Development of Vernonia amygdalina Leaf Extract Emulsion Formulations in Controlling Gray Mold Disease on Tomato (Lycopersicon esculentum Mill.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of V. amygdalina Crude Extract
2.2.1. Materials for Formulation Components
2.2.2. Preparation of Emulsion by Ternary Phase Diagram
2.3. Selection of Emulsion Formulation Composition from Ternary Phase Diagrams
2.4. Characterization of Emulsion Formulation
2.4.1. Centrifugation Test of Formulation
2.4.2. Storage Stability and Thermostability Test of Formulation
2.4.3. Particle Size and Zeta Potential Measurement
2.4.4. Surface Tension Analysis
2.4.5. Viscosity Analysis
2.5. In Situ Antifungal Activity of the Selected Nanoemulsion Formulations on Artificial Inoculated Tomato Fruits
2.5.1. Tomato Fruit Preparation and Treatment Application
2.5.2. Inoculation of B. cinerea on the Treated Tomato Fruit
2.6. Statistical Analysis
3. Results
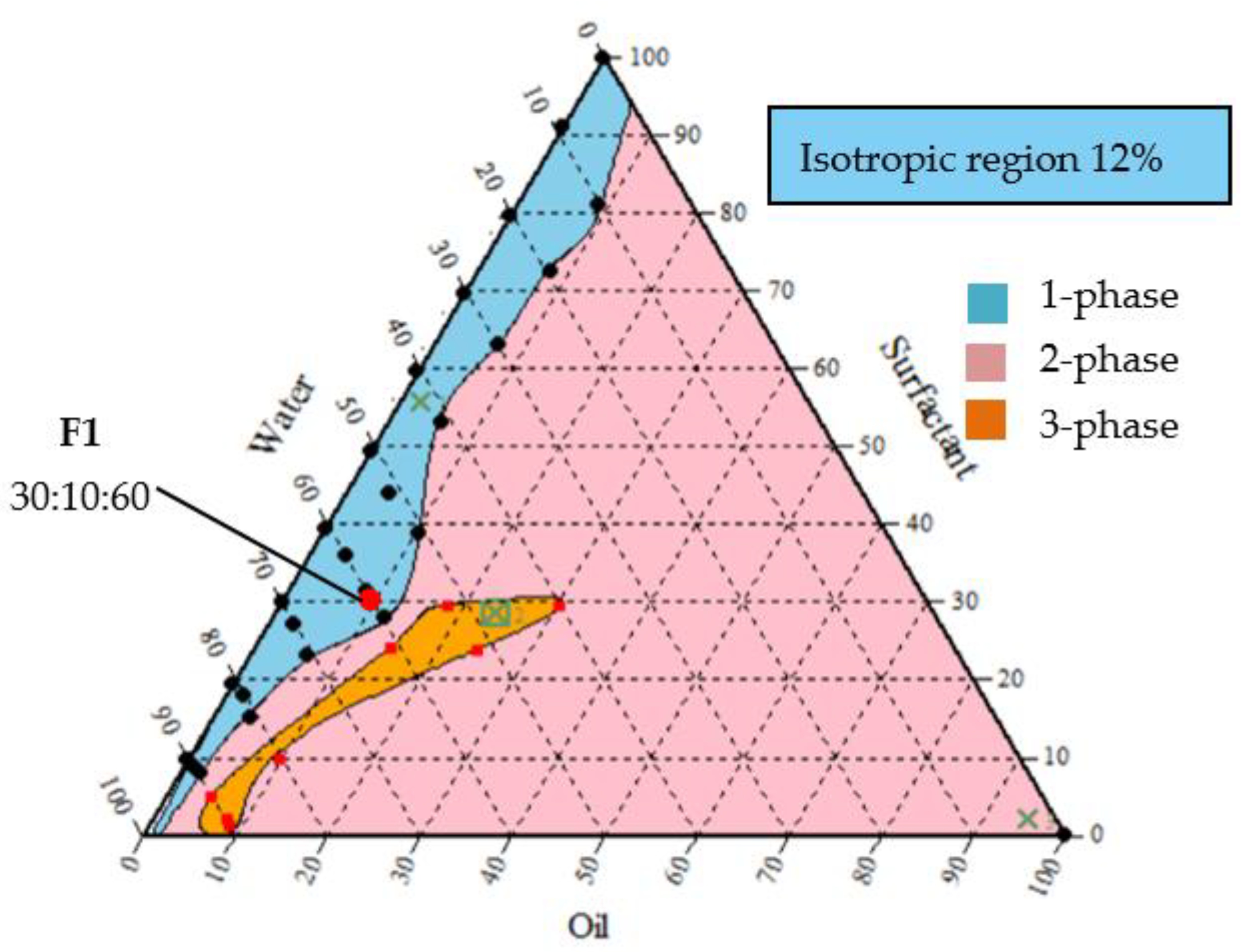
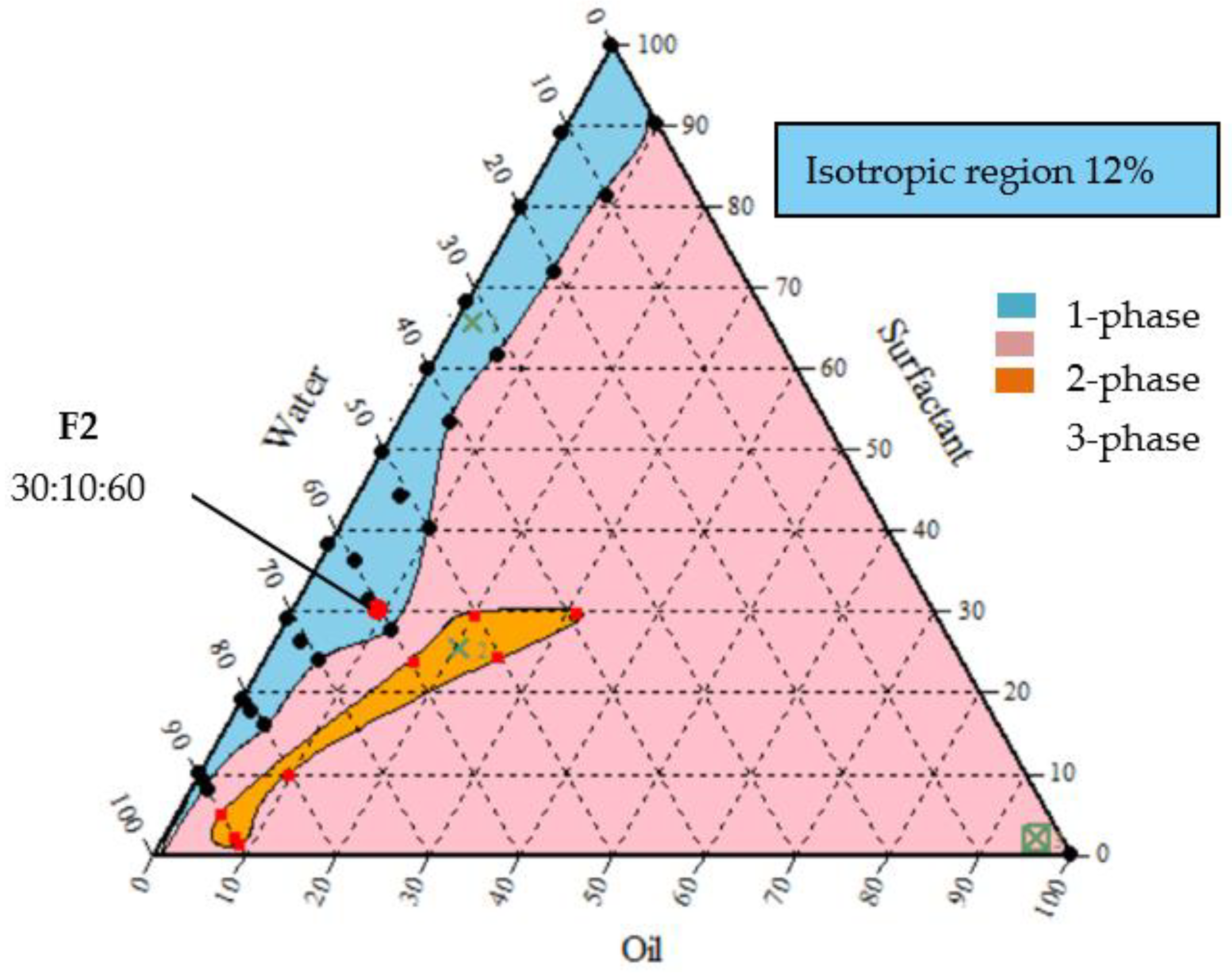
3.1. Ternary Phase Diagrams of Emulsion Formulations
3.2. Point of Selection
3.3. Characterization of Emulsion Formulations
3.4. Effects of Centrifugation, Storage Stability and Thermostability on Emulsion Formulation Homogeneity
3.5. Physical Characteristics of Emulsion Formulations
