Abstract
Small-holder banana fields are often intercropped with various annual crops to optimize land-use in East and Central Africa, a practice severely constrained by light availability under the banana canopy. Light availability is not a major constraint in newly established banana fields, giving a window of opportunity to target light-demanding annual crops before shifting to more shade-tolerant crops. This study investigated the performance of climbing and bush beans and the vegetable amaranth in banana fields with varying shade levels across three sites in the South Kivu province, DR Congo. These crops were selected for their highly nutritious and good market value and the added benefit of nitrogen fixation for the legumes. We show that both grain legumes and vegetable amaranth can achieve reasonable yields during a first annual cropping season in newly established banana fields, irrespective of the plant density. Declines in yield occurred during a second cropping season in more densely spaced banana fields (2 × 2 m and 2 × 3 m). A greater decline occurred in amaranth and its cultivation should be limited to the first annual cropping season or to less dense banana fields. The legumes could be extended to a second cropping season with reasonable yield. Significant variability in amaranth and legumes performance was observed across sites, with rapid yield declines occurring under more fertile soil conditions due to fast banana growth/canopy formation and under more vigorous cultivars. The choice of banana spacing will need to be tailored to the banana cultivar, soil conditions and the farmers’ objectives.
1. Introduction
Diversifying banana (Musa spp.) cropping systems is an important strategy for improving food and nutrition security, improving ecosystems health and building resilience of the smallholder cropping system. Most bananas, including plantains, in East and Central Africa are produced by small-holder farmers with land holdings varying between 0.5 to 2 ha [1]. However, in the last decades these banana production systems have been highly affected by soil degradation and declining soil fertility, drought events and an increasing prevalence of pests and diseases [2,3,4]. This has resulted in major yield gaps in these small land holdings, with lower average yields of 5–46 t/ha/year [5,6] compared to 60–70 t/ha/year achieved under controlled experiments [7]. Moreover, the average life span of the banana plantations has declined to 5–10 years compared with 25–50 years during the mid-20th century, mainly due to pests (e.g., the banana weevil) and diseases (e.g., Xanthomonas wilt of banana) and decreased soil fertility [4,7,8]. To increase overall productivity of banana fields and to make better use of the increasingly fragmented and small land holdings, farmers intercrop the banana fields with a variety of crop species such as beans, cassava, taro, sweet potato, leafy vegetables and yams [2]. Intercropping of banana with coffee is common but predominantly practiced on larger farms using wider banana spacing. Production of two or more crops using complementary resources thus limiting competition (e.g., by using crops with different rooting depths or nutrient requirements) can indeed allow for increased agricultural yield on a given piece of land [6,9,10]. However, continuous and intensified production puts the farm at risk of increased depletion of soil fertility [4] potentially leading to future crop failure. Sustainable management of these production systems is becoming increasingly challenging and urgent to ensure ecosystem stability and food, nutrition and livelihood security of the farmers.
Selection of appropriate crops for intercropping in banana fields will however depend on cultivation constraints. Light availability under the banana canopy has been identified as a major limiting factor for growing annual crops [4,11]. Light availability depends on the spatial distribution of the banana plants and banana plant density in the field and will decrease over time as the banana crop matures and the canopy becomes larger. A succession of crop types from light-demanding annual crops in newly established and open fields to more shade-tolerant crops in maturing banana fields would allow for optimal use of available land [4]. Most annual crops, however, only have low shade-tolerance and optimal crop selection for use during the limited timeframe when reasonable light is available in banana fields (e.g., in young banana fields or fields where plants or mats have been removed due to a pest or a disease) should be targeted.
Intercropping of bananas and herbaceous legumes (e.g., common bean and climbing bean) is common in the banana production systems of Central and East tropical Africa [11]. In addition, vegetable amaranth is also commonly grown on farms in Central Africa. These crops are highly valuable both in terms of the stability of the farmland (regarding soil erosion control and nutrient cycling) and the nutritional needs of the local community [12]. Both beans and amaranth are highly nutritious, protein-rich food sources [12,13]. Beyond carbohydrates and protein, beans are rich in iron, zinc and fibers [14]. Vegetable amaranth additionally is very rich in vitamins, including carotene, vitamin B6, vitamin C, riboflavin, folate and in dietary minerals including calcium and iron [12]. These types of high quality foods are of great value to the local population who are prone to nutritional deficiencies related to limited access and diversity in foods [15]. Additionally, the relatively high market value of both beans and amaranth [16] could provide important opportunities to increase the income of small-holder farmers. Legumes also biologically fix atmospheric nitrogen via symbiosis with rhizobia present in nodules on their roots [17] hence contributing to improved soil fertility and subsequent crop growth, through leaf fall and plant debris. Both the N transfer from root exudates and the N released from the turnover of below-ground residues could reduce or offset N depletion [18]. Intercropping with legumes in banana fields could be used as an agricultural strategy to stabilize soil and would, over time, increase crop yields for small-holder farmers, especially since inorganic fertilizers are scarce [4,19]. Both the N transfer from root exudates and the N released from the turnover of below-ground residues could reduce or offset N depletion [18]. Stabilizing or even increasing soil fertility is highly beneficial for the farmer and could increase the performance of the banana crop and subsequent intercrops.
However, limited information is available on the impact of various shade-levels on the yields [20,21] and the formation and efficiency of functional root nodules of legumes [22,23,24]. Moreover, vegetable amaranth has been an overlooked crop in scientific literature even though it is widely consumed in this region and is of great importance for diversifying available nutritional food [25]. While vegetable amaranth is generally grown in open spaces [25], a detailed assessment of potential cultivation within banana fields would allow local farmers to extend its production augmenting the availability for the local community.
The objective of this study carried out in South Kivu province, eastern DR Congo, was therefore to assess the performance of bush and climbing beans and vegetable amaranth, all sensitive to shading, when intercropped with bananas. The study specifically assessed which banana shade levels allow for reasonable annual crop yields. We hypothesize that the growth of important intercrop species is limited by the light availability under bananas, which will determine annual crop suitability.
2. Materials and Methods
2.1. Study Locations
The research was carried out at Kabare North Territory, South Kivu Province, eastern Democratic Republic of Congo between September 2014 and May 2018. The field trials were conducted at three sites, namely Katana (02°13.427′ S, 028°49.674′ E; 1647 masl), Kavumu (02°17.4′ S, 028°48.24′ E; 1744 masl) and the Institut National pour l’Etude et la Recherche Agronomique at Mulungu (INERA-Mulungu research station; 02°20.042′ S, 028°47.311′ E; 1707 masl). These three sites are located at close proximity (<15 km) to each other. This region is characterized by a mean annual rainfall of 1,656 ± 235 mm (2015–2018), with one main dry season from May till mid-September (monthly rainfall ≤ 150 mm) and a smaller dry season in January and February (monthly rainfall ~150 mm). The mean annual temperature varies between 16 °C and 20 °C. Katana has volcanic-derived granitic soils composed of clay but with a thick humus layer and are generally highly fertile [26,27]. Soils at INERA-Mulungu are volcanic-derived Andosols, which are reasonably fertile [28]. The soils at both tKatana and INERA-Mulungu have the same acidity (pH = 6.8) (Table 1) and are more fertile than the soils at the Kavumu site. Kavumu has slightly acidic heavy clay soils with a pronounced A horizon, with low organic matter, nitrogen and phosphorus levels.

Table 1.
Soil characteristics at Katana, Kavumu and INERA-Mulungu. For each site, soil pH, organic matter (OM), nitrogen content (N), phosphorus content (P) and calcium (Ca), magnesium (Mg) and potassium (K) are presented. The soil data presents average values of samples collected at the onset of the trials in 2014 and at the end of the trials in 2018.
2.2. Field Trials
The trials evaluated the performance of three annual crops, bush bean (‘Hm 21-7′), climbing bean (‘Namulenga’) and vegetable amaranth (‘Kichele’) at each of the three study sites. Each of the three annual crops were planted at the onset of a rainy season and harvested within 4 months. At each site, the crops were intercropped with banana plants at different growth stages and varying spacings/densities. This enabled the assessment of the performance of the annual crops under different shading and light accessibility levels.
For a first experimental trial at Katana, the annual crops were planted in between banana plants of three different spacings (i.e., 2 m × 2 m, 2 m × 3 m and 3 × 3 m), with each treatment replicated thrice. The beer banana cultivar (AAA-EAH, ‘Nshika’) was used at this site. Both banana plants and a first set of annual crops were planted in September 2014. A total of three annual cropping cycles was observed for the annual intercrops, with subsequent annual crops planted in March 2015 and September 2015. With consecutive cropping seasons, the annual crops were rotated with amaranth following bush bean, climbing bean following amaranth and bush bean following climbing bean. Each annual crop plot measured 2.5 m × 6 m (Table S1) and annual crops were planted up to 30 cm from a banana mat. Climbing beans were planted using a spacing of 50 cm × 25 cm and bush beans using 50 cm × 20 cm. Amaranth plants were planted at 15 cm within lines and 50 cm between the lines.
