germany. Soil Tillage Res. 1999, 52, 91–101. [Google Scholar] [CrossRef]
- Voltr, V.; Menšík, L.; Hlisnikovský, L.; Hruška, M.; Pokorný, E.; Pospíšilová, L. The Soil Organic Matter in Connection with Soil Properties and Soil Inputs. Agronomy 2021, 11, 779. [Google Scholar] [CrossRef]
- Murphy, B.W. Impact of soil organic matter on soil properties—A review with emphasis on Australian soils. Soil Res. 2015, 53, 605–635. [Google Scholar] [CrossRef]
- Coonan, E.C.; Richardson, A.E.; Kirkby, C.A.; Kirkegaard, J.A.; Amidy, M.R.; Strong, C.L. Soil fertility and nutrients mediate soil carbon dynamics following residue incorporation. Nutr. Cycl. Agroecosyst. 2020, 116, 205–221. [Google Scholar] [CrossRef]
- Maillard, F.; Leduc, V.; Bach, C.; Reichard, A.; Fauchery, L.; Saint-Andre, L.; Zeller, B.; Buee, M. Soil microbial functions are affected by organic matter removal in temperate deciduous forest. Soil Biol. Biochem. 2019, 133, 28–36. [Google Scholar] [CrossRef]
- Liu, C.; Lu, M.; Cui, J.; Li, B.; Fang, C. Effects of straw carbon input on carbon dynamics in agricultural soils: A meta-analysis. Glob. Chang. Biol. 2014, 20, 1366–1381. [Google Scholar] [CrossRef]
- Jezierska-Tys, S.; Wesołowska, S.; Gałązka, A.; Joniec, J.; Bednarz, J.; Cierpała, R. Biological activity and functional diversity in soil in different cultivation systems. Int. J. Environ. Sci. Technol. 2020, 17, 4189–4204. [Google Scholar] [CrossRef]
- Janušauskaite, D.; Kadžienė, G.; Auškalnienė, O. The Effect of Tillage System on Soil Microbiota in Relation to Soil Structure. Pol. J. Environ. Stud. 2013, 22, 1387–1391. [Google Scholar]
- Iriti, M.; Scarafoni, A.; Pierce, S.; Castorina, G.; Vitalini, S. Soil Application of Effective Microorganisms (EM) Maintains Leaf Photosynthetic Efficiency, Increases Seed Yield and Quality Traits of Bean (Phaseolus vulgaris L.) Plants Grown on Different Substrates. Int. J. Mol. Sci. 2019, 20, 2327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierce, S.; Quaglino, F.; Montagna, M.; Spada, A.; Casati, P.; Iriti, M. Evaluation of effective microorganisms® efficacy on ‘Candidatus Phytoplasma solani’-infected and healthy periwinkle plants. Mitt. Klosterneubg. Rebe Wein Obstbau Früchteverwert. 2016, 66, 89–92. [Google Scholar]
- Hu, C.; Qi, Y. Long-term effective microorganisms application promote growth and increase yields and nutrition of wheat in China. Eur. J. Agron. 2013, 46, 63–67. [Google Scholar] [CrossRef]
- Kotwica, K.; Jaskulska, I.; Gałęzewski, L.; Jaskulski, D.; Lamparski, R. The effect of tillage and management of post-harvest residues and biostymulant application on the yield of winter wheat in increasing monoculture. Acta Sci. Pol. Agric. 2014, 13, 65–76. [Google Scholar]
- Atlas, R.M. Handbook of Microbiological Media, 4th ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2010. [Google Scholar]
- Crawford, D.L.; Lynch, J.M.; Whipps, J.M.; Ousley, M.A. Isolation and characterization of actinomycetes antagonists of a fungal root pathogen. Appl. Environ. Microbiol. 1996, 59, 3899–3905. [Google Scholar] [CrossRef] [Green Version]
- Myśków, W.; Stachyra, A.; Zięba, S.; Masiak, D. Biological activity of soil as an index of its fertility. Soil Sci. Annu. 1996, 47, 89–99. [Google Scholar]
- PN-ISO 10390. Chemical and Agricultural Analysis—Determining Soil pH; Polish Standards Committee: Warszawa, Poland, 1997.