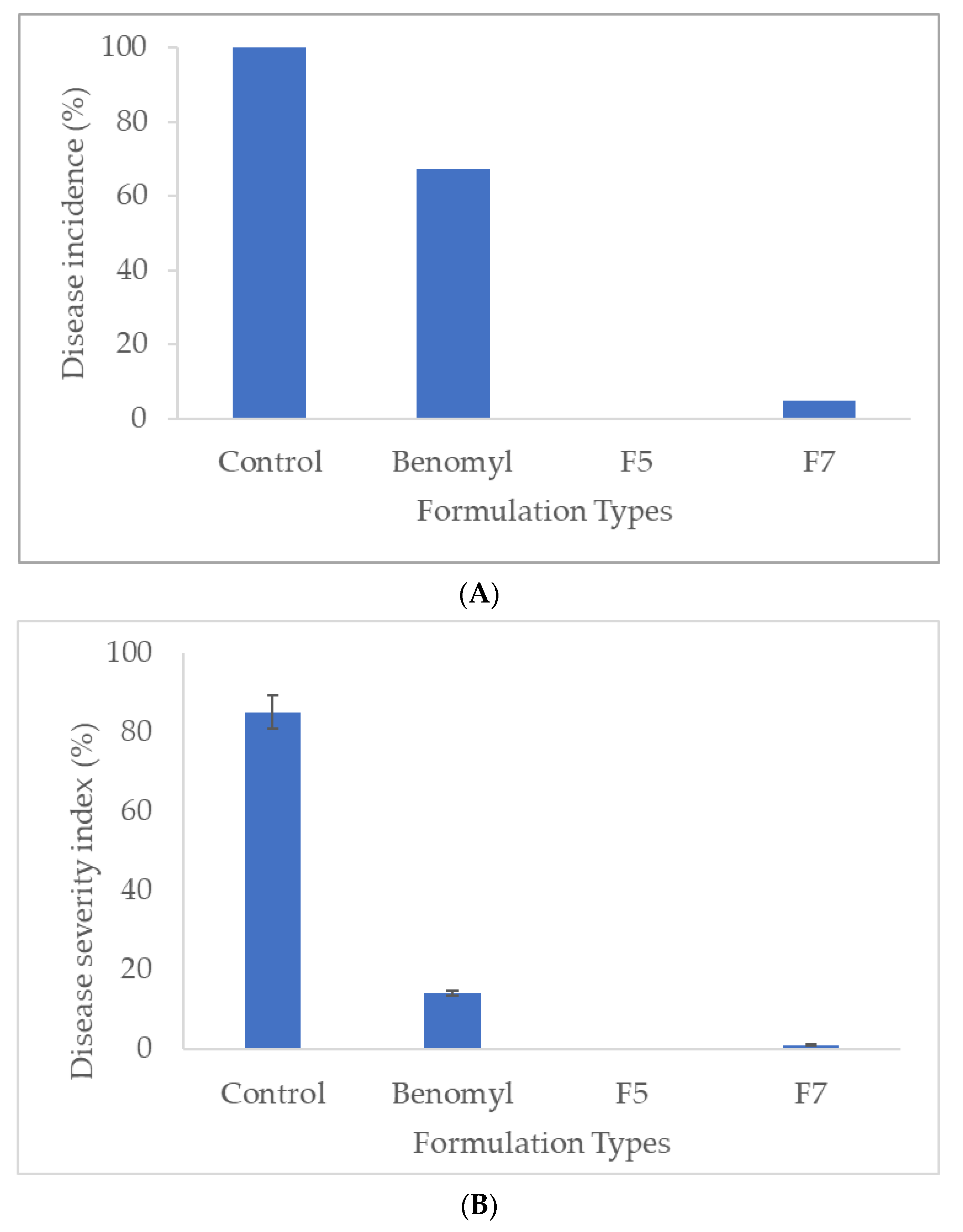
3.6. In Situ Antifungal Activity of V. amygdalina-derived Nanoemulsion Formulations Against B. cinerea on Tomato
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abbey, J.A.; Percival, D.; Abbey, L.; Asiedu, S.K.; Prithiviraj, B.; Schilder, A. Biofungicides as alternative to synthetic fungicide control of grey mould (Botrytis cinerea)—Prospects and challenges. Biocontrol Sci. Technol. 2019, 29, 207–228. [Google Scholar] [CrossRef]
- Zhao, S.; Guo, Y.; Wang, Q.; Luo, H.; He, C.; An, B. Expression of flagellin at yeast surface increases biocontrol efficiency of yeast cells against postharvest disease of tomato caused by Botrytis cinerea. Postharvest Biol. Technol. 2020, 162, 111112. [Google Scholar] [CrossRef]
- Zaker, M. Natural Plant Products as Eco-friendly Fungicides for Plant Diseases Control—A Review. Agriculture 2016, 14, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Borges, D.F.; Lopes, E.A.; Moraes, A.R.F.; Soares, M.S.; Visôtto, L.E.; Oliveira, C.R.; Valente, V.M.M. Formulation of botanicals for the control of plant-pathogens: A review. Crop. Prot. 2018, 110, 135–140. [Google Scholar] [CrossRef]
