The Effects of Fig Tree (Ficus carica L.) Leaf Aqueous Extract on Seed Germination and Seedling Growth of Three Medicinal Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Preparation of Aqueous Extracts Solutions
2.3. Seed Germination Experiment
2.4. Pot Experiment
2.5. Synthetical Allelopathic Effect Index (SE)
2.6. Statistical Analysis
3. Results
3.1. Effects of Fig Tree Leaf Aqueous Extract on Seed Germination of Mint, Dandelion, and Woad
3.2. Effects of Fig Tree Leaf Aqueous Extract on Seedling Growth of Mint, Dandelion, and Woad
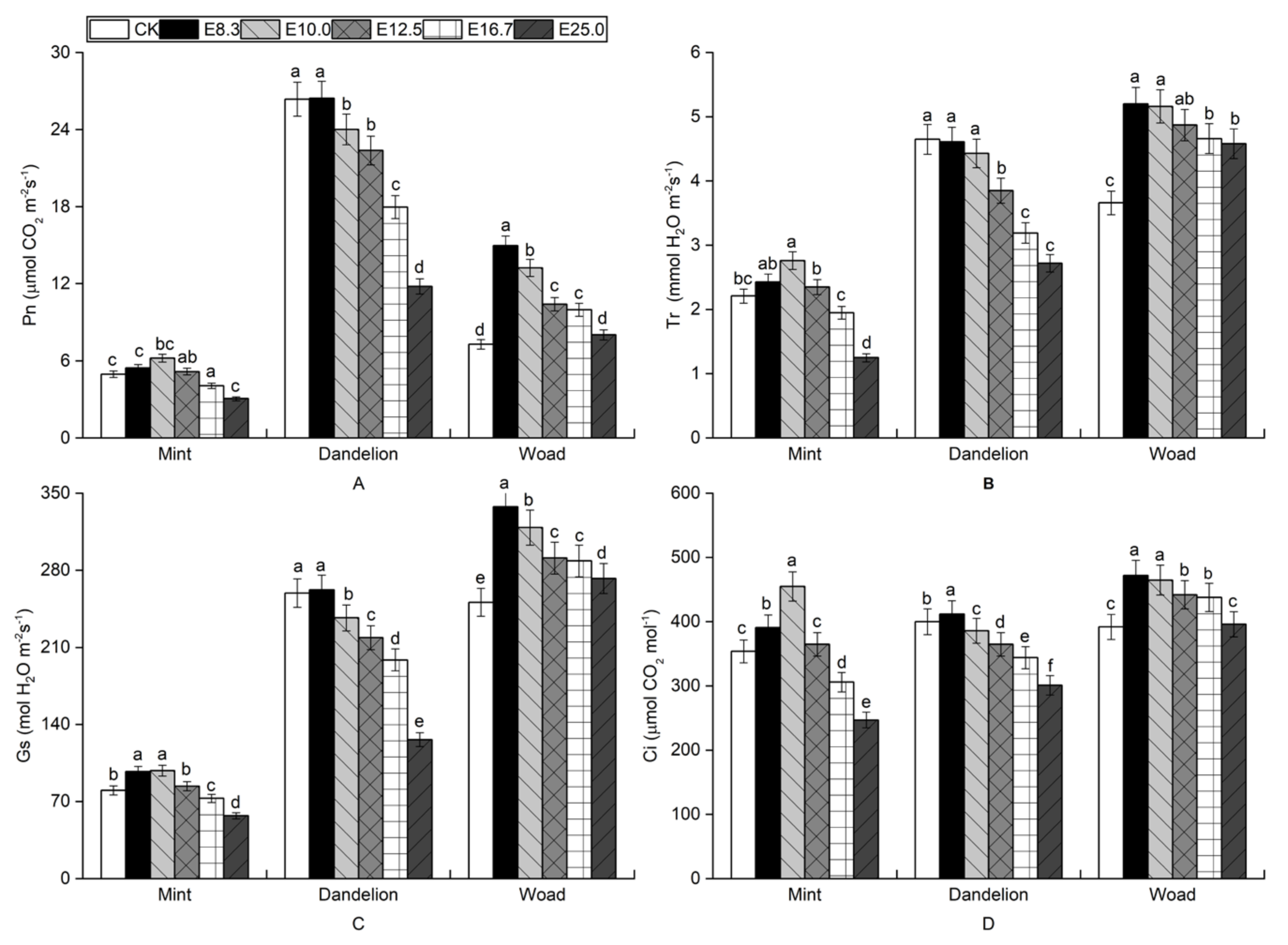
3.3. Effects of Fig Tree Leaf Aqueous Extract on Photosynthesis of Mint, Dandelion, and Woad
3.4. Effects of Fig Tree Leaf Aqueous Extract on Photosynthesis of Mint, Dandelion, and Woad
3.5. Allelopathic Effects of Fig Tree Leaf Aqueous Extract on Mint, Dandelion, and Woad
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaba, J.S.; Yamoah, F.A.; Acquaye, A. Towards sustainable agroforestry management: Harnessing the nutritional soil value through cocoa mix waste. Waste Manag. 2021, 124, 264–272. [Google Scholar] [CrossRef]
- Schoeneberger, M.M. Agroforestry: Working trees for sequestering carbon on agricultural lands. Agrofor. Syst. 2009, 75, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Verma, R.K.; Chauhan, A.; Verma, R.S.; Rahman, L.U.; Bisht, A. Improving production potential and resources use efficiency of peppermint (Mentha piperita L.) intercropped with geranium (Pelargonium graveolens L. Herit ex Ait) under different plant density. Ind. Crops Prod. 2013, 44, 577–582. [Google Scholar] [CrossRef]
- Sehgal, S. Growth and productivity of Ocimum basilicum influenced by the application of organic manures under Leucaena leucocephala hedgerows in western Himalayan mid hills. Range Manag. Agrofor. 2011, 32, 83–86. [Google Scholar]
- Cheng, H.; Wu, B.; Yu, Y.; Wang, S.; Wei, M.; Wang, C.; Du, D. The allelopathy of horseweed with different invasion degrees in three provinces along the Yangtze River in China. Physiol. Mol. Biol. Plants 2021, 27, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: New York, NY, USA, 1984; p. 422. [Google Scholar]
- Tseng, M.H.; Kuo, Y.H.; Chen, Y.M.; Chou, C.H. Allelopathic Potential of Macaranga tanarius (L.) Muell.–Arg. J. Chem. Ecol. 2003, 29, 1269–1286. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Hoque, A.T.M.R.; Hossain, M.K. Allelopathic effects of leaf litters of Eucalyptus camaldulensis on some forest and agricultural crops. J. For. Res. 2008, 19, 19–24. [Google Scholar] [CrossRef]