- PN-R-04023. Chemical and Agricultural Analysis—Determination of the Content of Available Phosphorus in Mineral Soils; Polish Standards Committee: Warszawa, Poland, 1996.
- PN-R-04022. Chemical and Agricultural Analysis—Determination of the Content Available Potassium in Mineral Soils; Polish Standards Committee: Warszawa, Poland, 1996.
- PN-R-04020. Chemical and Agricultural Analysis. Determination of the Content Available Magnesium; Polish Standards Committee: Warszawa, Poland, 1994.
- Statistica, Data Analysis Software System, Version 12; TIBCO Software Inc.: Palo Alto, CA, USA, 2019; Available online: https://www.tibco.com/products/data-science (accessed on 12 June 2021).
- Fuentes Llanillo, R.; Telles, T.; Soares Júnior, D.; Melo, T.; Friedrich, T.; Kassam, A. Expansion of no-tillage practice in conservation agriculture in Brazil. Soil Tillage Res. 2021, 208, 104877. [Google Scholar] [CrossRef]
- Lorite, I.J.; Ruiz-Ramos, M.; Gabaldon-Leal, C.; Cruz-Blanco, M.; Porras, R.; Santos, C. Water Scarcity and Sustainable Agriculture in Semiarid Environment: Tools, Strategies, and Challenges for Woody Crops. In Water Management and Climate Change in Semiarid Environments; Tejero, I.F.G., Zuazo, V.H.D., Eds.; Academic Press: London, UK, 2018; pp. 3–40. [Google Scholar]
- Pasricha, N.S. Conservation Agriculture Effects on Dynamics of Soil C and N under Climate Change Scenario. Adv. Agron. 2017, 145, 269–312. [Google Scholar]
- Rao, A.N.; Brainard, D.C.; Kumar, V.; Ladha, J.K.; Johnson, D.E. Preventive Weed Management in Direct-Seeded Rice: Targeting the Weed Seedbank. Adv. Agron. 2017, 144, 45–142. [Google Scholar]
- Jat, M.L.; Dagar, J.C.; Sapkota, T.B.; Govaerts, B.; Ridaura, S.L.; Saharawat, Y.S.; Sharma, R.K.; Tetarwal, J.P.; Jat, R.K.; Hobbs, H.; et al. Climate Change and Agriculture: Adaptation Strategies and Mitigation Opportunities for Food Security in South Asia and Latin America. Adv. Agron. 2016, 137, 127–235. [Google Scholar]
- Schjønning, P.; Jensen, J.L.; Bruun, S.; Jensen, L.S.; Christensen, B.T.; Munkholm, L.J.; Oelofse, M.; Baby, S.; Knudsen, L. The Role of Soil Organic Matter for Maintaining Crop Yields: Evidence for a Renewed Conceptual Basis. Adv. Agron. 2018, 150, 35–79. [Google Scholar]
- Gan, Y.; Siddique, K.H.M.; Turner, N.C.; Li, X.-G.; Niu, J.-Y.; Yang, C.; Liu, L.; Chai, Q. Ridge-Furrow Mulching Systems-An Innovative Technique for Boosting Crop Productivity in Semiarid Rain-Fed Environments. Adv. Agron. 2013, 118, 429–476. [Google Scholar]