- Miresmailli, S.; Isman, M.B. Botanical insecticides inspired by plant–herbivore chemical interactions. Trends Plant Sci. 2014, 19, 29–35. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Maggi, F.; Petrelli, R.; Nicoletti, M. Commentary: Making Green Pesticides Greener? The Potential of Plant Products for Nanosynthesis and Pest Control. J. Clust. Sci. 2016, 28, 3–10. [Google Scholar] [CrossRef]
- Rashid, T.S. Antimicrobial Activity of Rhus coriaria L. Fruit Extracts Against Selected Bacterial and Fungal Pathogens on Tomato. Ph.D. Thesis, University Putra Malaysia, Selangor, Malaysia, 12 May 2016. [Google Scholar]
- Haron, F.F. Antifungal Activity of Allamanda Spp. Extracts and Their Microemulsion Formulations Against Anthracnose (Colletotrichum Gloeosporioides) Disease of Papaya. Ph.D. Thesis, University Putra Malaysia, Selangor, Malaysia, October 2012. [Google Scholar]
- Pant, M.; Dubey, S.; Patanjali, P.K. Recent Advancements in Bio-Botanical Pesticide Formulation Technology Development. In Herbal Insecticides, Repellents and Biomedicines: Effectiveness and Commercialization; Vijay, V., Gopalakrishnan, R., Eds.; Springer International Publishing: New Delhi, India, 2016; pp. 117–126. [Google Scholar]
- Sharma, R.; Kumari, A.; Singh, N.S.; Singh, M.K.; Dubey, S.; Iqbal, N.; Patanjali, P.K. Development and stability enhancement of neem oil based microemulsion formulation using botanical synergist. J. Mol. Liq. 2019, 296, 112012. [Google Scholar] [CrossRef]
