Effects of Node Position and Electric Conductivity of Nutrient Solution on Adventitious Rooting of Nasturtium (Tropaeolum majus L.) Cuttings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Growth Conditions for Mother Plants
2.2. Treatments
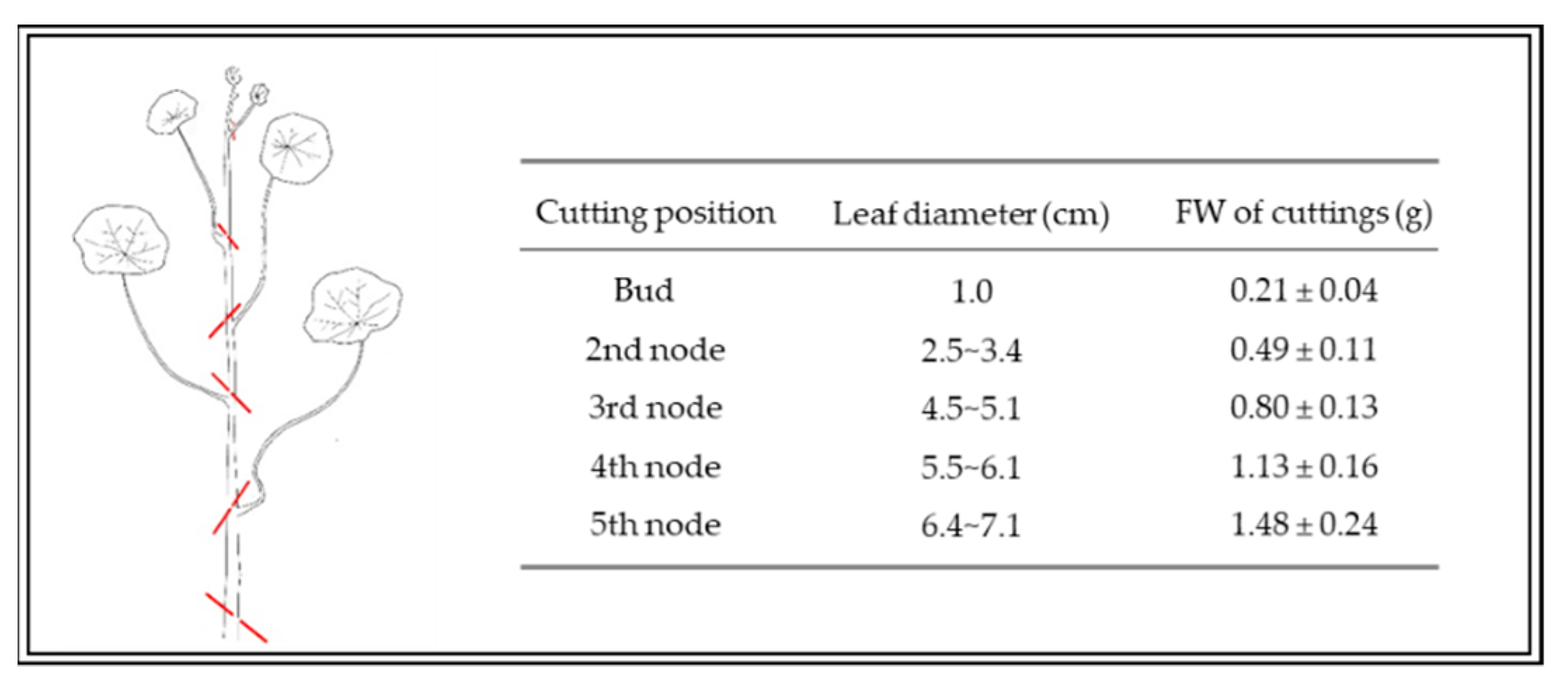
2.3. Measurement
2.3.1. Extraction and Analysis of Endogenous IAA
2.3.2. Measurement of Rooting Characteristics of Cuttings
2.4. Statistical Analysis
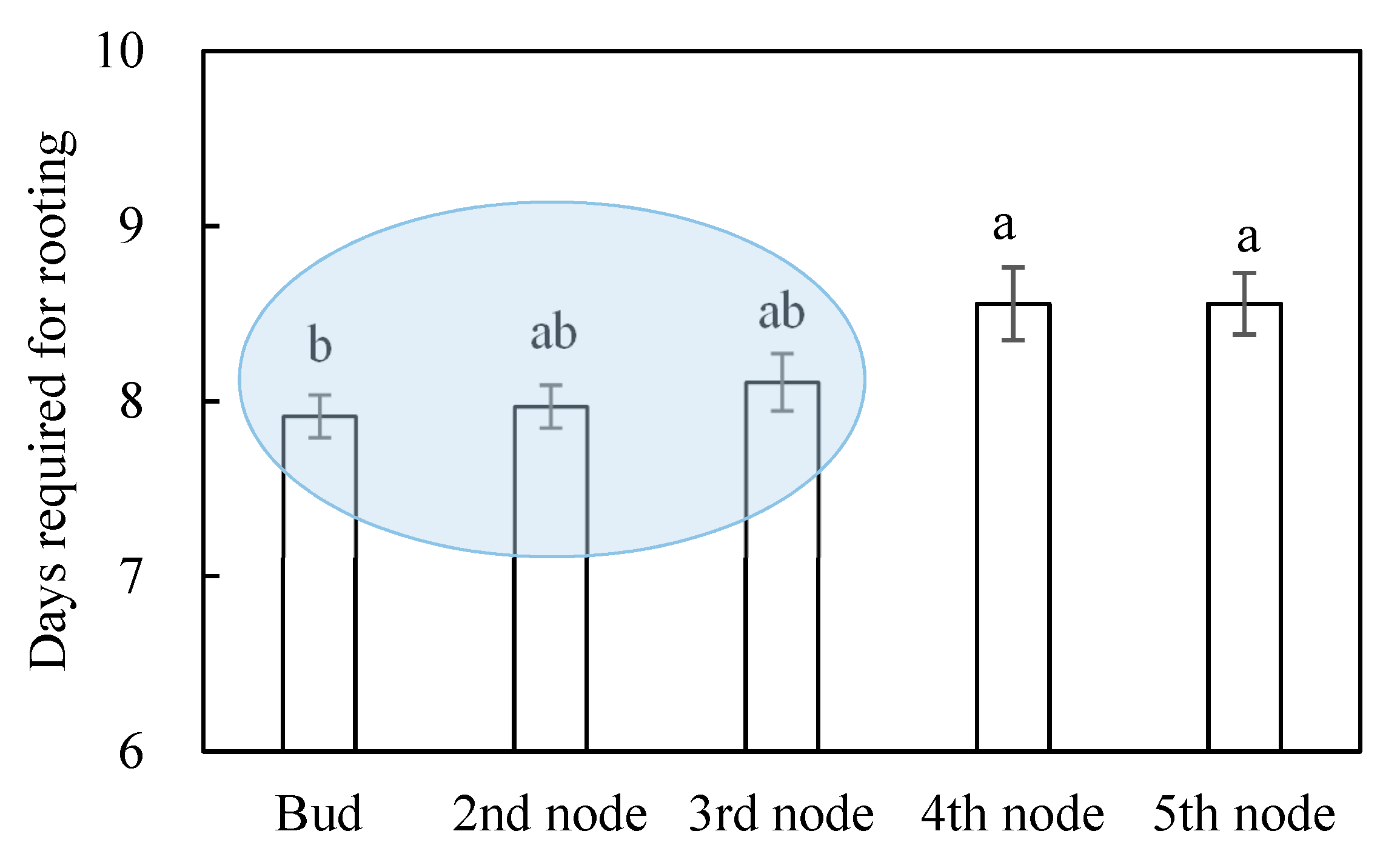
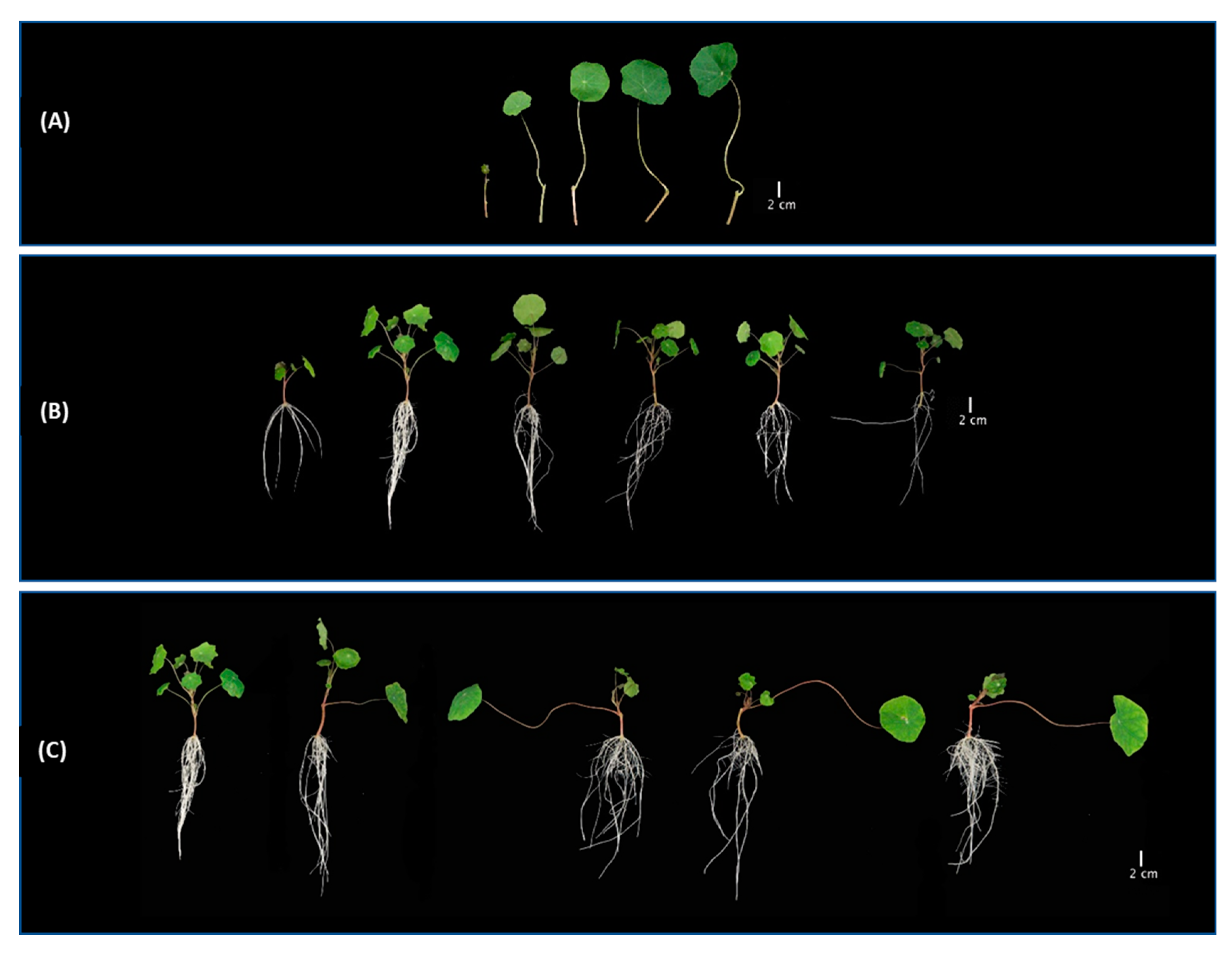
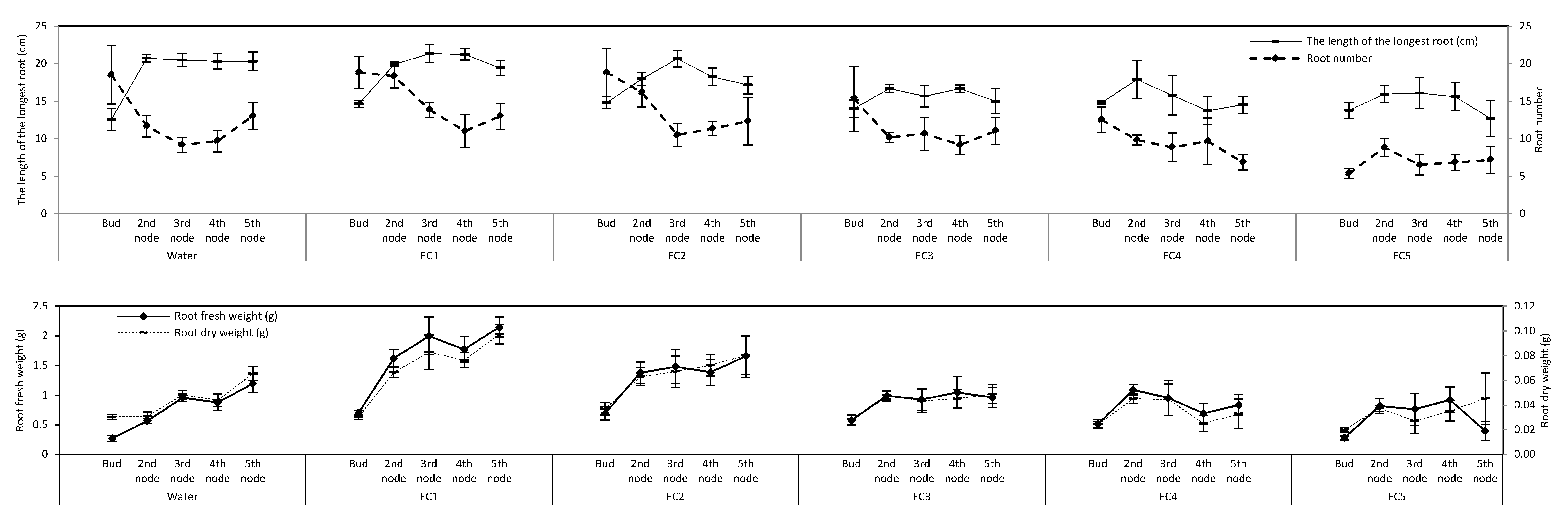
3. Results
4. Discussion
4.1. Effects of EC on Rooting of Nasturtium Cuttings
4.2. Effects of Node Position on Adventitious Rooting of Nasturtium Cuttings
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brondani, C.; Cuelho, F.; Helena, C.; Marangoni, D.; Guex, G.; Bonilha, D.F.; Manfron, P. Traditional usages, botany, phytochemistry, biological activity and toxicology of Tropaeolum majus L.—A review. Bol. Latinoam. Caribe Plant. Med. Aromat. 2016, 15, 264–273. [Google Scholar]
- Jakubczyk, K.; Janda, K.; Watychowicz, K.; Łukasiak, J.; Wolska, J. Garden nasturtium (Tropaeolum majus L.)—A source of mineral elements and bioactive compounds. Rocz. Panstw. Zakl. Hig. 2018, 69, 119–126. [Google Scholar]
- Garzón, G.A.; Wrolstad, R.E. Major anthocyanins and antioxidant activity of Nasturtium flowers (Tropaeolum majus). Food Chem. 2009, 114, 44–49. [Google Scholar] [CrossRef]