Standard banana field management practices such as de-suckering and de-trashing were adhered to throughout the cropping seasons. De-suckering of banana plants was carried out just before annual crop planting, with three plants retained per mat. In order to reduce shade levels for the intercrop, seven open functional leaves were kept on each banana plant during the annual cropping seasons in order to reduce shade (all excess older leaves were bent at the petiole; following Blomme et al. [29]. Additionally, and in line with farmer’s annual cropping practices and manure scarcity, decomposed goat manure was applied within the amaranth crop (60 t/ha of decomposed manure) at the onset of the second and third cropping cycles. The goat manure was applied in farrows and lightly covered with soil before planting amaranth plants to prevent direct root contact with the manure. One amaranth plant was planted per planting hole. The application of decomposed farmyard manure for bean cultivation is not practiced by farmers in the study region. Accordingly, no organic fertilizer was added in the bush and the climbing bean plots.
A second trial at Katana was performed in a newly established banana plot with a 3 m × 4 m spacing and with plot sizes of 10.5 m × 12 m (Table S1), replicated 3 times. Bananas were established during September 2015 using a mixture of Musa cultivars comprising ‘T6′ (AAB; plantain hybrid), ‘NARITA 2′ (previously ‘NSH20′) (AAA-EAH; cooking banana hybrid), ‘NARITA 27′ (previously ‘NSH42′) (AAA-EAH; beer banana hybrid) and ‘FHIA03′ (AABB; dessert banana hybrid). Irregular de-suckering was carried out before planting the annual crops. Beans and amaranth were planted in September 2015 and March 2016, using the same protocol as in the first trial. A randomized complete block design was used for all experiments across the three sites.
At Kavumu, one experimental trial was established in March 2015 in a newly established banana field (beer banana cultivar; AAA-EAH, ‘Nshika’) with a spacing of 3 m × 4 m. The beans and amaranth were grown in three replicate plots of 2 m × 4 m (Table S1) during four consecutive annual cropping cycles, starting in March 2015. The same protocols for crop planting, crop rotation and field management as in the Katana trials were used.
At INERA-Mulungu, two experimental trials were performed. In the first experiment, only bush beans were assessed during three cropping cycles (planted in March 2017, September 2017 and March 2018) under mature banana fields (cooking banana cultivar; AAA-EAH, ‘Barhabesha’; 3 replicates, field size of 20 m × 23 m) with two different spacings of 2 m × 2 m and 4 m × 4 m. In the second experiment, the three annual crops were assessed during two annual cropping cycles (planted in March 2016 and September 2016) under mature ‘Nshika’ banana plants spaced at 3 m × 4 m in three replicates and fields of 12 m × 21 m (Table S1). The annual crops were grown in plots of 7 m × 6 m and 12 m × 6 m in trials 1 and 2, respectively (Table S1), with 3 replicates each.
At each site, monocrops of the annual crops served as controls. Performance of these crops under monocropping was assessed during most cropping cycles of the intercrops at the three sites. However, note that no monocrops were available at Katana for the first cycle of September 2014 and at Mulungu during the cycles of March and September 2016 due to a lack of fields at the onset of the field trials.
2.3. Measurements
2.3.1. Light Intensity Measurements
An ACCUPAR photometer probe (Model LP-80, Decagon Devices, Pullman, WA, USA; Decagon Devices, 2004) was used to measure the photosynthetically active radiation (PAR, µmol/m2/s) received by the leguminous crops and amaranth under the different treatments. PAR values were assessed at 50 cm from a banana plant and in the center of each plot above the annual crops. Variation in shade intensity across the legume plots was in this way captured. Average PAR values (50 cm from a banana mat + at center of plot) were computed for each treatment and used in subsequent analysis. Measurements were carried out at a height of 30 cm above the annual crops. At least four PAR measurements were taken at legume flowering stage in each legume and amaranth intercrop treatment replicate (giving a total of at least 12 measurements per treatment). For the mono-cropped plots three PAR measurements at a height of 30 cm above the crops were also taken at legume flowering stage at the center of each replicate. Measurements were taken between 11.00 a.m. and 3.00 p.m.
2.3.2. Growth and Yield Assessments
Five bush/climbing bean plants were uprooted from the center of each plot at flowering stage for biomass and root nodule assessment. Roots were separated from the above ground tissue and fresh and dry weight of the aboveground plant biomass was measured. The number of nodules on the roots and their efficiency (red/brown for functional and white for non-functional nodules) were determined. The red/brown coloration of active root nodules is attributed to leghemoglobin that is crucial for symbiotic nitrogen fixation [30].
Fresh and dry legume grain yield was assessed in the net plot (covering both the area with limited and heavy shading and banana root interference) of each treatment (Table S1). Dry weights were obtained by air-drying for 72 h and subsequently drying in an oven (at 90 °C) for at least 48 h.
Amaranth was harvested before flower setting in the net plot of each treatment (Table S1). Amaranth plants were cut at 20 cm stem height during harvesting. Fresh and dry biomass weight was measured for the aboveground biomass, edible plant parts and the non-edible plant parts of the cut off plants.
2.3.3. Soil Sample Collection and Analysis
Composite soil samples were collected at the onset of trials and at the end of a trial from all 3 experimental locations. Soil samples were collected in each annual crop treatment and bulked for all replicates of the same crop species. Soils were sampled using a soil auger from the upper 30 cm soil layer and analyzed at the National Agricultural Research Organization (NARO), Kawanda, Uganda soils laboratory. The soil samples were analyzed using routine analytical methods for soil pH (soil acidity/alkalinity), soil organic matter content (OM), total nitrogen content (N), extractable phosphorus (P) and available bases (K, Ca and Mg). The available cations and available P were extracted using Mehlich 3 extraction method [31] with pH 2.5. Organic matter was analyzed colorimetrically at 600nm using potassium dichromate and sulphuric acid (Walkley Black method); nitrogen was analyzed using sulphuric/selenium digestion mixture, digested at 330 °C and later quantified colorimetrically using salicylate method. pH was read from a 1:2.5 soil—water extract. All the nutrients apart from OM and N analyzed in these soil samples are in available form.
2.4. Data Analysis
In the current study, annual crops were integrated within banana fields with different planting densities, canopy traits, growth stages, varying levels of de-suckering and leaf pruning. To cater for these differences, PAR was considered as the primary variable for comparison of the treatments. Analysis of variance was only carried out to compare within site treatments using the GenStat v. 11 statistical software [32]. The Least Significant Difference test at 5% probability level was used for mean separation. A simple linear regression model with PAR as the independent variable and grain or biomass yields as the dependent variables was used to determine the relationship between mean PAR and the grain/biomass yields within experiments using GenStat v. 11.
Three replicates were used across treatments and seasons, with yield and biomass from each replicate representing a data point in the regression analysis. For each regression analysis, 6 to 12 data points were used depending on the number of seasons included, if the analysis focused on comparing treatment performance across banana planting densities within one season or on comparing performance across seasons for one planting density, (Table S2).
3. Results
Both the bean and amaranth crops were able to achieve reasonable yields in young and sparsely spaced banana fields. Across sites, significant reductions in yields of the intercrops as compared to the monocrops were observed across consecutive cropping cycles as the banana field matured and the leaf canopy closed. This effect was stronger in more densely spaced banana fields. A high variability in legume and amaranth yields was also observed across sites and a uniform relation between available PAR under the banana canopy and the attainable yields was not observed. Accordingly, crop performance under various shading-levels showed a high site-dependence.
3.1. Bean Crop Performance across Sites
3.1.1. Katana 1
At Katana and in a first trial, a significant effect of banana spacing/plant densities and the age of the banana plants on the yields of legume intercrops (Figure 1 and Figure S1) was observed. During the first cropping cycle (September 2014) in which both bush and climbing beans were planted simultaneously with the banana plants, available PAR 50 cm from the banana plant was reduced to 60–70% in the 2 × 2 m formation, while available PAR ranged between 65–100% in the 2 × 3 m and 3 × 3 m plots compared to the monocrop.