- Campos, E.V.; Proença, P.L.; Oliveira, J.L.; Bakshi, M.; Abhilash, P.; Fraceto, L.F. Use of botanical insecticides for sustainable agriculture: Future perspectives. Ecol. Indic. 2019, 105, 483–495. [Google Scholar] [CrossRef] [Green Version]
- Kookana, R.S.; Boxall, A.B.A.; Reeves, P.T.; Ashauer, R.; Beulke, S.; Chaudhry, Q.; Cornelis, G.; Fernandes, T.F.; Gan, J.; Kah, M.; et al. Nanopesticides: Guiding Principles for Regulatory Evaluation of Environmental Risks. J. Agric. Food Chem. 2014, 62, 4227–4240. [Google Scholar] [CrossRef] [Green Version]
- Anton, N.; Vandamme, T.F. Nano-emulsions and Micro-emulsions: Clarifications of the Critical Differences. Pharm. Res. 2010, 28, 978–985. [Google Scholar] [CrossRef]
- Aboofazeli, R. Nanometric-scaled emulsions (nano-emulsions). Iran. J. Pharm. Res. 2010, 9, 325–326. [Google Scholar]
- Taylor, P. Ostwald ripening in emulsions. Adv. Colloid Interface Sci. 1998, 75, 107–163. [Google Scholar] [CrossRef]
- Kale, S.N.; Deore, S.L. Emulsion Micro Emulsion and Nano Emulsion: A Review. Syst. Rev. Pharm. 2016, 8, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Yusoff, S.F.; Haron, F.F.; Mohamed, M.T.M.; Asib, N.; Sakimin, S.Z.; Abu Kassim, F.; Ismail, S.I. Antifungal Activity and Phytochemical Screening of Vernonia amygdalina Extract against Botrytis cinerea Causing Gray Mold Disease on Tomato Fruits. Biology 2020, 9, 286. [Google Scholar] [CrossRef]