- Schreiner, M.; Krumbein, A.; Mewis, I.; Ulrichs, C.; Huyskens-Keil, S. Short-term and moderate UV-B radiation effects on secondary plant metabolism in different organs of nasturtium (Tropaeolum majus L.). Innov. Food Sci. Emerg. Technol. 2009, 10, 93–96. [Google Scholar] [CrossRef]
- Chisholm, M.J.; Hopkins, C.Y. Fatty Acids of Filbert Oil and Nasturtium Seed Oil. Can. J. Chem. 1953, 31, 1131–1137. [Google Scholar] [CrossRef] [Green Version]
- Melo, E.F.R.; dos Santos, O.S. Growth and production of nasturtium flowers in three hydroponic solutions. Hortic. Bras. 2011, 29, 584–589. [Google Scholar] [CrossRef] [Green Version]
- Pitzschke, A. Tropaeolum Tops Tobacco-Simple and Efficient Transgene Expression in the Order Brassicales. PLoS ONE 2013, 8, e73355. [Google Scholar] [CrossRef]
- Silva, T.P.; Finger, F.L. Ethylene and 1-methylcyclopropene action over senescence of nasturtium flowers. Hortic. Bras. 2015, 33, 453–458. [Google Scholar] [CrossRef] [Green Version]
- Hensel, A.; Brummell, D.A.; Hanna, R.; Maclachlan, G. Auxin-dependent breakdown of xyloglucan in cotyledons of germinating nasturtium seeds. Planta 1991, 183, 321–326. [Google Scholar] [CrossRef]
- Kleinwächter, M.; Hutter, I.; Schneider, C.; Schnug, E.; Selmar, D. Experimental field cultivation of in vitro propagated high-yield varieties of Tropaeolum majus L. J. Appl. Bot. Food Qual. 2008, 82, 55–59. [Google Scholar]
- Kleinwächter, M.; Hutter, I.; Schneider, C.; Schnug, E.; Selmar, D. Development of an in vitro system to propagate glucosinolate rich nasturtium (Tropaelum majus L.) varieties for agricultural use. Z. Arznei Gewurzpflanz. 2010, 15, 69–75. [Google Scholar]