- Vaneeckhaute, C.; Ghekiere, G.; Michels, E.; Vanrolleghem, P.A.; Tack, F.; Meers, E. Assessing nutrient use efficiency and environmental pressure of macronutrients in biobased mineral fertilizers: A review of recent advances and best practices at field scale. Adv. Agron. 2014, 128, 137–180. [Google Scholar]
- Özpinar, S.; Çay, A. Effects of minimum and conventional tillage systems on soil properties and yield of winter wheat (Triticum aestivum L.) in clay-loam in Çanakkale region. Turk. J. Agric. For. 2005, 29, 9–18. [Google Scholar]
- Krauss, M.; Berner, A.; Perrochet, F.; Frei, R.; Niggli, U.; Mäder, P. Enhanced soil quality with reduced tillage and solid manures in organic farming—A synthesis of 15 years. Sci. Rep. 2020, 10, 4403. [Google Scholar] [CrossRef] [Green Version]
- Koch, H.-J.; Stockfisch, N. Loss of soil organic matter upon ploughing under a loess soil after several years of conservation tillage. Soil Tillage Res. 2006, 86, 73–83. [Google Scholar] [CrossRef]
- Duan, Y.; Chen, L.; Zhang, J.; Li, D.; Han, X.; Zhu, B.; Li, J.; Zhao, B.; Huang, P. Long-term fertilisation reveals close associations between soil organic carbon composition and microbial traits at aggregate scales. Agric. Ecosyst. Environ. 2021, 306, 107169. [Google Scholar] [CrossRef]
- Coonan, E.C.; Kirkby, C.A.; Kirkegaard, J.A.; Amidy, M.; Strong, C.; Richardson, A. Microorganisms and nutrient stoichiometry as mediators of soil organic matter dynamics. Nutr. Cycl. Agroecosys. 2020, 117, 273–298. [Google Scholar] [CrossRef]
- Qin, J.; Liu, H.; Zhao, J.; Wang, H.; Zhang, H.; Yang, D.; Zhang, N. The Roles of Bacteria in Soil Organic Carbon Accumulation under Nitrogen Deposition in Stipa baicalensis Steppe. Microorganisms 2020, 8, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kallenbach, C.M.; Serita, D.; Frey, S.D.; Grandy, A.S. Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls. Nat. Commun. 2016, 7, 13630. [Google Scholar] [CrossRef]
- Chinta, Y.D.; Uchida, Y.; Araki, H. Roles of soil bacteria and fungi in controlling the availability of nitrogen from cover crop residues during the microbial hot moments. Appl. Soil Ecol. 2021, 168, 104135. [Google Scholar] [CrossRef]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The Role of Soil Microorganisms in Plant Mineral Nutrition-Current Knowledge and Future Directions. Front. Plant Sci. 2017, 8, 1617. [Google Scholar] [CrossRef] [Green Version]
- Grafe, M.; Goers, M.; von Tucher, S.; Baum, C.; Zimmer, D.; Leinweber, P.; Vestergaard, G.; Kublik, S.; Schloter, M.; Schulz, S. Bacterial potentials for uptake, solubilization and mineralization of extracellular phosphorus in agricultural soils are highly stable under different fertilization regimes. Environ. Microbiol. Rep. 2018, 10, 320–327. [Google Scholar] [CrossRef]