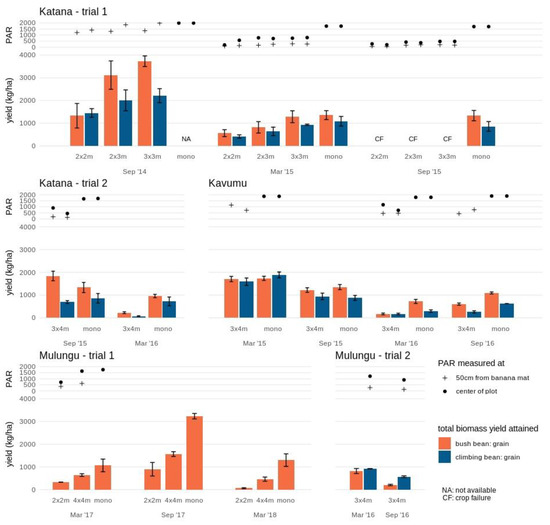
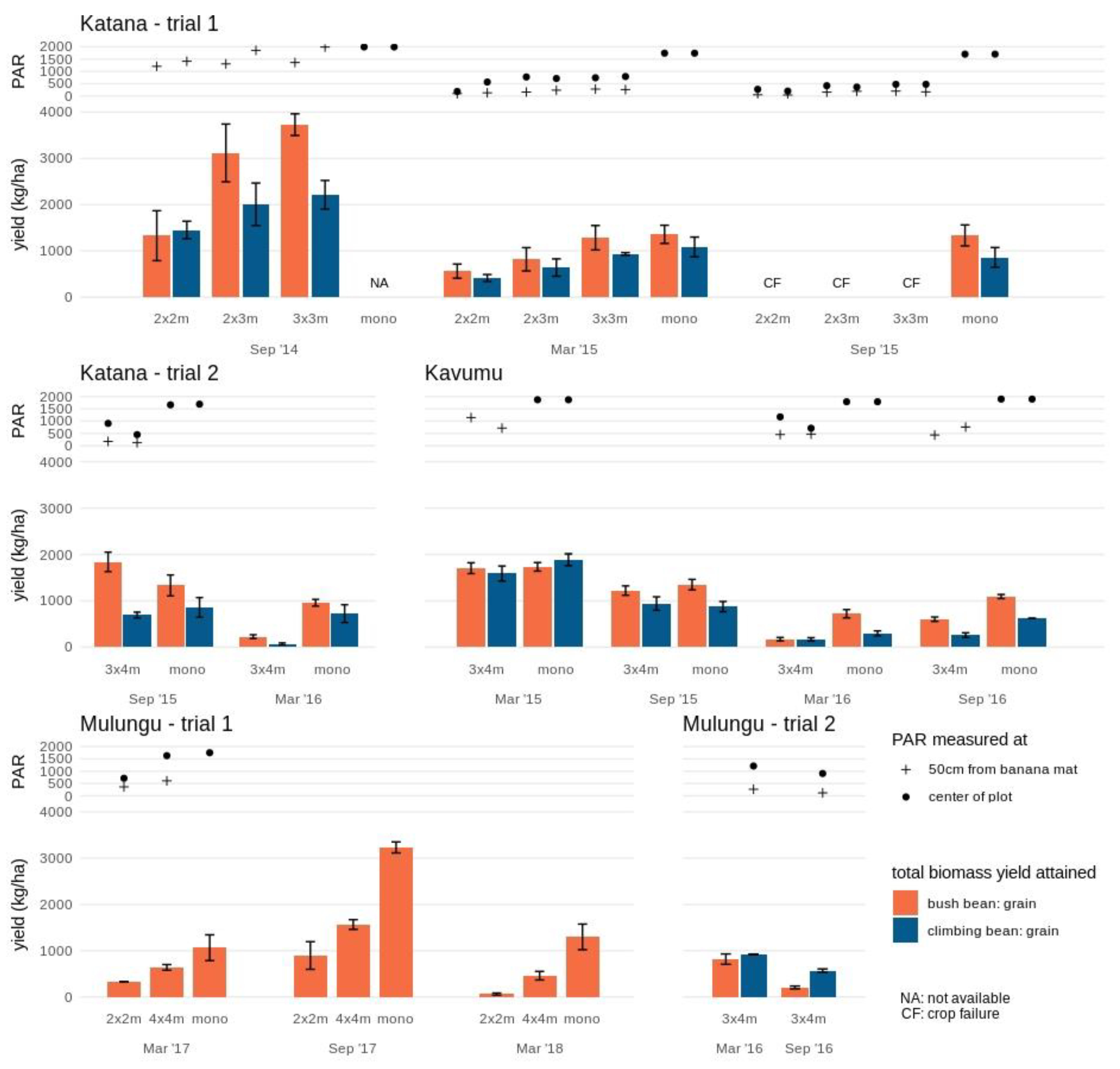
Figure 1.
Crop performance of bush bean and climbing bean in various intercrop formations and monocrops at the study sites Katana, Kavumu and Mulungu. Grain yield is indicated separately for bush bean (orange) and climbing bean (blue) at each intercrop formation and cropping season. At Katana, the crop failure (CF) in September 2015 is indicated. The annual crop in monocrop formation is not assessed (NA) at Katana in September 2014 and not available in trial 2 at Mulungu. Photosynthetic active radiation (PAR) measured at the center of the plot (full circle) and 50 cm from the banana mat (cross) is indicated for each annual intercrop and monocrop. Note that during some stages of the experimental trial PAR measurements are not available due to temporary equipment defects.
Accordingly, for bush bean a significantly lower grain yield was achieved during this first cropping cycle in the dense 2 × 2 m plots (1.3 ± 0.5 t/ha) compared to the more open 2 × 3 m and 3 × 3 m (3.1 ± 0.6 t/ha and 3.7 ± 0.2 t/ha, respectively) plots. Grain yield of the climbing bean was also lower in the 2×2m plot (1.5 ± 0.2 t/ha) compared to the more open 3 × 3 m plot (2.2 ± 0.3 t/ha; Figure 1). In this first annual cropping season, PAR only explained 18% and 38% of bush and climbing bean grain yields, respectively (Table S2). Total aboveground biomass yield remained equally high across banana plot densities for both bush (ranging from 5.7 ± 0.7 to 6.5 ± 0.8 y t/ha) and climbing (ranging from 4.0 ± 0.4 to 4.3 ± 0.6 t/ha) beans. PAR explained 7 and 12% of the variation in bush and climbing bean biomass yields, respectively.
During the second cropping season (March 2015), the maturing banana crops already significantly reduced the available PAR in the center of the plot with ~37–45% PAR retained in the 3 × 3 m and 2 × 3 m plots and only ~9–32% PAR in the 2 × 2 m plots compared to the monocrop. Spatial variability in PAR within the plots was observed, with even further reductions closer to the banana mats (on average 66% lower compared to the center of the plot). Accordingly, significant reductions in grain yields were found for both legumes. Compared to the grain yields in monocrop (1.4 ± 0.2 t/ha and 1.1 ± 0.2 t/ha for bush and climbing beans), bush bean grain yields declined by 59% and 40% in the 2 × 2 m and 2 × 3 m plots, while yields in the 3 × 3 m plots remained high (7% and a non-significant reduction). Grain yields for the climbing beans showed a greater reduction of 70% in the 2 × 2 m plots and 53% and 31% in respectively, the 2 × 3 m and 3 × 3 m plots. PAR accounted for 53–63% (R2 = 0.53–0.63) of the observed grain yield decline (Table S2). Similar trends in biomass yields to that of the grain yields were observed (Figure 1 and Figure S1) with PAR explaining 54–79% of the decline.
A third cropping cycle in September 2015 was unsuccessful for both legumes within the three banana intercropping formations. The PAR had reduced to ~14–30% across the banana plots compared to the monocrop, accounting for 94–98% of the grain and biomass yield reduction. Across all three seasons and within planting densities, PAR explained 67% to 94% of bush bean grain and biomass yield reductions compared to between 41% and 83% for the climbing beans (Table S2).
3.1.2. Katana 2
The second experimental trial in Katana albeit with a higher spacing (3 × 4 m) showed a similar pattern of reduced yields as the banana plants matured. During the first cropping cycle (September 2015) when the banana plants were small the climbing beans reached an equally high grain yield (0.7 ± 0.1 t/ha) in the 3×4m plot as compared to the yield in monocrop (0.9 ± 0.2 t/ha), while the bush beans even had a 38% higher grain yield (1.8 ± 0.2 t/ha compared to 1.3 ± 0.2 t/ha in monocrop) (Figure 1). However, during the second cycle (March 2016), both bush and climbing beans showed significant (P < 0.05) reductions in grain yield compared to the monocrop, with only 24% and 10% grain yield retention for bush and climbing beans, respectively. Across all the seasons, PAR explained 68 and 60% of the grain yields and 94% and 50% of the biomass yields in bush and climbing beans, respectively (Table S2).