- Matallana, L.; Kleinwächter, M.; Selmar, D. Sulfur is limiting the glucosinolate accumulation in nasturtium in vitro plants (Tropaeolum majus L.). J. Appl. Bot. Food Qual. 2005, 80, 1–5. [Google Scholar]
- Kevers, C.; Hausman, J.F.; Faivre-Rampant, O.; Evers, D.; Gaspar, T. Hormonal control of adventitious rooting: Progress and questions. J. Appl. Bot. 1997, 71, 71–79. [Google Scholar]
- Caboni, E.; Tonelli, M.G.; Lauri, P.; Iacovacci, P.; Kevers, C.; Damiano, C.; Gaspar, T. Biochemical aspects of almond microcuttings related to in vitro rooting ability. Biol. Plant. 1997, 39, 91–97. [Google Scholar] [CrossRef]
- Xu, M.; Zhu, L.; Shou, H.; Wu, P. A PIN1 Family Gene, OsPIN1, involved in Auxin-dependent Adventitious Root Emergence and Tillering in Rice. Plant Cell Physiol. 2005, 46, 1674–1681. [Google Scholar] [CrossRef]
- Solikin, S. Effect of nodes position on the growth and yield of stem cutting of Sambiloto (Andrographis paniculata). Nusant. Biosci. 2018, 10, 226–231. [Google Scholar] [CrossRef]
- Caplan, D.; Stemeroff, J.; Dixon, M.; Zheng, Y. Vegetative propagation of cannabis by stem cuttings: Effects of leaf number, cutting position, rooting hormone, and leaf tip removal. Can. J. Plant Sci. 2018, 98, 1126–1132. [Google Scholar] [CrossRef]
- Shibuya, T.; Taniguchi, T.; Tsukuda, S.; Shiozaki, S.; Itagaki, K. Adventitious root formation of Japanese cedar (Cryptomeria japonica D. Don) cuttings is stimulated by soaking basal portion of cuttings in warmed water while cooling their apical portion. New For. 2014, 45, 589–602. [Google Scholar] [CrossRef]