- Siti Fairuz, Y.; Ismail, S.I.; Farah Farhanah, H.; Mahmud, T.M.M. Phytochemical Composition in Hexane and Methanolic Leaf Extract of Vernonia amygdalina. Malays. Appl. Biol. 2019, 48, 11–17. [Google Scholar]
- Rahmani, M.; Haron, F.F.; Sijam, K.; Omar, D. Bioassay-guided Isolation of Antifungal Plumericin from Allamanda Species (Apocynaceae). J. Biol. Sci. 2013, 13, 158–162. [Google Scholar] [CrossRef] [Green Version]
- Choupanian, M.M.C.; Omar, D.D.O.; Basri, M.M.B.; Asib, N.N.A. Preparation and characterization of neem oil nanoemulsion formulations against Sitophilus oryzae and Tribolium castaneum adults. J. Pestic. Sci. 2017, 42, 158–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asib, N.; Omar, D.; Awang, R.M.; Ashikin, N.; Abdullah, P. Preparation, characterization and toxicity of nano-emulsion formulations of rotenone extract of Derris Elliptica. J. Chem. Biol. Phys. Sci. 2015, 5, 3989–3997. [Google Scholar]
- Department of Agriculture (DoA). Maklumat Racun Perosak. Available online: http://www.doa.gov.my/index/resources/aktiviti_sumber/sumber_awam/maklumat_racun_perosak/slaid/slaid3.pdf (accessed on 17 November 2020).