- Li, J.X.; Ye, J.W.; Liu, D.H. Allelopathic effects of Miscanthus floridulus on seed germination and seedling growth of three crops. J. Appl. Ecol. 2020, 31, 2219–2226. [Google Scholar]
- Zhang, Y.J.; Tang, S.M.; Liu, K.S.; Li, X.F.; Huang, D.; Wang, K. The allelopathic effect of Potentilla acaulis on the changes of plant community in grassland, northern China. Ecol. Res. 2015, 30, 41–47. [Google Scholar] [CrossRef]
- Ning, L.; Yu, F.H.; van Kleunen, M. Allelopathy of a native grassland community as a potential mechanism of resistance against invasion by introduced plants. Biol. Invasions 2016, 18, 3481–3493. [Google Scholar] [CrossRef] [Green Version]
- Islam, A.; Kato-Noguchi, H. Mentha sylvestris: A potential allelopathic medicinal plant. Int. J. Agric. Biol. 2013, 15, 1313–1318. [Google Scholar]
- Abdel-Aty, A.M.; Hamed, M.B.; Salama, W.H.; Ali, M.M.; Fahmy, A.S.; Mohamed, S.A. Ficus carica, Ficus sycomorus and Euphorbia tirucalli latex extracts: Phytochemical screening, antioxidant and cytotoxic properties. Biocatal. Agric. Biotechnol. 2019, 20, 101199. [Google Scholar] [CrossRef]
- Raafat, K.; Wurglics, M. Phytochemical analysis of Ficus carica L. active compounds possessing anticonvulsant activity. J. Tradit. Complement. Med. 2018, 9, 263–270. [Google Scholar] [CrossRef]
- Zhao, J.Y.; Gong, L.M.; Wu, L.L.; She, S.Q.; Liao, Y.; Zheng, H.; Zhao, Z.F.; Liu, G.; Yan, S. Immunomodulatory effects of fermented fig (Ficus carica L.) fruit extracts on cyclophosphamide-treated mice. J. Funct. Foods 2020, 75, 104219. [Google Scholar] [CrossRef]
- Derwich, E.; Benziane, Z.; Taouil, R.; Senhaji, O.; Touzani, M. Aromatic plants of morocco: GC/MS analysis of the essential oils of leaves of mentha piperita. Adv. Environ. Biol. 2010, 4, 80–85. [Google Scholar]
- Dhawan, S.S.; Mishra, A.; Gupta, P.; Bahl, J.R.; Bansal, R.P. Phylogentic relationship of cold tolerant Mentha arvensis variety ’CIM Kranti’ with some released varieties as assessed through physiological and molecular analysis. J. Appl. Res. Med. Aromat. Plants 2018, 10, 67–74. [Google Scholar] [CrossRef]
- Tanticharakunsiri, W.; Mangmool, S.; Wongsariya, K.; Ochaikul, D. Characteristics and upregulation of antioxidant enzymes of kitchen mint and oolong tea kombucha beverages. J. Food Biochem. 2021, 45, e133574. [Google Scholar] [CrossRef]
- Bodalska, A.; Kowalczyk, A.; Wodarczyk, M.; Fecka, I. Analysis of Polyphenolic Composition of a Herbal Medicinal Product-Peppermint Tincture. Molecules 2019, 25, 69. [Google Scholar] [CrossRef] [Green Version]
- Grzeszczuk, M.; Jadczak, D. Estimation of biological value of some species of mint (Mentha, L.). Herba Pol. 2009, 55, 193–199. [Google Scholar]