- Zhen, Z.; Liu, H.; Wang, N.; Guo, L.; Meng, J.; Ding, N.; Wu, G.; Jiang, G. Effects of Manure Compost Application on Soil Microbial Community Diversity and Soil Microenvironments in a Temperate Cropland in China. PLoS ONE 2014, 9, e108555. [Google Scholar] [CrossRef] [PubMed]
- Rousk, J.; Bååth, E. Fungal and bacterial growth in soil with plant materials of different C/N ratios. FEMS Microbiol Ecol. 2007, 62, 258–267. [Google Scholar] [CrossRef] [Green Version]
- Han, S.H.; An, J.Y.; Hwang, J.; Kim, S.B.; Park, B.B. The effects of organic manure and chemical fertilizer on the growth and nutrient concentrations of yellow poplar (Liriodendron tulipifera Lin.) in a nursery system. Forest Sci. Technol. 2016, 12, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Kaur, K.; Kapoor, K.K.; Gupta, A.P. Impact of organic manure with and without mineral fertilizers on soil chemical biological properties under tropical conditions. J. Plant. Nutr. Soil Sci. 2005, 168, 117–122. [Google Scholar] [CrossRef]
- Akhtar, K.; Wang, W.; Ren, G.; Khan, A.; Feng, Y.; Yang, G. Changes in soil enzymes, soil properties, and maize crop productivity under wheat straw mulching in Guanzhong, China. Soil Tillage Res. 2018, 182, 94–102. [Google Scholar] [CrossRef]
- Pérez-Valera, E.; Kyselková, M.; Ahmed, E.; Sladecek, F.; Goberna, M.; Elhottová, D. Native soil microorganisms hinder the soil enrichment with antibiotic resistance genes following manure applications. Sci. Rep. 2019, 9, 6760. [Google Scholar] [CrossRef] [Green Version]
- Jamieson, R.; Gordon, R.; Sharples, K.E.; Madani, G. Movement and persistence of fecal bacteria in agricultural soils and subsurface drainage water: A review. Can. Biosyst. Eng. 2002, 44, 1.1–1.9. [Google Scholar]
- Guber, A.K.; Shelton, D.R.; Pachepsky, Y.A. Transport and Retention of Manure-Borne Coliforms in Soil. Vadose Zone J. 2005, 4, 828–837. [Google Scholar] [CrossRef] [Green Version]
- Thomsen, I.K.; Christensen, B.T. Yields of wheat and soil carbon and nitrogen contents following long-term incorporation of barley straw and ryegrass catch crops. Soil Use Manag. 2004, 20, 432–438. [Google Scholar] [CrossRef]
- Mühlbachová, G.; Růžek, P.; Kusá, H.; Vavera, R.; Káš, M. Winter Wheat Straw Decomposition under Different Nitrogen Fertilizers. Agriculture 2021, 11, 83. [Google Scholar] [CrossRef]