- World Health Organization and Food Agricultural Organization. Manual on Development and Use of FAO and WHO Specifications for Pesticides, 1st ed.; WHO Press: Rome, Italy, 2016; 144p. [Google Scholar]
- Rosero-Hernández, E.D.; Moraga, J.; Collado, I.G.; Echeverri, F. Natural Compounds That Modulate the Development of the Fungus Botrytis cinerea and Protect Solanum lycopersicum. Plants 2019, 8, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benita, S. Microencapsulation: Methods and Industrial Applications, 2nd ed.; CRC Press: New York, NY, USA, 2005; 355p. [Google Scholar]
- Kumar, S.; Nehra, M.; Dilbaghi, N.; Marrazza, G.; Hassan, A.A.; Kim, K.-H. Nano-based smart pesticide formulations: Emerging opportunities for agriculture. J. Control. Release 2019, 294, 131–153. [Google Scholar] [CrossRef] [PubMed]
- Kah, M.; Hofmann, T. Nanopesticide research: Current trends and future priorities. Environ. Int. 2014, 63, 224–235. [Google Scholar] [CrossRef]
- Chandrasekaran, N.; Sugumar, S.; Mukherjee, A. Nanoemulsion formation and characterization by spontaneous emulsification: Investigation of its antibacterial effects on Listeria monocytogenes. Asian J. Pharm. 2015, 9, 23. [Google Scholar] [CrossRef]
- Jiang, L.C.; Basri, M.; Omar, D.; Rahman, M.B.A.; Salleh, A.B.; Rahman, R.N.Z.R.A.; Selamat, A. Green nano-emulsion intervention for water-soluble glyphosate isopropylamine (IPA) formulations in controlling Eleusine indica (E. indica). Pestic. Biochem. Physiol. 2012, 102, 19–29. [Google Scholar] [CrossRef]
- Kamarudin, N. Oil Nano-emulsion Formulation of Azadirachtin for Control of Bemisia Tabaci Gennadius. Ph.D. Thesis, University Putra Malaysia, Selangor, Malaysia, July 2013. [Google Scholar]
- Zheng, L.; Cao, C.; Chen, Z.; Cao, L.; Huang, Q.; Song, B. Evaluation of emulsion stability by monitoring the interaction between droplets. LWT 2020, 132, 109804. [Google Scholar] [CrossRef]
- Costa, C.; Medronho, B.; Filipe, A.; Mira, I.; Lindman, B.; Edlund, H.; Norgren, M. Emulsion Formation and Stabilization by Biomolecules: The Leading Role of Cellulose. Polymers 2019, 11, 1570. [Google Scholar] [CrossRef] [Green Version]
- Barzegar, A.; Moosavi-Movahedi, A.A. Intracellular ROS Protection Efficiency and Free Radical-Scavenging Activity of Curcumin. PLoS ONE 2011, 6, e26012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lechuga, M.; Fernández-Serrano, M.; Jurado, E.; Núñez-Olea, J.; Ríos, F. Acute toxicity of anionic and non-ionic surfactants to aquatic organisms. Ecotoxicol. Environ. Saf. 2016, 125, 1–8. [Google Scholar] [CrossRef]
- Agarwal, S.P.; Rajesh, K. Physical Pharmacy, 2nd ed.; CBS Publisher: Delhi, India, 2007; pp. 177–186. [Google Scholar]
- Maphosa, Y.; Jideani, V.A.; Adeyi, O. Effect of soluble dietary fibres from Bambara groundnut varieties on the stability of orange oil beverage emulsion. Afr. J. Sci. Technol. Innov. Dev. 2017, 9, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control. Release 2017, 252, 28–49. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Miao, S. Structuring Food Emulsions to Improve Nutrient Delivery During Digestion. Food Eng. Rev. 2015, 7, 439–451. [Google Scholar] [CrossRef]
- Payet, L.; Terentjev, E.M. Emulsification and Stabilization Mechanisms of O/W Emulsions in the Presence of Chitosan. Langmuir 2008, 24, 12247–12252. [Google Scholar] [CrossRef]
- Chanamai, R.; McClements, D.J. Impact of Weighting Agents and Sucrose on Gravitational Separation of Beverage Emulsions. J. Agric. Food Chem. 2000, 48, 5561–5565. [Google Scholar] [CrossRef]
- Jiao, J.; Burgess, D.J. Ostwald ripening of water-in-hydrocarbon emulsions. J. Colloid Interface Sci. 2003, 264, 509–516. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions: Principles, Practices, and Techniques, 3rd ed.; CRC Press: New York, NY, USA, 2015; pp. 289–373. [Google Scholar]
- Sjöblom, J.; Stenius, P.; Simon, S.; Grimes, B.A. Emulsion Stabilization. In Encyclopedia of Colloid and Interface Science, 1st ed.; Tadros, T., Ed.; Springer International Publishing: Heidelberg, Germany, 2013; pp. 415–454. [Google Scholar]
- Gurpret, K.; Singh, S.K. Review of Nanoemulsion Formulation and Characterization Techniques. Indian J. Pharm. Sci. 2018, 80, 781–789. [Google Scholar] [CrossRef]
- Da Costa, S.; Basri, M.; Shamsudin, N.; Basri, H. Stability of Positively Charged Nanoemulsion Formulation Containing Steroidal Drug for Effective Transdermal Application. J. Chem. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Murphy, M.J.; Yu, H.; Bagaria, H.G.; Yoon, K.Y.; Neilson, B.M.; Bielawski, C.W.; Johnston, K.P.; Huh, C.; Bryant, S.L. Investigation of Nanoparticle Adsorption During Transport in Porous Media. SPE J. 2015, 20, 667–677. [Google Scholar] [CrossRef]
- Glare, T.; Caradus, J.; Gelernter, W.; Jackson, T.; Keyhani, N.; Köhl, J.; Marrone, P.; Morin, L.; Stewart, A. Have biopesticides come of age? Trends Biotechnol. 2012, 30, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Bera, B. Nanoporous silicon prepared by vapour phase strain etch and sacrificial technique. Int. J. Comput. Appl. 2015, 975, 8887. [Google Scholar]
- Nobbmann, U.L. Polydispersity–What Does It Mean for DLS and Chromatography. Available online: http://www.materials-talks.com/blog/2014/10/23/polydispersity-what-does-it-mean-for-dls-and-chromatography (accessed on 18 November 2020).