- Nguyen, D.T.P.; Lu, N.; Kagawa, N.; Takagaki, M. Optimization of photosynthetic photon flux density and root-zone temperature for enhancing secondary metabolite accumulation and production of coriander in plant factory. Agronomy 2019, 9, 224. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.T.P.; Lu, N.; Kagawa, N.; Kitayama, M.; Takagaki, M. Short-term root-zone temperature treatment enhanced the accumulation of secondary metabolites of hydroponic coriander (Coriandrum sativum L.) grown in a plant factory. Agronomy 2020, 10, 413. [Google Scholar] [CrossRef] [Green Version]
- López-Bucio, J.; Cruz-Ramírez, A.; Herrera-Estrella, L. The role of nutrient availability in regulating root architecture. Curr. Opin. Plant Biol. 2003, 6, 280–287. [Google Scholar] [CrossRef]
- Loach, K. Water relations and adventitious rooting. In Adventitious Root Formation in Cuttings; Davis, T.D., Haissing, B.E., Sankhla, N., Eds.; Dioscorides Press: Portland, OR, USA, 1988; pp. 102–116. [Google Scholar]
- Puri, S.; Thompson, F.B. Relationship of water to adventitious rooting in stem cuttings of Populus species. Agrofor. Syst. 2003, 58, 1–9. [Google Scholar] [CrossRef]
- Lu, N.; Bernardo, E.L.; Tippayadarapanich, C.; Takagaki, M.; Kagawa, N.; Yamori, W. Growth and accumulation of secondary metabolites in perilla as affected by photosynthetic photon flux density and electrical conductivity of the nutrient solution. Front. Plant Sci. 2017, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Bertram, L. Vegetative propagation of Hibiscus rosa-sinensis L. in relation to nutrient concentration of the propagation medium. Sci. Hortic. 1991, 48, 131–139. [Google Scholar] [CrossRef]