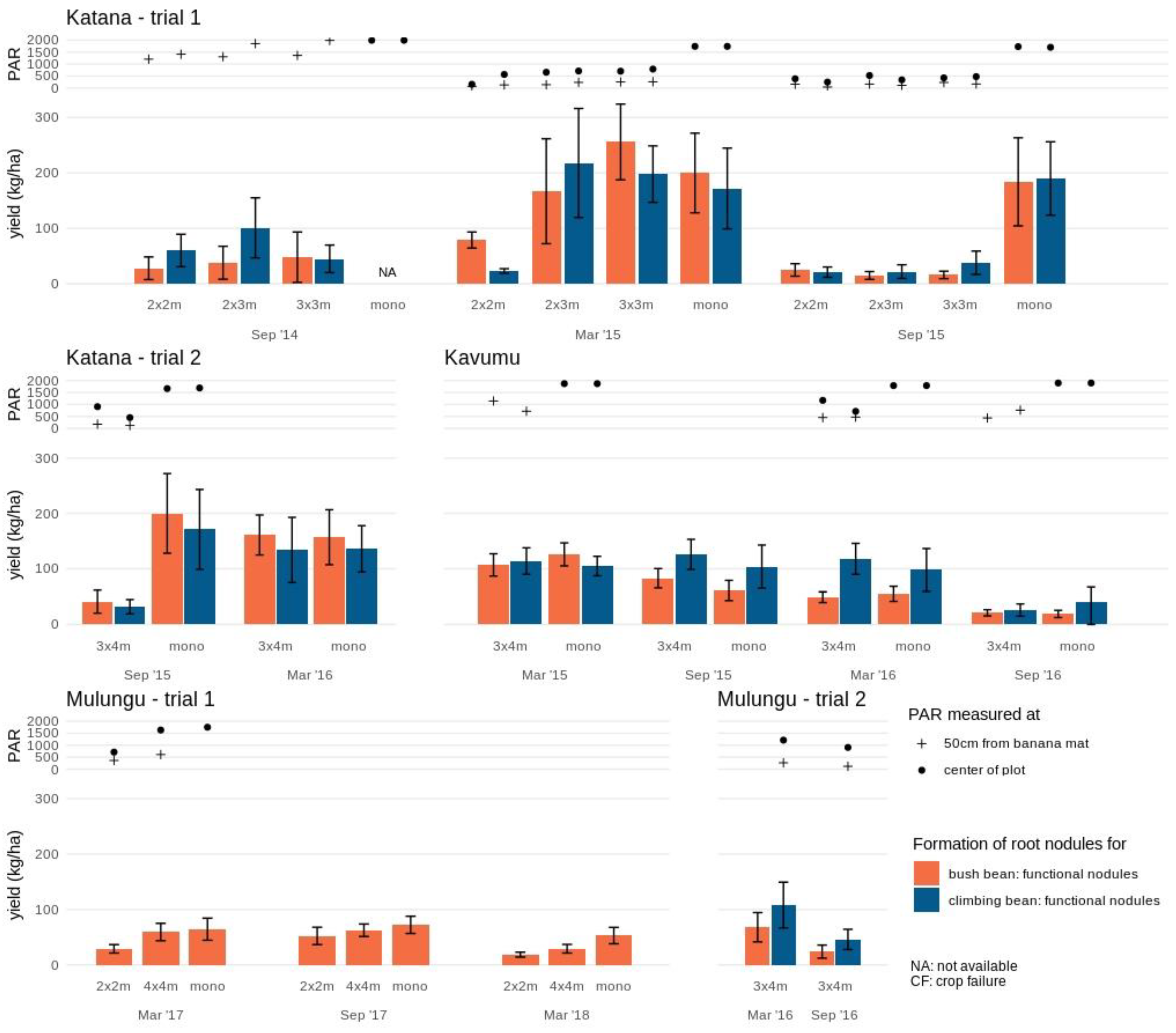
Nodule formation (Figure 2 and Figure S2) however did not follow the same patterns as aboveground yields in both experimental trials at Katana. During the first cropping cycle, when PAR levels and aboveground yields were high, only a limited number of functional root nodules were formed in each intercrop formation (2 × 2 m up to 3 × 4 m). In trial 2, the nodule formation for bush beans and climbing beans, respectively, was 21% and 19% of 200 ± 72 and 171 ± 72 per plant observed in the monocrop during this first cropping cycle (for September 2015). In trial 1 similarly low numbers of functional root nodules were formed during the first annual cropping season in each intercrop formation (2 × 2 m, 2 × 3 m and 3 × 3 m), with ~15–25% and ~25–56% of the mean root nodules per plant in the bush bean (i.e., 192 ± 54) and climbing bean (i.e., 181 ± 50) monocrops observed, respectively. During the second cropping cycle with lower PAR and aboveground yields, a high number of functional root nodules (comparable to the monocrop) was formed in the 2 × 3 m, 3 × 3 m and 3 × 4 m plots, whilst values remained low in the 2 × 2 m plots (40% and 13% of the monocrop yield in the bush and climbing beans, respectively). No strong associations (R2 = 0.09–0.25) were observed between number of root nodules formed for both bush and climbing beans with PAR levels, grain and aboveground biomass. During the third cropping cycle of the first trial, a small number of nodules were still formed, even though the bean crops attained no aboveground yield.
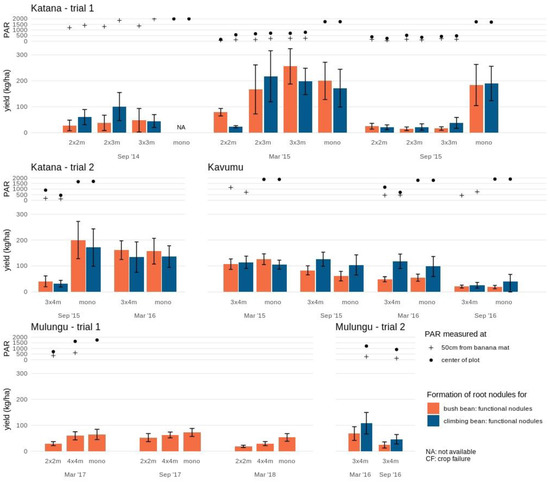
Figure 2.
Root nodule formation of bush bean and climbing bean in various intercrop formations and monocrops at the study sites Katana, Kavumu and Mulungu. Functional nodules are indicated separately for bush bean (orange) and climbing bean (blue) at each intercrop formation and cropping season. The annual crop in monocrop formation is not assessed (NA) at Katana in September 2014 and not available in trial 2 at Mulungu. Photosynthetic active radiation (PAR) measured at the center of the plot (full circle) and 50 cm from the banana mat (cross) is indicated for each annual intercrop and monocrop. Note that during some stages of the experimental trial PAR measurements are not available due to temporary equipment defects.
3.1.3. Kavumu
At Kavumu, both legumes grown as monocrops showed significant decreases in grain yield with consecutive cropping cycles, ranging from 1.9 ± 0.1 ton/ha to 0.30 ± 0.05 ton/ha for climbing beans and from 1.8 ± 0.1 ton/ha to 0.7 ± 0.1 ton/ha for bush beans (Figure 1). The yield reduction in the mono-cropped plots could be attributed to the close proximity of the monocrops to the banana fields. As the banana plants matured, the monocrop plots experienced partial shading from the neighboring banana plots. During the first two cropping cycles (March and September 2015) both legumes grown intercropped in 3×4m banana spacing plots attained similar grain yields as the monocrops. In addition to the wide spacing, soils in Kavumu are less fertile (low OM, N and K) and the banana plants grew relatively slowly during 2015, resulting in a slower increase in shade level.
During the third cropping cycle however, the yield of bush bean showed a significant reduction of 76% compared to the monocrop, while this reduction was limited to 45% in the fourth cycle. The climbing bean showed a significant but lower yield reduction of 44–58% in the last two cropping cycles compared to the monocrop. PAR explained 20% to 30% and 28% to 39% of the observed variation in grain and biomass yields, respectively (Table S2).
Nodule numbers in both the bush bean monocrop and intercrop (3 × 4 m) reduced with consecutive cropping cycles, with a steady decrease from 126 ± 20 to 19 ± 6 functional nodules per plant observed for the monocrop (Figure 2). The climbing bean nevertheless did attain a high number of nodules for the first three consecutive cycles in both intercrop and monocrop (ranging 98 ± 39–126 ± 27 functional nodules per plant), which reduced during the last cycle (26 ± 11–41 ± 26 functional nodules per plant).
3.1.4. INERA-Mulungu
At Mulungu trial 1, grain yields for bush beans significantly declined in both the 2 × 2 m and 4 × 4 m intercrops of mature banana fields (Figure 1). In the 2 × 2 m plots, grain yield was reduced by 69% to 95% (depending on cropping cycle) compared to the monocrop, while in the 4 × 4 m plots grain yield declined by 40% to 65%. In the 2 × 2 m plots, grain yield did vary spatially within the plots, with limited yield retained closer to the banana mat. This spatial variation was not significant in the 4 × 4 m plots. Moreover, a significant change in yield according to cropping cycle was found. Even though similar PAR values are expected since banana plants were mature from trial initiation, a higher yield was attained during the cropping cycle of September 2017 as compared to those in March 2017 and 2018, both in monocrop and in intercrop formations. This higher yield for crops planted in September could have resulted from higher and more uniformly distributed rainfall over this cropping cycle (Sept-Dec) as compared to the second cycle (March-May). PAR accounted for 79% to 90% and 78% to 91% of the variation in bush bean grain and biomass yields, respectively within the different cropping seasons. Across the seasons, PAR accounted for 50% to 64% and 21% to 49% of the grain and biomass yields, respectively (Table S2).
At Mulungu trial 2, whilst no comparison is available with monocropping, the 3 × 4 m intercropped bush beans and climbing beans reached similar grain yields of 0.8 ± 0.1 and 0.92 ± 0.01 ton/ha during the first cropping cycles. Significant grain yield reductions of 75% and 38% were recorded for bush and climbing during the second cropping cycle. Accordingly, PAR values were already reduced by 28% during this second cropping cycle.
Nodule formation on average was lower at the INERA-Mulungu site compared to Katana and Kavumu (Figure 2 and Figure S2), with an average of 63 ± 3 functional nodules per plant in monocrop in trial 1. The 4 × 4 m spaced banana fields had a higher number of root nodules compared to the 2 × 2 m fields across all seasons. The number of functional root nodules increased in the second cropping season before declining in the third season for both the 2 × 2 m and 4 × 4 m plots. An 8–45% reduction in root nodules relative to the monocrop was observed for the 4x4m plot across the seasons compared with a 28–65% reduction for the 2 × 2 m plots.
At Mulungu trial 2, whilst no reference comparison in monocrop was available, functional nodule formation in the 3 × 4 m intercrop reduced for both bush and climbing beans from the first to the second cropping season by 65% and 57%, respectively in response to a 28% reduction in PAR. The climbing beans did show a higher number of functional nodules compared to the bush beans.