- Baboota, S.; Shakeel, F.; Ahuja, A.; Ali, J.; Shafiq, S. Design, development and evaluation of novel nanoemulsion formulations for transdermal potential of celecoxib. Acta Pharm. 2007, 57, 315–332. [Google Scholar] [CrossRef] [Green Version]
- Smole, M.S.; Hribernik, S.; Kurečič, M.; Krajnc, A.U.; Kreže, T.; Kleinschek, K.S. Surface Properties of Non-conventional Cellulose Fibres, 1st ed.; Springer International Publishing: Cham, Switzerland, 2019; 85p. [Google Scholar]
- Tadros, T. Ostwald Ripening. In Encyclopedia of Colloid and Interface Science, 1st ed.; Tadros, T., Ed.; Springer International Publishing: Heidelberg, Germany, 2013; 820p. [Google Scholar]
- Sharma, N.; Bansal, M.; Visht, S.; Sharma, P.K.; Kulkarni, G.T. Nanoemulsion: A new concept of delivery system. Chron. Young Sci. 2010, 1, 2. [Google Scholar]
- Dasgupta, N.; Ranjan, S. Nanoemulsion in Food. In An Introduction to Food Grade Nano-Emulsions; Dasgupta, N., Ranjan, S., Eds.; Springer: Singapore, 2018; Volume 13, 35p. [Google Scholar]
- Tadros, T.F. Agrochemicals, Paints and Coatings and Food Colloids. In Formulation Science and Technology, 1st ed.; Tadros, T.F., Ed.; Deutsche Nationalbibliothek: Berlin, Germany, 2018; Volume 4, pp. 245–254. [Google Scholar]
- Silva, H.D.; Cerqueira, M.; Vicente, A.A. Nanoemulsions for Food Applications: Development and Characterization. Food Bioprocess Technol. 2012, 5, 854–867. [Google Scholar] [CrossRef] [Green Version]
- Sepúlveda-Rivas, S.; Fritz, H.F.; Valenzuela, C.; Santiviago, C.A.; Morales, J.O. Development of Novel EE/Alginate Polyelectrolyte Complex Nanoparticles for Lysozyme Delivery: Physicochemical Properties and In Vitro Safety. Pharm. 2019, 11, 103. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Ahmed, K.; Li, Y.; McClements, D.J.; Xiao, H. Nanoemulsion- and emulsion-based delivery systems for curcumin: Encapsulation and release properties. Food Chem. 2012, 132, 799–807. [Google Scholar] [CrossRef]
- Chuacharoen, T.; Prasongsuk, S.; Sabliov, C.M. Effect of Surfactant Concentrations on Physicochemical Properties and Functionality of Curcumin Nanoemulsions Under Conditions Relevant to Commercial Utilization. Molecules 2019, 24, 2744. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Severity Scale | Description | Inference |
|---|---|---|
| 0 | No visible symptoms of fruits | No infection |
| 1 | 1–25% inoculated area covered with slight necrotic | Mild infection |
| 2 | 26–50% of inoculated area covered with necrotic and white mycelia of fungal | Moderate infection |
| 3 | 51–75% of fruits are necrotic with spore mass appeared | Severe infection |
| 4 | >76% necrotic tissue appears soft, watery and decay | Very severe/devastating |