- Bradáčová, K.; Florea, A.S.; Bar-Tal, A.; Minz, D.; Yermiyahu, U.; Shawahna, R.; Kraut-Cohen, J.; Zolti, A.; Erel, R.; Dietel, K.; et al. Microbial Consortia versus Single-Strain Inoculants: An Advantage in PGPM-Assisted Tomato Production? Agronomy 2019, 9, 105. [Google Scholar] [CrossRef] [Green Version]
- Javaid, A. Effects of biofertilizers combined with different soil amendments on potted rice plants. Chil. J. Agric. Res. 2011, 71, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Daiss, N.; Lobo, M.G.; Socorro, A.R.; Brückner, U.; Heller, J.; Gonzalez, M. The effect of three organic pre-harvest treatments on Swiss chard (Beta vulgaris L. var cycla L.) quality. Eur. Food Res. Technol. 2008, 226, 345–353. [Google Scholar] [CrossRef]
- Szymanek, M.; Dziwulska-Hunek, A.; Zarajczyk, J.; Michałek, S.; Tanaś, W. The Influence of Red Light (RL) and Effective Microorganism (EM) Application on Soil Properties, Yield, and Quality in Wheat Cultivation. Agronomy 2020, 10, 1201. [Google Scholar] [CrossRef]

| Levels of Soil Management (A) | Levels of Organic Input (B) | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Mean | |
| bacteria (106 cfu g−1) | |||||
| 1 | 51.3 bc | 53.3 c | 49.3 bc | 48.7 b | 50.7 c |
| 2 | 46.7 c | 48.7 cd | 43.3 bc | 41.7 b | 45.1 cd |
| 3 | 44.0 c | 39.7 d | 40.3 c | 41.7 b | 41.4 d |
| 4 | 115.0 a | 118.3 a | 100.0 a | 104.0 a | 109.3 a |
| 5 | 61.3 b | 72.7 b | 53.3 b | 46.7 b | 58.5 b |
| Mean | 63.7 AB | 66.5 A | 57.2 B | 56.6 B | |
| LSD for: Factor A = 6.1; Factor B = 8.5; Interaction A/B = 11.6; B/A = 12.7 | |||||
| Actinomycetes (105 cfu g−1) | |||||
| 1 | 28.7 b | 36.0 b | 24.0 b | 22.7 b | 27.8 bc |
| 2 | 26.0 b | 27.3 c | 29.0 b | 24.3 b | 26.7 c |
| 3 | 26.7 b | 36.0 b | 22.0 b | 21.7 b | 26.6 c |
| 4 | 47.3 a | 44.7 a | 46.7 a | 44.0 a | 45.7 a |
| 5 | 32.3 b | 32.3 bc | 28.3 b | 21.0 b | 31.1 b |
| Mean | 32.2 AB | 35.3 A | 30.0 BC | 26.7 C | |
| LSD for: Factor A = 4.2; Factor B = 4.1; Interaction A/B = 7.6; B/A = 7.4 | |||||
| Fungi (104 cfu g−1) | |||||
| 1 | 26.0 b | 28.7 b | 26.7 b | 25.3 b | 26.7 c |
| 2 | 19.3 c | 24.0 c | 18.0 c | 15.3 d | 19.2 d |
| 3 | 20.0 c | 23.3 c | 18.0 c | 14.7 d | 19.0 d |
| 4 | 33.3 a | 31.3 b | 31.7 a | 32.7 a | 32.3 a |
| 5 | 34.3 a | 36.0 a | 28.7 ab | 20.0 c | 29.7 b |
| Mean | 26.6 AB | 28.7 A | 24.6 BC | 21.6 C | |
| LSD for: Factor A = 1.9, Factor B = 3.3; Interaction A/B = 4.3; B/A = 4.9 | |||||
| Levels of Soil Management (A) | Levels of Organic Input (B) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Mean | |||||
| Count | Wk | Count | Wk | Count | Wk | Count | Wk | ||