- Hu, C. Taraxacum: Phytochemistry and health benefits. Chin. Herb. Med. 2018, 10, 353–361. [Google Scholar] [CrossRef]
- Bao, J.; Chen, L.; Liu, T. Dandelion polysaccharide suppresses lipid oxidation in Antarctic krill (Euphausia superba). Int. J. Biol. Macromol. 2019, 133, 1164–1167. [Google Scholar] [CrossRef]
- Choi, B.R.; Cho, I.J.; Jung, S.J.; Kim, J.K.; Lee, D.G.; Ku, S.K.; Park, K.M. Lemon Balm and Dandelion Leaf Extracts Synergistically Protect against Carbon Tetrachloride-Induced Acute Liver Injury in Mice. Appl. Sci. 2021, 11, 390. [Google Scholar] [CrossRef]
- Forner, C.; Zeidler, C.; Stein, P.; Stössel, E.; Wefelmeier, L.; Peukert, N.; Isermann, D.; Ständer, S. Woad extract containing cream improves significantly dry, irritated, and pruritic skin. Dermatol. Ther. 2019, 32, e12939. [Google Scholar] [CrossRef] [PubMed]
- Su, J.H.; Diao, R.G.; Lv, S.G.; Mou, X.D.; Li, K. Modes of Antiviral Action of Chemical Portions and Constituents from Woad Root Extract against Influenza Virus A FM1. Evid. Based Complement. Altern. Med. Ecam. 2016, 2016, 2537294. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Z.Z. The complete chloroplast genome sequence of the medicinal and economic plant woad Isatis indigotica (Brassicaceae). Mitochondrial DNA Part B 2017, 2, 514–515. [Google Scholar] [CrossRef]
- Peng, X.B.; Zhang, Y.Y.; Cai, J.; Jiang, Z.M.; Zhang, S.X. Photosynthesis, growth and yield of soybean and maize in a tree-based agroforestry intercropping system on the Loess Plateau. Agrofor. Syst. 2009, 76, 569–577. [Google Scholar] [CrossRef]
- Kumar, B.; Verma, S.K.; Singh, H.P. Effect of temperature on seed germination parameters in Kalmegh (Andrographis paniculata Wall ex Nees.). Ind. Crops Prod. 2011, 34, 1241–1244. [Google Scholar] [CrossRef]
- Moori, S.; Ahmadi-Lahijani, M.J. Hormopriming instigates defense mechanisms in Thyme (Thymus vulgaris L.) seeds under cadmium stress. J. Appl. Res. Med. Aromat. Plants 2020, 19, 100268. [Google Scholar] [CrossRef]
- Saffari, P.; Majd, A.; Jonoubi, P.; Najafi, F. Effect of treatments on seed dormancy breaking, seedling growth, and seedling antioxidant potential of Agrimonia eupatoria L. J. Appl. Res. Med. Aromat. Plants 2021, 20, 100282. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Wang, N.S.; Hu, X.J.; Lin, X.Y.; Feng, Y.; Chong, W.J. Nitrate nutrition enhances nickel accumulation and toxicity in Arabidopsisplants. Plant Soil 2013, 371, 105–115. [Google Scholar] [CrossRef]