| Formulation | Component Composition Ratio |
|---|---|
| F1 | 30 Glucopon 225: 10 Agnique AMD810: 60 water |
| F2 | 30 Glucopon 215: 10 Agnique AMD810: 60 water |
| F3 | 20 mix Agnique MBL510 and MBL530: 10 Agnique AMD810: 70 water |
| F4 | 30 mix Agnique MBL510 and MBL530: 10 Agnique AMD810: 60 water |
| F5 | 30 Agnique MBL530: 10 Agnique AMD810: 60 water |
| F6 | 15 Agnique MBL530: 75 Agnique AMD810: 10 water |
| F7 | 20 Agnique MBL510: 10 Agnique AMD810: 70 water |
| F8 | 15 Agnique MBL510: 5 Agnique AMD810: 80 water |
| Formulation | Composition Ratio (% w/w) | |||||||
|---|---|---|---|---|---|---|---|---|
| Glucopon 225 | Glucopon 215 | Mix Agnique MBL510 + MBL530 | Agnique MBL530 | Agnique MBL510 | Agnique AMD810 | Water | DVLE | |
| F1 | 18 | - | - | - | - | 6 | 36 | 40 |
| F2 | - | 18 | - | - | - | 6 | 36 | 40 |
| F3 | - | - | 12 | - | - | 6 | 42 | 40 |
| F4 | - | - | 18 | - | - | 6 | 36 | 40 |
| F5 | - | - | - | 18 | - | 6 | 36 | 40 |
| F6 | - | - | - | 9 | - | 45 | 6 | 40 |
| F7 | - | - | - | - | 12 | 6 | 42 | 40 |
| F8 | - | - | - | - | 9 | 3 | 48 | 40 |
| Formulation | Particle Size (nm) | Polydispersity Index (PdI) | Viscosity (cP) | Surface Tension (mN/m) | Zeta Potential (mV) |
|---|---|---|---|---|---|
| F1 | 182.37c z | 0.45bcd z | 4.30bc z | 29.18d z | −26.83b z |
| F2 | 977.27a | 0.07e | 4.38bc | 26.24f | −32.70a |
| F3 | 188.07c | 0.49bc | 4.32bc | 29.20d | −16.43d |
| F4 | 171.13c | 0.51b | 4.49b | 25.62g | −22.80c |
| F5 | 66.44e | 0.41d | 4.20c | 27.62c | −32.70a |
| F6 | 899.37b | 0.51b | 4.23bc | 29.52b | −13.57d |
| F7 | 139.63d | 0.40cd | 4.37bc | 26.41e | −31.70a |
| F8 | 45.77e | 0.91a | 7.50a | 29.85a | −33.87a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yusoff, S.F.; Haron, F.F.; Asib, N.; Mohamed, M.T.M.; Ismail, S.I. Development of Vernonia amygdalina Leaf Extract Emulsion Formulations in Controlling Gray Mold Disease on Tomato (Lycopersicon esculentum Mill.). Agronomy 2021, 11, 373. https://doi.org/10.3390/agronomy11020373
Yusoff SF, Haron FF, Asib N, Mohamed MTM, Ismail SI. Development of Vernonia amygdalina Leaf Extract Emulsion Formulations in Controlling Gray Mold Disease on Tomato (Lycopersicon esculentum Mill.). Agronomy. 2021; 11(2):373. https://doi.org/10.3390/agronomy11020373
Chicago/Turabian StyleYusoff, Siti Fairuz, Farah Farhanah Haron, Norhayu Asib, Mahmud Tengku Muda Mohamed, and Siti Izera Ismail. 2021. "Development of Vernonia amygdalina Leaf Extract Emulsion Formulations in Controlling Gray Mold Disease on Tomato (Lycopersicon esculentum Mill.)" Agronomy 11, no. 2: 373. https://doi.org/10.3390/agronomy11020373