| 1 | 54.5 bc | 1.23 | 57.2 c | 1.29 | 52.0 bc | 1.18 | 51.2 b | 1.16 | 53.7 c |
| 2 | 49.5 c | 1.12 | 51.6 cd | 1.17 | 46.4 bc | 1.05 | 44.3 b | 1.00 | 47.9 cd |
| 3 | 46.9 c | 1.06 | 43.5 d | 0.98 | 42.7 c | 0.97 | 44.0 b | 0.99 | 44.3 d |
| 4 | 120.1 a | 2.71 | 123.1 a | 2.78 | 105.0 a | 2.37 | 108.8 a | 2.46 | 114.2 a |
| 5 | 64.9 b | 1.47 | 76.3 b | 1.72 | 56.5 b | 1.28 | 49.0 b | 1.11 | 61.6 b |
| Mean | 67.2 AB | 70.3 A | 60.5 B | 59.4 B | |||||
| LSD for: Factor A = 6.1; Factor B = 8.4; Interaction A/B = 11.7; B/A = 12.7 | |||||||||
| Levels of Soil Management (A) | Levels of Organic Input (B) | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Mean | |
| 1 | 176.9 c | 151.1 b | 168.4 b | 177.4 d | 168.5 b |
| 2 | 240.3 a | 175.1 a | 236.7 a | 270.9 b | 230.7 a |
| 3 | 218.7 b | 181.2 a | 248.9 a | 312.6 a | 240.3 a |
| 4 | 135.2 d | 140.9 b | 145.9 c | 148.3 e | 142.6 c |
| 5 | 135.9 d | 120.4 c | 152.8 c | 217.5 c | 156.7 b |
| Mean | 181.4 BC | 153.7 C | 190.5 AB | 225.3 A | |
| LSD for: Factor A = 12.75. Factor B = 35.61; Interaction A/B = 32.131; B/A = 45.368 | |||||
| Levels of Soil Management (A) | Levels of Organic Input (B) | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Mean | |
| 1 | 0.364 ab | 0.395 ab | 0.204 b | 0.179 b | 0.285 b |
| 2 | 0.332 b | 0.374 bc | 0.196 b | 0.164 b | 0.266 b |
| 3 | 0.278 c | 0.324 c | 0.127 c | 0.124 b | 0.213 c |
| 4 | 0.406 a | 0.444 a | 0.351 a | 0.361 a | 0.391 a |
| 5 | 0.357 ab | 0.396 ab | 0.175 bc | 0.160 b | 0.272 b |
| Mean | 0.347 A | 0.387 A | 0.211 B | 0.198 B | |
| LSD for: Factor A = 0.045, Factor B = 0.081; Interaction A/B = 0.053; B/A = 0.079 | |||||
| Level of Soil Management (A) | Level of Organic Input (B) | Mean | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||||||
| Content | Wz | Content | Wz | Content | Wz | Content | Wz | ||
| pH | |||||||||
| 1 | 7.0 | 0.91 | 7.5 | 0.97 | 7.2 | 0.98 | 7.3 | 0.96 | 7.3 |
| 2 | 7.4 | 0.99 | 7.3 | 1.00 | 7.5 | 1.03 | 7.4 | 1.00 | 7.4 |
| 3 | 7.1 | 0.93 | 7.2 | 0.96 | 7.4 | 0.99 | 7.5 | 0.96 | 7.3 |
| 4 | 7.5 | 0.97 | 7.3 | 0.99 | 7.5 | 0.99 | 7.5 | 0.99 | 7.5 |
| 5 | 6.9 | 0.88 | 7.2 | 0.95 | 7.1 | 0.96 | 7.3 | 0.94 | 7.1 |
| Mean | 7.2 | 7.3 | 7.3 | 7.4 | |||||
| Organic Carbon—OC (g C kg−1 d.m. soil) | |||||||||
| 1 | 20.0 | 1.01 | 16.3 | 1.03 | 21.7 | 1.00 | 29.9 | 1.01 | 21.9 |
| 2 | 35.1 | 1.02 | 27.8 | 1.02 | 40.4 | 0.99 | 25.9 | 0.98 | 32.3 |
| 3 | 38.9 | 1.02 | 36.9 | 1.03 | 30.8 | 0.99 | 36.5 | 0.98 | 35.8 |
| 4 | 29.5 | 1.06 | 27.6 | 1.05 | 32.4 | 1.02 | 23.8 | 1.02 | 23.3 |