- Zhang, C.P.; Li, Y.C.; Yuan, F.G.; Hu, S.J.; Liu, H.Y.; He, P. Role of 5-aminolevulinic acid in the salinity stress response of the seeds and seedlings of the medicinal plant Cassia obtusifolia L. Bot. Stud. 2013, 54, 18. [Google Scholar] [CrossRef] [Green Version]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Hodges, D.M.; Delong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Williamson, G.B.; Richardson, D. Bioassays for allelopathy: Measuring treatment responses with independent controls. J. Chem. Ecol. 1988, 14, 181. [Google Scholar] [CrossRef]
- Kala, C.P. Medicinal and aromatic plants: Boon for enterprise development. J. Appl. Res. Med. Aromat. Plants 2015, 2, 134–139. [Google Scholar] [CrossRef]
- Abbas, A.; Huang, P.; Hussain, S.; Saqib, M.; Du, D. Application of allelopathic phenomena to enhance growth and production of camelina (Camelina sativa (L.). Appl. Ecol. Env. Res. 2021, 19, 453–469. [Google Scholar] [CrossRef]
- Pannacci, E.; Pettorossi, D.; Tei, F. Phytotoxic effects of aqueous extracts of sunflower on seed germination and growth of Sinapis alba L., Triticum aestivum L. and Lolium multiflorum Lam. Allelopath. J. 2013, 32, 23–36. [Google Scholar]
- An, Y.; Ma, Y.Q.; Shui, J.F.; Zhong, W.J. Switchgrass (Panicum virgatum L.) has ability to induce germination of Orobanche cumana. J. Plant Interact. 2015, 10, 142–151. [Google Scholar] [CrossRef] [Green Version]
- Sitthinoi, P.; Lertmongkol, S.; Chanprasert, W.; Vajrodaya, S. Allelopathic effects of jungle rice (Echinochloa colona (L.) Link) extract on seed germination and seedling growth of rice. Agric. Nat. Resour. 2017, 51, 74–78. [Google Scholar] [CrossRef]
- Batish, D.R.; Singh, H.P.; Kaur, S. Crop Allelopathy and Its Role in Ecological Agriculture. J. Crops Prod. 2001, 4, 121–161. [Google Scholar] [CrossRef]
- Kwiecińska-Poppe, E.; Kraska, P.; Pałys, E. The influence of water extracts from Galium aparine L. and Matricaria maritima subspinodora (L.) dostál on germination of winter rye and triticale. Acta Sci. Pol. Agric. 2011, 10, 75–85. [Google Scholar]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence-a practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Colom, M.R.; Vazzana, C. Photosynthesis and PSII functionality of drought-resistant and drought-sensitive weeping lovegrass plants. Environ. Exp. Bot. 2003, 49, 135–144. [Google Scholar] [CrossRef]
- Jones, H.G. Partitioning stomatal and non-stomatal limitations to photosynthesis. Plant Cell Environ. 1985, 8, 95–104. [Google Scholar] [CrossRef]