- Dewir, Y.H.; Chakrabarty, D.; Ali, M.B.; Hahn, E.J.; Paek, K.Y. Effects of hydroponic solution EC, substrates, PPF and nutrient scheduling on growth and photosynthetic competence during acclimatization of micropropagated Spathiphyllum plantlets. Plant Growth Regul. 2005, 46, 241–251. [Google Scholar] [CrossRef]
- Kozai, T.; Niu, G. Role of the plant factory with artificial lighting (PFAL) in urban areas. In Plant Factory—An Indoor Vertical Farming System for Efficient Quality Food Production; Kozai, T., Niu, G., Takagaki, M., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 7–33. [Google Scholar]
- Durgbanshi, A.; Arbona, V.; Pozo, O.; Miersch, O.; Sancho, J.V.; Gómez-Cadenas, A. Simultaneous determination of multiple phytohormones in plant extracts by liquid chromatography-electrospray tandem mass spectrometry. J. Agric. Food Chem. 2005, 53, 8437–8442. [Google Scholar] [CrossRef] [PubMed]
- Lisar, S.Y.S.; Motafakkerazad, R.; Hossain, M.M.; Rahman, I.M.M. Water Stress in Plants: Causes, Effects and Responses. In Water Stress; Rahman, I.M.M., Hasegawa, H., Eds.; Academic Press: Cambridge, MA, USA; InTech: London, UK, 2012; pp. 1–14. [Google Scholar]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2008, 103, 551–560. [Google Scholar] [CrossRef] [Green Version]
- Geiss, G.; Gutierrez, L.; Bellini, C. Adventitious Root Formation: New Insights and Perspectives. Root Dev. 2009, 37, 127–156. [Google Scholar] [CrossRef]