3.2. Amaranth Crop Performance across Sites
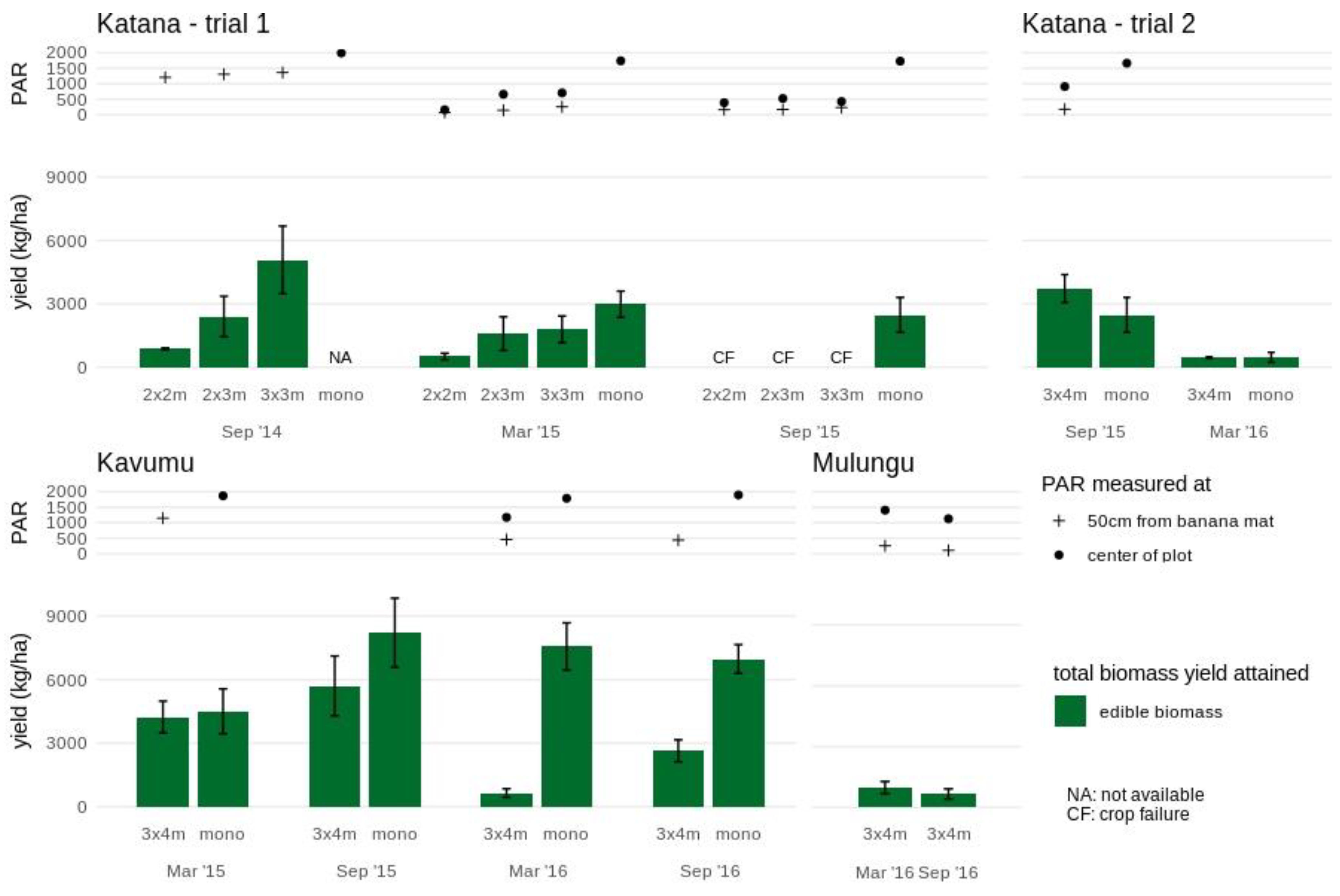
Amaranths yields were generally lower at Katana compared with Kavumu (Figure 3 and Figure S3). In monocrop, an average yield of edible leaves of 2.7 ± 0.1 ton/ha was found. The crop performance was significantly lower in the 2 × 2 m intercrop even during the first two cropping cycles, with an average yield reduction of 75% (average edible yield of 0.7 ± 0.1 ton/ha). Amaranths yields were higher in plots with an increased banana spacing. During the first cropping cycle (September 2014), edible yields attained in the 3 × 3 m and 2 × 3 m intercrops (5.1 ± 1.6 ton/ha and 2.4 ± 1.0 ton/ha, respectively) were similar to the average monocrop yields. During the second cropping cycle (March 2015), edible yields in the 3 × 3 m intercrop reduced to 1.8 ± 0.6 ton/ha which, although lower, did not significantly different from average monocrop yields. On the contrary, the edible yield in the 2 × 3 m intercrop of 1.6 ± 0.8 ton/ha was significantly lower than the monocrop yield. The amaranth crop failed during the third cropping cycle (September 2015) across all intercrops (2 × 2 m, 2 × 3 m and 3 × 3 m) potentially related to the closing of the banana canopy.
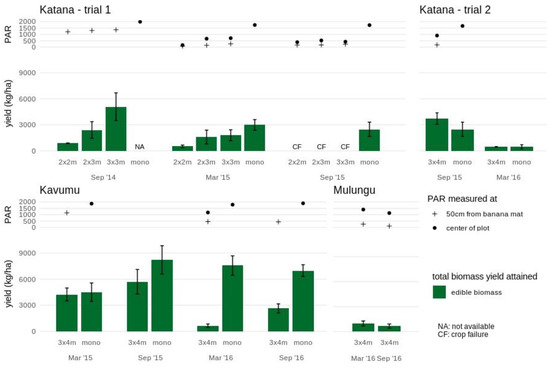
Figure 3.
Crop performance of amaranth in various intercrop formations and monocrops at the study sites Katana, Kavumu and Mulungu. Edible aboveground biomass yields are indicated for each intercrop formation and cropping season. At Katana, the crop failure (CF) in September 2015 is indicated. The annual crop in monocrop formation is not assessed (NA) at Katana in September 2014 and not available in trial 2 at Mulungu. Photosynthetic active radiation (PAR) measured at the center of the plot (full circle) and 50 cm from the banana mat (cross) is indicated for each annual intercrop and monocrop. Note that during some stages of the experimental trial PAR measurements are not available due to temporary equipment defects.
In the 3 × 4 m intercrop trial at Katana, lower amaranth yields were attained in the second cropping cycle (March 2016) in both the monocrop and the 3 × 4 m intercrop. Additional environmental circumstances other than light reduction may have contributed to the reduced yield.
The highest yields of amaranth were attained in the monocrop plot in Kavumu (Figure 3). The initial high edible yield of 4.5 ± 1.1 ton/ha achieved during the first cropping season in monocrop even increased significantly during the subsequent three cropping seasons to an average of 7.6 ± 0.2 ton/ha. In the 3 × 4 m intercrop, a similar edible yield of 4.3 ± 0.7 ton/ha was achieved as the monocrop during the first cropping cycle. During the second cropping cycle, the edible yield remained equally high (5.7 ± 1.4 ton/ha), although the increase as seen in the monocrop was not found. During the third cropping cycle, the edible yield dropped to 0.6 ± 0.2 ton/ha in the 3 × 4 m intercrop, in which a 35% reduction of PAR compared to the monocrop was also recorded. The edible yield remained low during the fourth cropping cycle (2.6 ± 0.5 ton/ha, not significantly different from the low yield in the third cycle) during which further reductions in light availability accompanying the maturing of the banana could be assumed.
In Mulungu, relatively low amaranths yields were achieved in the intercrop in the mature 3 × 4 m banana fields when compared to intercrops in the younger banana fields in both Kavumu and Katana (Figure 3). The amaranth edible yield was similar during the two cropping cycles (1.0 ± 0.3 ton/ha and 0.7 ± 0.2 ton/ha).
4. Discussion
Intercropping of banana with different annual crops is common to ensure optimal use of the available land space by smallholder farmers in East and Central Africa. Banana shade is a major limiting factor to this practice in the region. In this study we investigate the performance of climbing and bush beans and the vegetable amaranth under various banana shading levels.
4.1. Beans Intercrop Performance
Intercropping herbaceous legumes in young and sparsely spaced banana fields often works well and is a common practice by small-holder farmers in Eastern DR Congo [11,19]. Indeed, in this study both climbing and bush beans had high grain and biomass yields (c.f. Figure 1 and Figure S1) during the first cropping cycle when they were planted simultaneously with the banana crop. The beans intercrop yields declined with subsequent cropping seasons as the shade levels increased. These findings are consistent with other studies [6,11,20], that showed increasing banana shade intensity to affect the performance of a range of legume crops including beans. Competition for nutrients and water in addition to light could have also contributed to the above-mentioned yield declines [6]. Within each trial, legume crop performance decreased more rapidly with consecutive cropping cycles in more densely spaced banana fields. This was most clear in the first trial at Katana, where reductions in legume crop yields were most severe across cropping cycles in the 2 × 2 m fields, compared to the 2 × 3 m and the 3 × 3 m fields.
At this site, the banana canopy developed rapidly due to the high soil fertility.
In this trial, all three planting densities attained zero yields in the third cropping season. Eliakira et al. [33] also reported grain yield of beans intercropped with maize to vary with plant density and spatial arrangement of the crops. In the first trial at Katana, all three planting densities attained zero yields in the third cropping season. Thus, for farmers with smaller land holding and to whom intercropping with beans is inevitable for meeting their nutritional needs, lower banana planting densities (or larger crop spacings) are recommended to enable intercropping for multiple seasons. Other more shade-tolerant annual crop species and varieties (e.g., Taro—Colocasia esculenta; Yam—Dioscorea sp., Chili pepper—Capsicum annuum; and Mucuna—Mucuna pruriens) could also be targeted for integration into banana systems once large leaf canopies have been developed [4,34]. The climbing beans generally performed better than the bush beans from one cropping season to another, possibly due to their more robust nature and their ability to climb higher on staking materials or the banana plants to access light that was limiting in the later seasons. This is consistent with findings of Ocimati et al. [6] that showed climbing beans to offer a higher competition to the banana crops. Thus, a manipulation of both the banana spacing density with integration of different climbing bean types on varying lengths of staking materials could be explored for effective banana-climbing bean intercropping.