- Collatz, G.J. Physiological and environmental regulation of stomatal conductance, photosynthesis and transpiration: A model that includes a laminar boundary layer. Agri. For. Meteorol. 1991, 54, 107–136. [Google Scholar] [CrossRef]
- Li, J.X.; Chen, L.; Chen, Q.H.; Miao, Y.H.; Peng, Z.; Huang, B.S.; Guo, L.P.; Liu, D.H.; Du, H.Z. Allelopathic effect of Artemisia argyi on the germination and growth of various weeds. Sci. Rep. 2021, 11, 4303. [Google Scholar] [CrossRef]
- Yu, J.Q.; Ye, S.F.; Zhang, M.F.; Hu, W.H. Effects of root exudates and aqueous root extracts of cucumber (Cucumis sativus) and allelochemicals, on photosynthesis and antioxidant enzymes in cucumber. Biochem. Syst. Ecol. 2003, 31, 129–139. [Google Scholar] [CrossRef]
- Ahmed, I.M.; Cao, F.; Zhang, M.; Chen, X.H.; Zhang, G.P.; Wu, F.B. Difference in Yield and Physiological Features in Response to Drought and Salinity Combined Stress during Anthesis in Tibetan Wild and Cultivated Barleys. PLoS ONE 2013, 8, e77869. [Google Scholar]
- Baniasadi, F.; Saffari, V.R.; Moud, A.A.M. Physiological and growth responses of Calendula officinalis L. plants to the interaction effects of polyamines and salt stress. Sci. Hortic. 2018, 234, 312–317. [Google Scholar] [CrossRef]
- Liu, J.X.; Hu, H.B.; Lei, R.X. Physiological and biochemical mechanism of allelopathy of Peganum multisectum Bobr on alfalfa. Chin. J. Grassl. 2007, 29, 72–79. [Google Scholar]
- Morgan, E.C.; Overholt, W.A. Potential allelopathic effects of Brazilian pepper (Schinus terebinthifolius Raddi, Anacardiaceae) aqueous extract on germination and growth of selected Florida native plants. J. Torrey Bot. Soc. 2005, 132, 11–15. [Google Scholar] [CrossRef]
- Zeng, R.S.; Luo, S.M.; Shi, Y.H.; Shi, M.B.; Tu, C.Y. Physiological and Biochemical Mechanism of Allelopathy of Secalonic Acid F on Higher Plants. Agron. J. 2001, 93, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Liu, Q.; Feng, J.; Lv, J.P.; Xie, S.L. Effect of high-doses pyrogallol on oxidative damage, transcriptional responses and microcystins synthesis in Microcystis aeruginosa TY001 (Cyanobacteria). Ecotoxicol. Environ. Saf. 2016, 134, 273–279. [Google Scholar] [CrossRef]
- Harun, M.A.Y.A.; Robinson, R.W.; Johnson, J.; Uddin, M.N. Allelopathic potential of Chrysanthemoides monilifera subspmonilifera (boneseed): A novel weapon in the invasion processes. South Afr. J. Bot. 2014, 93, 157–166. [Google Scholar]
- Jin, Y.H.; Tao, D.L.; Hao, Z.Q.; Ye, J.; Du, Y.J.; Liu, H.L.; Zhou, Y.B. Environmental Stresses and Redox Status of Ascorbate. Acta Bot. Sin. 2003, 45, 795–800. [Google Scholar]