- Nakai, A.; Yurugi, Y.; Kisanuki, H. Stress responses in Salix gracilistyla cuttings subjected to repetitive alternate flooding and drought. Trees Struct. Funct. 2010, 24, 1087–1095. [Google Scholar] [CrossRef]
- Kelen, M.; Ozkan, G. Relationships between rooting ability and changes of endogenous IAA and ABA during the rooting of hardwood cuttings of some grapevine rootstocks. Eur. J. Hortic. Sci. 2003, 68, 8–13. [Google Scholar]
- Bartels, D.; Sunkar, R. Drought and Salt Tolerance in Plants. Crit. Rev. Plant Sci. 2005, 24, 23–58. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef] [Green Version]
- Ludwig-Müller, J.; Cohen, J.D. Identification and quantification of three active auxins in different tissues of Tropaeolum majus. Physiol. Plant. 2002, 115, 320–329. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Lu, N.; Kikuchi, M.; Takagaki, M. Effects of Node Position and Electric Conductivity of Nutrient Solution on Adventitious Rooting of Nasturtium (Tropaeolum majus L.) Cuttings. Agronomy 2021, 11, 363. https://doi.org/10.3390/agronomy11020363
Xu W, Lu N, Kikuchi M, Takagaki M. Effects of Node Position and Electric Conductivity of Nutrient Solution on Adventitious Rooting of Nasturtium (Tropaeolum majus L.) Cuttings. Agronomy. 2021; 11(2):363. https://doi.org/10.3390/agronomy11020363
Chicago/Turabian StyleXu, Wenshuo, Na Lu, Masao Kikuchi, and Michiko Takagaki. 2021. "Effects of Node Position and Electric Conductivity of Nutrient Solution on Adventitious Rooting of Nasturtium (Tropaeolum majus L.) Cuttings" Agronomy 11, no. 2: 363. https://doi.org/10.3390/agronomy11020363