The performance across annual cropping seasons was also strongly influenced by the characteristics of the soils at the different trial locations and the banana growth traits. Kanyenga et al. [35] reported differences in the growth habit of intercrops and variations in environmental factors (soil and rainfall), from site to site and from one cropping season to another. The soils at Katana and Mulungu were, respectively more fertile than the soils at Kavumu (Table 1). Thus, banana plants at Katana and Mulungu were more robust while growth and formation of canopy was slower at Kavumu, resulting in slower yield declines at Kavumu, even for the same planting densities. In addition, the ‘Nshika’ cultivar grown at Kavumu has a smaller canopy structure compared to the mixed cultivars in the 3 × 4 m Katana plot. A successful integration of beans (that are sensitive to light) in banana over several annual cropping seasons will therefore require the identification of more optimal combinations of banana planting densities for different soil conditions, banana cultivars (as influenced by their morphological and growth traits) and their management practices (e.g., leaf pruning and de-suckering) and bean cultivars (of varying traits) and their management (e.g., staking vs. no staking). For example, under highly fertile soil conditions a 3 × 4 m banana mat spacing with regular de-suckering to maintain three to four plants per mat is advised for the integration of shade-sensitive annual crops. Whereas in poor soils a 3 × 3 m (and above) banana mat/plant spacing in combination with sucker management is recommended. These banana spacing arrangements allow for annual crop growth over at least three annual cropping seasons.
4.2. Nodule Formation and Soil Fertility
The number of active root nodules formed in legumes had no strong association with the amount of PAR received and was not correlated to grain and biomass yields. This suggests a potential role of several factors in influencing nodule formation including the effect of shade and site characteristics. A broad range of factors including soil acidity, water, drought stress, nutrition, light availability have been reported to affect root nodulation and N fixation [36,37]. At Katana and Mulungu functional root nodules were observed to increase and thereafter decline with increasing banana canopy cover while there was a general decline with subsequent seasons for bush beans and a near constant nodulation for climbing beans at Kavumu over the first three seasons.
The initial low nodule formation (during the first cropping cycle) across all banana plant densities at Katana and Mulungu compared with Kavumu can be attributed to the difference in soil attributes and the production history of the fields. The Mulungu and Katana sites were more fertile with a higher soil nitrogen content compared to the Kavumu site (cf. Table 1) and the high initial N availability could have reduced the need for nodule formation. High amounts of plant available soil nitrogen have been reported to decrease the number of root nodules and N fixation [36,37,38,39]. McKenzie et al. [40] also reported low soil pH to have a negative effect on nodule formation and nodule efficiency. Though soils at Katana and Mulungu had a higher soil pH (i.e., 6.8) compared to Kavumu with a pH of 6.1 (cf. Table 1), these pH values fall within the optimum range of 6.0 to 7.5 for bean production [41,42]. Thus, the observed differences were not due to differences in soil pH. The site at Mulungu recorded the least number of root nodules across seasons compared with Katana and Kavumu and this could possibly be partially explained by the land-use history of the fields. Hultman [37] observed a higher root nodulation in fields with a previous history of legume production compared to fields with no such previous history. The field at Mulungu was previously cropped to banana monocrops whereas beans had been previously planted at the Katana and Kavumu sites.
Nodule formation did increase significantly during the second cropping cycle. Here, an increased N intake from the banana crop and a subsequent reduction in plant available N in soil could have stimulated the formation of N-fixing nodules in the subsequent second cropping cycle. During the third cropping cycle, nodule formation dropped significantly, possibly due to the significant decline in light reaching the legume crops. This can be supported by the fact that the canopy at the Kavumu site that had a lower soil fertility level closed more slowly compared to Katana and Mulungu, as such resulting in a more gradual decline in root nodules with increasing shade level in the bush bean crop at Kavumu. In climbing beans, a similar number of nodules in intercrop as in monocrop was observed in the second and third seasons, possibly due to their robust nature and ability to climb the stakes to access more light. During the fourth cropping cycle at Kavumu (the only site where a fourth cycle was assessed) a reduced number of nodules was however formed in the climbing beans possibly due to an overall increase in soil N content and shading. In maize-legume intercropping, increased shading has been reported to reduced nodule formation [43]. Schubert [36] also reported light deficiency to negatively affect nitrogen fixation. Deprivation of N and a high irradiance has been reported to enhance root growth at the expense of the above ground growth [44]. Several studies [36,45,46] have shown a significant decline in root nodulation in different legumes species under shaded conditions. Chu and Robertson [45] also observed a reduction in nodule weight in white clover (Trifolium repens L.) under shade, due to dominance of empty nodule ‘hulls,’ that is, nodules not colonized by Rhizobia spp.
4.3. Amaranth Intercrop Performance
The investigated variety of vegetable amaranth showed good potential to be intercropped in sparsely spaced (2 × 3 m and wider spaced) newly established banana fields and yields comparable to the monocrop could generally be achieved during one or two cropping cycles when the banana spacing was not limiting (c.f. Figure 3). This indicates the potential to expand crop production of the light-demanding amaranth crop beyond the general production in open fields [25]. However, beyond the second season, shading significantly reduced amaranth yield across the study sites. These findings are consistent with that of Wadud et al. [47] who explored the suitability of red amaranth (Amaranthus gangeticus) for inclusion in agroforestry systems. Wadud et al. [47] observed any reduction in PAR to negatively affect yield and all morphological traits. They observed a 75%, 50% and 25% decline in PAR to reduce amaranth yield by 75%, 55% and 25%, respectively. Reductions were also observed in number of leaves, leaf size and stem girth while the proportion of the stem to the leaves increased, thus concluding that amaranth was unsuitable for tree-agroforestry systems. Similar findings were reported by Ufoegbune and Eruola [48], who observed significant reductions in plant height, number of leaves, leaf width and overall yield of amaranths under shaded conditions in comparison with open spaces.
The higher declines in crop yields in dense banana fields in the current study and severe yield reductions under the maturing banana fields confirm that amaranth is highly sensitive to shading. Thus, its integration under the banana crop is only recommended during the first two seasons when the banana canopies have not closed. Yield variability was also observed across sites. The higher yields in the less fertile soils at Kavumu (cf. Table 1) compared to Katana and Mulungu could be attributed to the slower growth rate and delayed canopy cover formation at Kavumu. With amaranth leaf yields being highly variable across sites, the yields achieved in monocrop at each site could already give an indication of the expected and attainable yields in young and/or sparsely spaced banana fields with limited shade levels.
4.4. Variability in Crop Performance across Sites
In the current study, annual crops were integrated within fields of banana plants with different planting densities, canopy traits, growth stages, varying levels of de-suckering and leaf pruning. For example, the level of de-suckering will highly affect the canopy openness and shade-level of the field. At Katana in the mixed banana cultivar field (3 × 4 m), no regular de-suckering of the banana mats was performed which allowed the mats to become larger and consequently increasing shade levels and reducing the performance of the annual intercrops to values lower than that in the 3 × 3 m plots that had been regularly de-suckered. Similarly, moderate leaf pruning at the onset of intercropping, a common practice in the region, increases light availability for the intercrop and improving the agronomic efficiency of the system [6,11]. To cater for these differences, PAR was considered as the primary variable for comparing against treatments.
Although the study primarily focused on the effect of PAR on the performance of the annual crops, high variability was observed in bean and amaranth establishment and performance across the different sites even under similar PAR levels. This can be attributed to the differences in soil characteristics and competition with the banana crop for nutrients. Overall, soils at Katana were more fertile compared with intermediately fertile and poor soils at INERA-Mulungu and Kavumu, respectively (Table 1). The banana canopy at Katana, was observed to form more rapidly due to the higher soil fertility, resulting in a more rapid decline in the performance of the annual crops. In contrast, banana plants at INERA-Mulungu were already mature and thus could have offered a higher competition for soil nutrients. In contrast to Katana, banana growth was slow at the less fertile Kavumu site, resulting in a slower decline in annual crop yields.
The beans showed less variability across sites. With the legume crops being (slightly) more shade-tolerant than amaranth, they could cope with small increases in competition for light related to developing banana plants. Additionally, legumes were able to perform better on the less fertile soils compared to other annual crops possibly due to their ability to biologically fix nitrogen.