- Inderjit Wardle, D.A.; Karban, R.; Callaway, R.M. The ecosystem and evolutionary contexts of allelopathy. Trends Ecol. Evol. 2011, 26, 655–662. [Google Scholar] [CrossRef] [Green Version]
- Song, R. Growth promotion of maize by soybean root exudates. Legume Res. 2012, 35, 226–230. [Google Scholar]
- Yeo, H.H.T.; Chong, K.Y.; Yee, A.T.K.; Giam, X.; Corlett, R.T.; Tan, H.T.W. Leaf litter depth as an important factor inhibiting seedling establishment of an exotic palm in tropical secondary forest patches. Biol. Invasions 2014, 16, 381–392. [Google Scholar] [CrossRef]
- Mohsin, N.; Tariq, M.; Zaki, M.J.; Abbasi, M.W.; Imran, M. Allelopathic effect of Ficus benghalensis L. leaves extract on germination and early seedling growth of maize, mung bean and sunflower. Int. J. Biol. Res. 2016, 4, 34–38. [Google Scholar]
- Ashti, S.A.; Hero, F.H.; Dlshad, A.O.; Nawroz, A.T. Response of some plant species towards the allelopathy of two types of chickpea (Cicer arietinum L.) seed extracts. Appl. Ecol. Environ. Res. 2018, 16, 8119–8129. [Google Scholar] [CrossRef]
- Ren, K.; Hayat, S.; Qi, X.; Liu, T.; Cheng, Z. The garlic allelochemical DADS influences cucumber root growth involved in regulating hormone levels and modulating cell cycling. J. Plant Physiol. 2018, 230, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.C.; Kong, C.H.; Chen, L.C.; Wang, P.; Wang, S.L. A broadleaf species enhances an autotoxic conifers growth through belowground chemical interactions. Ecology 2016, 97, 2283–2292. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.C.; Yu, S.X. Arbuscular mycorrhizal fungi protect native woody species from novel weapons. Plant Soil 2019, 440, 39–52. [Google Scholar] [CrossRef]
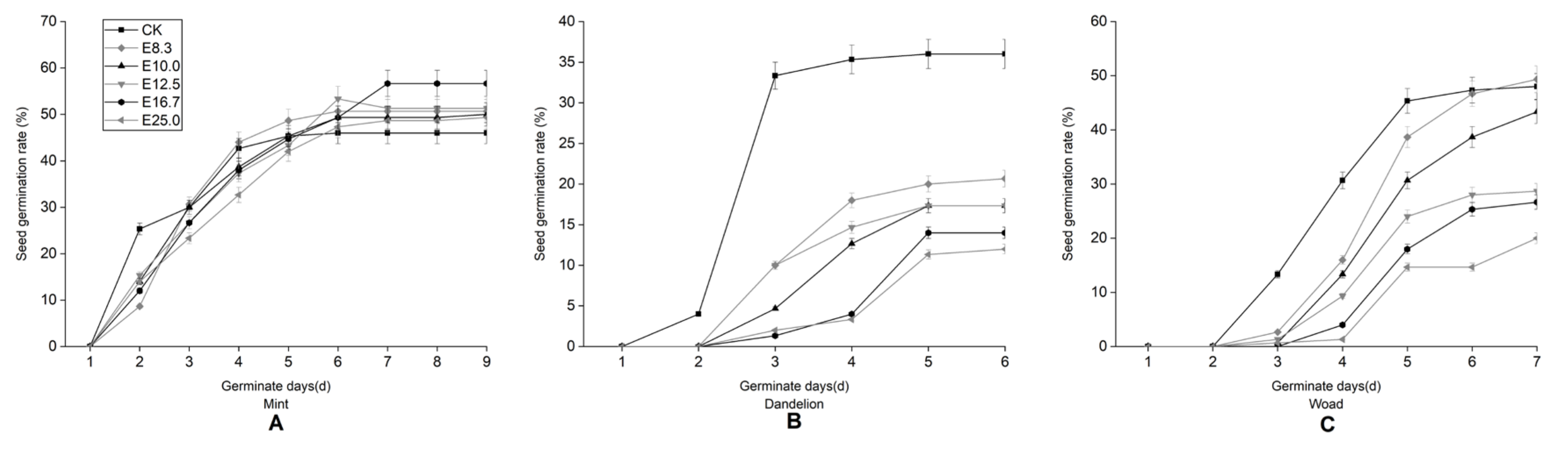




| Plant Species | Concentration (g/L) | GR (%) | GP (%) | GI | SVI |
|---|---|---|---|---|---|
| Mint | 0.0 | 46.00 ± 2.00b | 30.00 ± 12.17a | 33.75 ± 4.85a | 3.23 ± 0.06b |
| 8.3 | 50.67 ± 4.16ab | 30.67 ± 12.22a | 31.46 ± 4.37a | 3.37 ± 0.29b | |
| 10.0 | 50.00 ± 6.05ab | 30.00 ± 8.72a | 30.73 ± 5.37a | 3.58 ± 0.39ab | |
| 12.5 | 51.33 ± 4.16ab | 26.67 ± 9.87a | 31.45 ± 9.01a | 3.96 ± 0.08a | |
| 16.7 | 56.67 ± 5.03a | 26.67 ± 6.11a | 31.51 ± 5.39a | 3.94 ± 0.26a | |
| 25.0 | 49.33 ± 2.31ab | 23.33 ± 11.37a | 28.88 ± 1.86a | 3.22 ± 0.20b | |
| Dandelion | 0.0 | 36.00 ± 8.00a | 35.33 ± 9.02a | 17.57 ± 2.05a | 0.46 ± 0.13a |
| 8.3 | 20.67 ± 3.06a | 18.00 ± 4.00b | 7.64 ± 1.02b | 0.24 ± 0.04b | |
| 10.0 | 17.33 ± 2.31a | 12.67 ± 3.05bc | 6.14 ± 1.75bc | 0.19 ± 0.02bc | |
| 12.5 | 17.33 ± 3.06a | 14.67 ± 5.03b | 6.68 ± 1.79b | 0.18 ± 0.02bc | |
| 16.7 | 14.00 ± 4.00a | 4.00 ± 2.00c | 3.29 ± 0.76cd | 0.14 ± 0.02bc | |