| 5 | 40.4 | 1.03 | 39.4 | 1.03 | 22.1 | 1.01 | 34.5 | 1.01 | 34.1 |
| Mean | 32.9 | 29.6 | 29.5 | 30.1 | |||||
| Total Nitrogen—TN (g N kg−1 d.m. soil) | |||||||||
| 1 | 1.59 | 1.05 | 1.35 | 1.04 | 1.53 | 1.03 | 2.12 | 1.01 | 1.65 |
| 2 | 2.26 | 0.94 | 2.04 | 1.03 | 2.60 | 0.97 | 2.07 | 1.04 | 2.24 |
| 3 | 2.56 | 1.04 | 2.46 | 1.04 | 2.25 | 0.93 | 2.39 | 0.98 | 2.42 |
| 4 | 1.54 | 0.86 | 1.38 | 0.93 | 2.29 | 0.89 | 1.74 | 1.10 | 1.73 |
| 5 | 2.67 | 0.99 | 2.71 | 1.10 | 1.86 | 1.22 | 2.37 | 1.04 | 2.40 |
| Mean | 2.12 | 1.99 | 2.11 | 2.14 | |||||
| Phosphorus—Pa (mg P kg−1 d.m. soil) | |||||||||
| 1 | 172.7 | 0.99 | 173.5 | 1.00 | 82.8 | 1.01 | 79.8 | 0.97 | 127.2 |
| 2 | 217.1 | 0.98 | 218.4 | 0.98 | 99.4 | 0.97 | 99.4 | 0.95 | 158.6 |
| 3 | 176.1 | 0.95 | 182.3 | 0.98 | 173.5 | 0.96 | 173.9 | 0.95 | 176.5 |
| 4 | 248.5 | 1.41 | 239.8 | 1.36 | 233.3 | 1.22 | 239.8 | 1.25 | 240.3 |
| 5 | 194.0 | 1.01 | 196.2 | 1.02 | 202.7 | 0.99 | 198.4 | 0.97 | 197.8 |
| 201.7 | 202.0 | 158.3 | 158.3 | ||||||
| Potassium—Ka (mg K kg−1 d.m. soil) | |||||||||
| 1 | 220.8 | 1.18 | 223.4 | 1.16 | 224.8 | 1.06 | 225.8 | 1.07 | 223.7 |
| 2 | 236.6 | 1.14 | 214.9 | 1.13 | 218.3 | 1.02 | 220.6 | 1.06 | 222.6 |
| 3 | 218.9 | 1.16 | 218.5 | 1.17 | 184.3 | 1.04 | 188.3 | 1.05 | 202.5 |
| 4 | 307.8 | 1.49 | 306.2 | 1.49 | 260.8 | 1.33 | 261.3 | 1.33 | 284.0 |
| 5 | 233.8 | 1.17 | 232.5 | 1.17 | 212.1 | 1.04 | 211.4 | 1.06 | 222.5 |
| Mean | 243.6 | 239.1 | 220.1 | 221.5 | |||||
| Magnesium—Mg (mg Mg kg−1 d.m. soil) | |||||||||
| 1 | 28.3 | 0.84 | 28.1 | 0.83 | 55.3 | 0.91 | 55.3 | 0.92 | 41.7 |
| 2 | 27.5 | 0.86 | 27.1 | 0.85 | 40.7 | 0.89 | 41.3 | 0.89 | 34.2 |
| 3 | 37.9 | 0.90 | 37.9 | 0.90 | 26.4 | 0.87 | 37.1 | 0.83 | 34.8 |
| 4 | 57.0 | 1.03 | 58.1 | 1.05 | 42.3 | 1.03 | 38.4 | 1.01 | 48.9 |
| 5 | 39.4 | 0.88 | 31.2 | 0.86 | 21.6 | 0.87 | 34.6 | 0.87 | 31.7 |
| Mean | 38.0 | 36.5 | 37.3 | 41.3 | |||||
| Levels of Soil Management (A) | Levels of Organic Input (B) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Mean | 1 | 2 | 3 | 4 | Mean | |
| Autumn | Spring | |||||||||
| N-NH4 [mg kg−1] | ||||||||||
| 1 | 15.4 | 12.7 | 17.6 | 18.8 | 16.1 | 12.9 | 16.9 | 15.0 | 11.1 | 13.9 |
| 2 | 18.6 | 11.1 | 19.1 | 19.3 | 17.0 | 19.1 | 19.2 | 19.3 | 10.2 | 16.9 |
| 3 | 19.6 | 18.0 | 23.2 | 24.1 | 21.2 | 21.4 | 25.5 | 18.2 | 17.0 | 20.5 |
| 4 | 24.6 | 17.9 | 25.5 | 26.8 | 23.7 | 24.1 | 25.4 | 22.6 | 16.5 | 22.1 |
| 5 | 11.3 | 10.7 | 17.0 | 11.8 | 12.7 | 11.9 | 11.7 | 12.3 | 10.8 | 11.7 |
| Mean | 17.9 | 14.1 | 20.5 | 20.2 | 17.9 | 19.7 | 17.5 | 13.1 | ||
| N-NO3 [mg kg−1] | ||||||||||