5. Conclusions
Both grain legumes (climbing bean and bush bean) and vegetable amaranth can achieve reasonable yields when intercropped in newly established banana fields, irrespective of the plant density. However, as of the second annual cropping season, yield declines are observed with increasing canopy formation, with higher declines in the denser 2 × 2 m and 2 × 3 m banana plots. Higher declines also occur under high soil fertility conditions and for banana cultivars with a more robust structure. The choice of banana spacing will thus depend on farmers objectives, soil conditions and type of banana crop. For example, for highly fertile soils, a banana plant spacing of 2 × 2 m and 2 × 3 m is not recommended for banana-legume intercropping whereas similar spacings can return favorable yields under less fertile soils. For the high-density banana crop and later banana crop cycles, a shift to more shade-tolerant crops (e.g., taro, cocoyam and yam) is advisable [34]. In addition, management practices, such as timely de-suckering and cutting/bending of excess outer leaves can also help to prolong the time-window for intercropping with shade-sensitive annual crops. The vegetable amaranth was observed to be more sensitive to shade than the bush and climbing beans. Its cultivation could thus be limited to the first annual cropping season or to less dense banana fields. For small-holder farmers in Central and East Africa, where banana-legume intercropping is crucial for soil restoration and ecosystem stability and meeting household nutritional needs, targeted intercropping with beans beyond the first and second seasons is highly recommended. Studies to explore the interactions of the banana-legume intercrops with different soil scenarios, banana cultivars with varying growth and canopy characteristics and management options is recommended for optimal banana-legume intercropping. In this study, one variety of each of the three annual crops was evaluated. The evaluation of a wider range of bean and amaranth varieties could potentially pinpoint more shade-tolerant varieties and is hence recommended. The evaluation of staking arrangements under different shade levels for the climbing bean types is also recommended.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4395/11/2/368/s1, All data of crop yields and legume formation can be found in detail in Supplementary Information. Figure S1: Crop performance of bush bean and climbing bean in various intercrop formations and monocrops at the study sites Katana, Kavumu and Mulungu. The total aboveground biomass yield represents the sum of the grain and non-edible aboveground yield and is indicated separately for bush bean (orange) and climbing bean (blue) at each intercrop formation and cropping season. At Katana, the crop failure (CF) in September 2015 is indicated. The annual crop in monocrop formation is not assessed (NA) at Katana in September 2014 and not available in trial 2 at Mulungu. Photosynthetic active radiation (PAR) measured at the center of the plot (full circle) and 50 cm from the banana mat (cross) is indicated for each annual intercrop and monocrop. Note that during some stages of the experimental trial PAR measurements are not available due to temporary equipment defects, Figure S2: Root nodule formation of bush bean and climbing bean in various intercrop formations and monocrops at the study sites Katana, Kavumu and Mulungu. Non-functional root nodules are presented for bush bean (orange) and climbing bean (blue) at each intercrop formation and cropping season. The annual crop in monocrop formation is not assessed (NA) at Katana in September 2014 and not available in trial 2 at Mulungu. Photosynthetic active radiation (PAR) measured at the center of the plot (full circle) and 50cm from the banana mat (cross) is indicated for each annual intercrop and monocrop. Note that during some stages of the experimental trial PAR measurements are not available due to temporary equipment defects, Figure S3: Crop performance of amaranth in various intercrop formations and monocrops at the study sites Katana, Kavumu and Mulungu. The total aboveground biomass yield represents the sum of the edible and non-edible aboveground biomass. All yields are indicated for each intercrop formation and cropping season. At Katana, the crop failure (CF) in September 2015 is indicated. The annual crop in monocrop formation is not assessed (NA) at Katana in September 2014 and not available in trial 2 at Mulungu. Photosynthetic active radiation (PAR) measured at the center of the plot (full circle) and 50 cm from the banana mat (cross) is indicated for each annual intercrop and monocrop. Note that during some stages of the experimental trial PAR measurements are not available due to temporary equipment defects, Table S1: Experiment description of all inter- and mono-cropped trials. ‘L,’ ‘H,’ ‘M’ and ‘N,’ respectively denote low, high, moderate and no shade. ‘M’ denotes monocrop, Table S2: The regression (R2) values between Photosynthetically Active Radiation (PAR) with (i) grain yields and (ii) biomass yields of bush and climbing beans.
Author Contributions
G.B. and W.O. conceived and developed the research concept, contributed to data analysis and contributed to writing and editing the manuscript. J.N. contributed to the development of the research concept, conducted the field experiments, collected and analyzed the data and contributed to manuscript writing and editing. E.K. supported data analysis and interpretation and the writing and editing of the manuscript. N.S., L.B. and D.A. contributed to shaping the research concept, supported the establishment of experiments, data collection and data interpretation. A.K.L. and B.W. contributed to data interpretation and manuscript editing. G.B. acquired funding for the study. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by funds from the Directorate General for Development, Belgium through the Consortium for Improving Agriculture-based Livelihoods in Central Africa (CIALCA) and from the CGIAR Research Program on Roots, Tubers and Bananas and the CGIAR Fund Donors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available upon request.
Acknowledgments
We would like to thank the Directorate General for Development, Belgium for funding this research through the Consortium for Improving Agriculture-based Livelihoods in Central Africa (CIALCA). We would also like to acknowledge the financial contribution of the CGIAR Research Program on Roots, Tubers and Bananas and the CGIAR Fund Donors.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as or result in a potential conflict of interest.
References
- Jagwe, J.; Ouma, E.; van Asten, P.; Abele, S. Banana Marketing in Rwanda, Burundi and South Kivu CIALCA Project Survey Report. 2014. Available online: https://test.cialca.org/wp-content/uploads/2018/06/6.-Banana_market_report.pdf (accessed on 15 January 2020).
- Ocimati, W.; Karamura, D.; Rutikanga, A.; Sivirihauma, C.; Ndungo, V.; Ntamwira, J.; Kamira, M.; Kanyaruguru, J.P. Agronomic practices for Musa across different agro-ecological zones in Burundi, eastern Democratic Republic of Congo and Rwanda. In Banana Systems in the Humid Highlands of sub-Saharan Africa: Enhancing Resilience and Productivity; CABI Publishing: Wallingford, Oxfordshire, UK, 2013; pp. 175–190. [Google Scholar]
- Tinzaara, W.; Stoian, D.; Ocimati, W.; Kikulwe, E.; Otieno, G.; Blomme, G. Challenges and opportunities for smallholders in banana value chains. In Understanding the Behaviour and Improving the Welfare of Pigs; Burleigh Dodds Science Publishing: Cambridge, UK, 2018; Volume 1, pp. 65–90. [Google Scholar]
- Blomme, G.; Ocimati, W.; Groot, J.; Ntamwira, J.; Bahati, L.; Kantungeko, D.; Remans, R.; Tittonell, P. Agroecological integration of shade- and drought-tolerant food/feed crops for year-round productivity in banana-based systems under rain-fed conditions in Central Africa. Acta Hortic. 2018, 41–54. [Google Scholar] [CrossRef]
- Okumu, M.O.; van Asten, P.J.A.; Kahangi, E.; Okech, S.H.; Jefwa, J.; Vanlauwe, B. Production gradi-ents in smallholder banana (cv. Giant Cavendish) farms in central Kenya. Sci. Hortic. 2011, 127, 475–481. [Google Scholar] [CrossRef]
- Ocimati, W.; Ntamwira, J.; Groot, J.; Taulya, G.; Tittonell, P.; Dhed’A, B.; Van Asten, P.; Vanlauwe, B.; Ruhigwa, B.; Blomme, G. Banana leaf pruning to facilitate annual legume intercropping as an intensification strategy in the East African highlands. Eur. J. Agron. 2019, 110, 125923. [Google Scholar] [CrossRef]
- van Asten, P.J.A.; Gold, C.S.; Okech, S.H.O.; Gaidashova, S.V.; Tushemereirwe, W.K.; De Waele, D. Soil quality problems in East African banana systems and their relation with other yield loss factors. InfoMusa 2004, 13, 20–25. [Google Scholar]
- Gold, C.S.; Bagabe, M.I.; Sendege, R. Banana weevil, Cosmopolites sordidus (Germar) (Coleoptera: Curculionidae): Tests for suspected resistance to carbofuran and dieldrin in the Masaka District, Uganda. Afr. Entomol. 1999, 7, 189–196. [Google Scholar]
- Sileshi, G.; Akinnifesi, F.K.; Ajayi, O.C.; Chakeredza, S.; Kaonga, M.; Matakala, P.W. Contributions of agroforestry to ecosystem services in the Miombo eco-region of eastern and southern Africa. Afr. J. Environ. Sci. Technol. 2007, 1, 68–80. [Google Scholar]
- Pypers, P.; Sanginga, J.; Kasereka, B.; Walangululu, M.; Vanlauwe, B. Increased productivity through integrated soil fertility management in cassava–legume inter-cropping systems in the highlands of Sud-Kivu, DR Congo. Field Crops Res. 2010, 120, 76–85. [Google Scholar] [CrossRef]
- Ntamwira, J.; Pypers, P.; van Asten, P.; Vanlauwe, B.; Ruhigwa, B.; Lepoint, P.; Dhed’a, B.; Monde, T.; Kamira, M.; Blomme, G. Effect of banana leaf pruning on banana and legume yield under intercropping in farm-ers’ fields in eastern Democratic Republic of Congo. J. Hortic. For. 2014, 6, 72–80. [Google Scholar] [CrossRef]
- Venskutonis, P.R.; Kraujalis, P. Nutritional Components of Amaranth Seeds and Vegetables: A Review on Composition, Properties, and Uses. Compr. Rev. Food Sci. Food Saf. 2013, 12, 381–412. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Khalil, I.A.; Ateeq, N.; Khan, M.S. Nutritional quality of important food legumes. Food Chem. 2006, 97, 331–335. [Google Scholar] [CrossRef]
- Mulambu, J.; Andersson, M.; Palenberg, M.; Pfeiffer, W.; Saltzman, A.; Birol, E.; Oparinde, A.; Boy, E.; Asare-Marfo, D.; Lubobo, A.; et al. Iron Beans in Rwanda: Crop Development and Delivery Experience. In African Journal of Food, Agriculture, Nutrition and Development; Published by the African Scholarly Science Communications Trust (ASSCAT): London, UK, 2017; Volume 17, pp. 12026–12050. [Google Scholar]
- Oniang’O, R.K.; Mutuku, J.M.; Malaba, S.J. Contemporary African food habits and their nutritional and health implications. Asia Pac. J. Clin. Nutr. 2003, 12, 231–236. [Google Scholar]
- PABRA. Better Beans for Africa. 2006. Available online: www.ciat.cgiar.org/africa/pabra.htm. (accessed on 10 October 2020).