| 25.0 | 12.00 ± 5.29a | 3.33 ± 2.31c | 2.88 ± 1.90d | 0.10 ± 0.02c | |
| Woad | 0.0 | 48.00 ± 16.00a | 45.33 ± 20.13a | 17.96 ± 4.62a | 1.71 ± 0.05c |
| 8.3 | 49.33 ± 12.22a | 38.67 ± 8.33a | 13.72 ± 3.70ab | 3.04 ± 0.06a | |
| 10.0 | 43.33 ± 22.12a | 30.67 ± 22.75a | 11.16 ± 6.19abc | 2.57 ± 0.10b | |
| 12.5 | 28.67 ± 17.24a | 24.00 ± 21.17a | 8.17 ± 1.77bc | 1.37 ± 0.15d | |
| 16.7 | 26.67 ± 18.04a | 18.00 ± 7.21a | 6.32 ± 2.40bc | 1.29 ± 0.05d | |
| 25.0 | 20.00 ± 17.44a | 14.67 ± 15.14a | 4.40 ± 0.30c | 0.9 ± 0.12e |
| Plant Species | Concentration (g/L) | ODW (g/plant) | UDW (g/plant) | TDW (g/plant) |
|---|---|---|---|---|
| Mint | 0.0 | 0.33 ± 0.25ab | 0.28 ± 0.02ab | 0.62 ± 0.03ab |
| 8.3 | 0.37 ± 0.18a | 0.33 ± 0.03a | 0.70 ± 0.02a | |
| 10.0 | 0.34 ± 0.03ab | 0.27 ± 0.02ab | 0.61 ± 0.05ab | |
| 12.5 | 0.32 ± 0.03abc | 0.26 ± 0.01bc | 0.58 ± 0.03b | |
| 16.7 | 0.29 ± 0.05bc | 0.21 ± 0.06cd | 0.50 ± 0.10bc | |
| 25.0 | 0.25 ± 0.04c | 0.18 ± 0.03d | 0.43 ± 0.07c | |
| Dandelion | 0.0 | 0.96 ± 0.10b | 0.31 ± 0.03b | 1.28 ± 0.11bc |
| 8.3 | 1.24 ± 0.05a | 0.36 ± 0.01a | 1.60 ± 0.05a | |
| 10.0 | 1.14 ± 0.01a | 0.23 ± 0.04c | 1.37±0.03b | |
| 12.5 | 1.01 ± 0.0 2b | 0.22 ± 0.03c | 1.23 ± 0.04c | |
| 16.7 | 0.83 ± 0.05c | 0.19 ± 0.02c | 1.02 ± 0.06d | |
| 25.0 | 0.53 ± 0.04d | 0.14 ± 0.01d | 0.67 ± 0.04e | |
| Woad | 0.0 | 2.20 ± 0.09ab | 0.85 ± 0.10bc | 3.06 ± 0.19bc |
| 8.3 | 2.36 ± 0.20a | 1.17 ± 0.08a | 3.53 ± 0.27a | |
| 10.0 | 2.07 ± 0.07c | 1.03 ± 0.02ab | 3.09 ± 0.09b | |
| 12.5 | 1.82 ± 0.13cd | 0.88 ± 0.12b | 2.70 ± 0.13cd | |
| 16.7 | 1.67 ± 0.19d | 0.85 ± 0.08bc | 2.53 ± 0.27d | |
| 25.0 | 1.27 ± 0.73e | 0.67 ± 0.13c | 1.94 ± 0.14e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Yang, X.; Tian, Y.; Yu, M.; Shi, S.; Qiao, B.; Zhao, C.; Mao, L. The Effects of Fig Tree (Ficus carica L.) Leaf Aqueous Extract on Seed Germination and Seedling Growth of Three Medicinal Plants. Agronomy 2021, 11, 2564. https://doi.org/10.3390/agronomy11122564
Li C, Yang X, Tian Y, Yu M, Shi S, Qiao B, Zhao C, Mao L. The Effects of Fig Tree (Ficus carica L.) Leaf Aqueous Extract on Seed Germination and Seedling Growth of Three Medicinal Plants. Agronomy. 2021; 11(12):2564. https://doi.org/10.3390/agronomy11122564
Chicago/Turabian StyleLi, Chunying, Xue Yang, Yao Tian, Meiting Yu, Sen Shi, Bin Qiao, Chunjian Zhao, and Liang Mao. 2021. "The Effects of Fig Tree (Ficus carica L.) Leaf Aqueous Extract on Seed Germination and Seedling Growth of Three Medicinal Plants" Agronomy 11, no. 12: 2564. https://doi.org/10.3390/agronomy11122564