| 1 | 24.8 | 16.7 | 26.4 | 31.5 | 24.9 | 28.2 | 29.2 | 26.7 | 24.3 | 27.1 |
| 2 | 26.0 | 19.9 | 30.2 | 31.0 | 26.8 | 28.2 | 30.0 | 26.9 | 26.2 | 27.8 |
| 3 | 24.3 | 14.2 | 29.8 | 41.3 | 27.4 | 28.7 | 31.2 | 28.2 | 20.7 | 27.2 |
| 4 | 30.5 | 32.5 | 30.5 | 47.1 | 35.1 | 32.2 | 29.4 | 28.2 | 26.1 | 28.9 |
| 5 | 17.3 | 19.2 | 24.4 | 23.2 | 21.0 | 8.0 | 16.7 | 14.7 | 8.1 | 11.9 |
| Mean | 24.6 | 20.5 | 28.3 | 34.8 | 25.1 | 27.3 | 24.9 | 21.1 | ||
| TMN | ||||||||||
| 1 | 40.2 ab | 29.4 b | 44.0 a | 50.2 bc | 40.9 bc | 41.1 c | 46.2 b | 41.7 b | 35.4 ab | 41.1 c |
| 2 | 44.5 a | 31.0 b | 49.3 a | 50.3 bc | 43.8 b | 47.3 bc | 49.2 ab | 46.2 ab | 29.6 b | 43.1 c |
| 3 | 43.9 ab | 32.2 b | 53.0 a | 65.4 ab | 48.6 b | 50.0 ab | 56.7 a | 46.4 ab | 37.7 ab | 47.7 b |
| 4 | 55.1 a | 50.4 a | 55.9 a | 73.9 a | 58.8 a | 56.3 a | 54.8 a | 50.8 a | 42.6 a | 51.1 a |
| 5 | 28.6 b | 29.9 b | 41.4 a | 35.0 c | 33.7 c | 19.9 d | 28.4 c | 27.0 c | 18.9 c | 23.6 d |
| Mean | 42.5 B | 34.6 BC | 48.7 AB | 55.0 A | 42.9 B | 47.0 A | 42.4 B | 32.8 C | ||
| LSD for: Factor A = 9.2; Factor B = 8.5; Interaction A/B = 15.5; B/A = 14.7 | LSD for: Factor A = 2.8; Factor B = 3.6 Interaction A/B = 8.1 B/A = 8.2; | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotwica, K.; Breza-Boruta, B.; Bauza-Kaszewska, J.; Kanarek, P.; Jaskulska, I.; Jaskulski, D. The Cumulative Effect of Various Tillage Systems and Stubble Management on the Biological and Chemical Properties of Soil in Winter Wheat Monoculture. Agronomy 2021, 11, 1726. https://doi.org/10.3390/agronomy11091726
Kotwica K, Breza-Boruta B, Bauza-Kaszewska J, Kanarek P, Jaskulska I, Jaskulski D. The Cumulative Effect of Various Tillage Systems and Stubble Management on the Biological and Chemical Properties of Soil in Winter Wheat Monoculture. Agronomy. 2021; 11(9):1726. https://doi.org/10.3390/agronomy11091726
Chicago/Turabian StyleKotwica, Karol, Barbara Breza-Boruta, Justyna Bauza-Kaszewska, Piotr Kanarek, Iwona Jaskulska, and Dariusz Jaskulski. 2021. "The Cumulative Effect of Various Tillage Systems and Stubble Management on the Biological and Chemical Properties of Soil in Winter Wheat Monoculture" Agronomy 11, no. 9: 1726. https://doi.org/10.3390/agronomy11091726
APA StyleKotwica, K., Breza-Boruta, B., Bauza-Kaszewska, J., Kanarek, P., Jaskulska, I., & Jaskulski, D. (2021). The Cumulative Effect of Various Tillage Systems and Stubble Management on the Biological and Chemical Properties of Soil in Winter Wheat Monoculture. Agronomy, 11(9), 1726. https://doi.org/10.3390/agronomy11091726