- Wortmann, C.; Sengooba, T. The banana-bean intercropping system—bean genotype × cropping system interactions. Field Crop. Res. 1993, 31, 19–25. [Google Scholar] [CrossRef]
- Sierra, J.; Desfontaines, L. Role of root exudates and root turnover in the below-ground N transfer from Canavalia ensiformis (jackbean) to the associated Musa acuminata (banana). Crop. Pasture Sci. 2009, 60, 289–294. [Google Scholar] [CrossRef]
- Ouma, G. Intercropping and its application to banana production in East Africa: A review. J. Plant Breed. Crop Sci. 2009, 1, 013–015. [Google Scholar]
- Wortmann, C.S.; Sengooba, T.; Kyamanywa, S. Banana and Bean Intercropping: Factors Affecting Bean Yield and Land Use Efficiency. Exp. Agric. 1992, 28, 287–294. [Google Scholar] [CrossRef]
- Akyeampong, E.; Hitimana, L.; Torquebiau, E.; Munyemana, P.C. Multistrata Agroforestry with Beans, Bananas and Grevillea robusta in the Highlands of Burundi. Exp. Agric. 1999, 35, 357–369. [Google Scholar] [CrossRef]
- Kawaka, F.; Dida, M.M.; Opala, P.A.; Ombori, O.; Maingi, J.M.; Osoro, N.; Muthini, M.; Amoding, A.; Mukaminega, D.; Muoma, J. Symbiotic Efficiency of Native Rhizobia Nodulating Common Bean (Phaseolus vulgaris L.) in Soils of Western Kenya. Int. Sch. Res. Not. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Pommeresche, R.; Hansen, S. Examining Root Nodule Activity on Legumes. Fertil Crop Technical Note. 2017. Available online: https://orgprints.org/31344/1/tn-wp5-root-nodules_final_2017.pdf (accessed on 11 September 2020).
- Isidra-Arellano, M.C.; Reyero-Saavedra, M.D.R.; Sánchez-Correa, M.D.S.; Pingault, L.; Sen, S.; Joshi, T.; Girard, L.; Castro-Guerrero, N.A.; Mendoza-Cozatl, D.G.; Libault, M.; et al. Phosphate Deficiency Negatively Affects Early Steps of the Symbiosis between Common Bean and Rhizobia. Genes 2018, 9, 498. [Google Scholar] [CrossRef]
- National Research Council. Lost Crops of Africa: Volume II: Vegetables; The National Academies Press: Washington, DC, USA, 2006. [Google Scholar] [CrossRef]
- Lunze, L. Effects of the traditional cropping system on soil fertility in South Kivu. In Proceedings of the Soil Fertility Research for Bean Cropping Systems in Africa, Addis Ababa, Ethiopia, 5–9 September 1988. [Google Scholar]
- Lunze, L. Possibilités de Gestion de la Fertilité des Sols au Sud-Kivu Montagneux. In Cahiers du Centre D’études et de Recherche Pour la Promotion Rurale et la paix; CERPRU: Bukavu, Democratic Republic of the Congo, 2000; Volume 14, pp. 28–34. [Google Scholar]
- Kempers, A.; Zweers, A. Ammonium determination in soil extracts by the salicylate method. Commun. Soil Sci. Plant Anal. 1986, 17, 715–723. [Google Scholar] [CrossRef]
- Blomme, G.; Ocimati, W.; Sivirihauma, C.; Vutseme, L.; Mariamu, B.; Kamira, M.; Van Schagen, B.; Ekboir, J.; Ntamwira, J. A control package revolving around the removal of single diseased banana stems is effective for the restoration of Xanthomonas wilt infected fields. Eur. J. Plant Pathol. 2017, 149, 385–400. [Google Scholar] [CrossRef]
- Ott, T.; van Dongen, J.T.; Guünther, C.; Krusell, L.; Desbrosses, G.; Vigeolas, H.; Bock, V.; Czechowski, T.; Geigenberger, P.; Udvardi, M.K. Symbiotic Leghemoglobins Are Crucial for Nitrogen Fixation in Legume Root Nodules but Not for General Plant Growth and Development. Curr. Biol. 2005, 15, 531–535. [Google Scholar] [CrossRef]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- VSN International Ltd. GenStat, 12th ed. 2009. Available online: www.vsni.co.uk (accessed on 12 June 2020).
- Eliakira, K.N.; Baijukya, F.; Ndakidemi, P. Productivity of intercropping with maize and common bean over five cropping seasons on smallholder farms of Tanzania. Eur. J. Agron. 2020, 113, 125964. [Google Scholar] [CrossRef]
- Blomme, G.; Ntamwira, J.; Kearsley, E.; Bahati, L.; Amini, D.; Safari, N.; Ocimati, W. Sensitivity and Tolerance of Different Annual Crops to Different Levels of Banana Shade and Dry Season Weather. Front. Sustain. Food Syst. 2020, 4, 545926. [Google Scholar] [CrossRef]
- Kanyenga, L.A.; Kasongo, L.M.; Kizungu, V.; Nachigera, M.; Kalonji, M. Effect of climate change on common bean (Phaseolus vulgaris) crop production: Determination of the optimum planting period in midlands and highlands zones of the Democratic Republic of Congo. Glob. J. Agric. Res. Rev. 2016, 4, 390–399. [Google Scholar]
- Schubert, S. Nitrogen assimilation by legumes—processes and ecological limitations. Nutr. Cycl. Agroecosyst. 1995, 42, 99–107. [Google Scholar] [CrossRef]
- Hultman, C. Abundance of Root Nodules on Common Bean, Phaseolus Vulgaris. 2018. Available online: https://stud.epsilon.slu.se/13194/ (accessed on 4 April 2020).
- Vargas, M.A.T.; Mendes, I.C.; Hungria, M. Benefits of inoculation of the common bean (Phaseolus vulgaris) crop with efficient and competitive Rhizobium. Biol. Fertil. Soils 2003, 32, 228–233. [Google Scholar] [CrossRef]
- Tarekegn, M.A.; Kibret, K. Effects of rhizobium, nitrogen and phosphorus fertilizers on growth, nodu-lation, yield and yield attributes of soybean at Pawe Northwestern Ethiopia. World Sci. News. 2017, 67, 201–218. [Google Scholar]
- McKenzie, B.A.; Hill, G.D.; Gabiana, C. Root growth and nodulation of pinto bean (Phaseolus vulgaris L.) cv. Othello. Proc. Agron. Soc. NZ 1996, 26, 75–78. [Google Scholar]
- Thung, M. Bean agronomy in monoculture. In Common Beans: Re-Search for Crop Improvement; Schoonhoven, A., van Voysest, O., Eds.; Antony Rowe Ltd.: Chippenham, UK, 1991; pp. 737–834. [Google Scholar]
- Salcedo, J.M. Regeneration guidelines: Common bean. In Crop Specific Regeneration Guidelines [CD-ROM]; Dulloo, M.E., Thormann, I., Jorge, M.A., Han-son, J., Eds.; CGIAR System-wide Genetic Resource Pro-gramme: Rome, Italy, 2008; p. 9. [Google Scholar]
- Lemlem, A. The effect of intercropping maize with cowpea and lablab on crop yield. Herald. J. Agric. Food Sci. Res. 2013, 2, 156–170. [Google Scholar]
- Grechi, I.; Vivin, P.; Hilbert, G.; Milin, S.; Robert, T.; Gaudillère, J.-P. Effect of light and nitrogen supply on internal C:N balance and control of root-to-shoot biomass allocation in grapevine. Environ. Exp. Bot. 2007, 59, 139–149. [Google Scholar] [CrossRef]
- Chu, A.C.P.; Robertson, A.G. The effects of shading and defoliation on nodulation and nitrogen fixa-tion by white clover. Plant Soil 1974, 41, 509–519. [Google Scholar] [CrossRef]
- Butler, G.W.; Greenwood, R.M.; Soper, K. Effects of Shading and Defoliation on the Turnover of Root and Nodule Tissue of Plants of Trifolium repens, Trifolium pratense, and Lotus uliginosus. N. Z. J. Agric. Res. 1959, 2, 415–426. [Google Scholar] [CrossRef]
- Wadud, M.A.; Rahman, G.M.M.; Chowdhury, M.J.U.; Mahboob, M.G. Performance of Red Amaranth under Shade Condition for Agroforestry Systems. J. Biol. Sci. 2002, 2, 765–766. [Google Scholar] [CrossRef]
- Ufoegbune, G.; Eruola, K.O. Performance of Amaranthus species under two different environmental conditions in derived savannah agroecology, Southwestern Nigeria. Afr. J. Agric. Technol. Environ. 2016, 4, 33–45. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).