Recent Advances in Onion Genetic Improvement
Abstract
1. Introduction
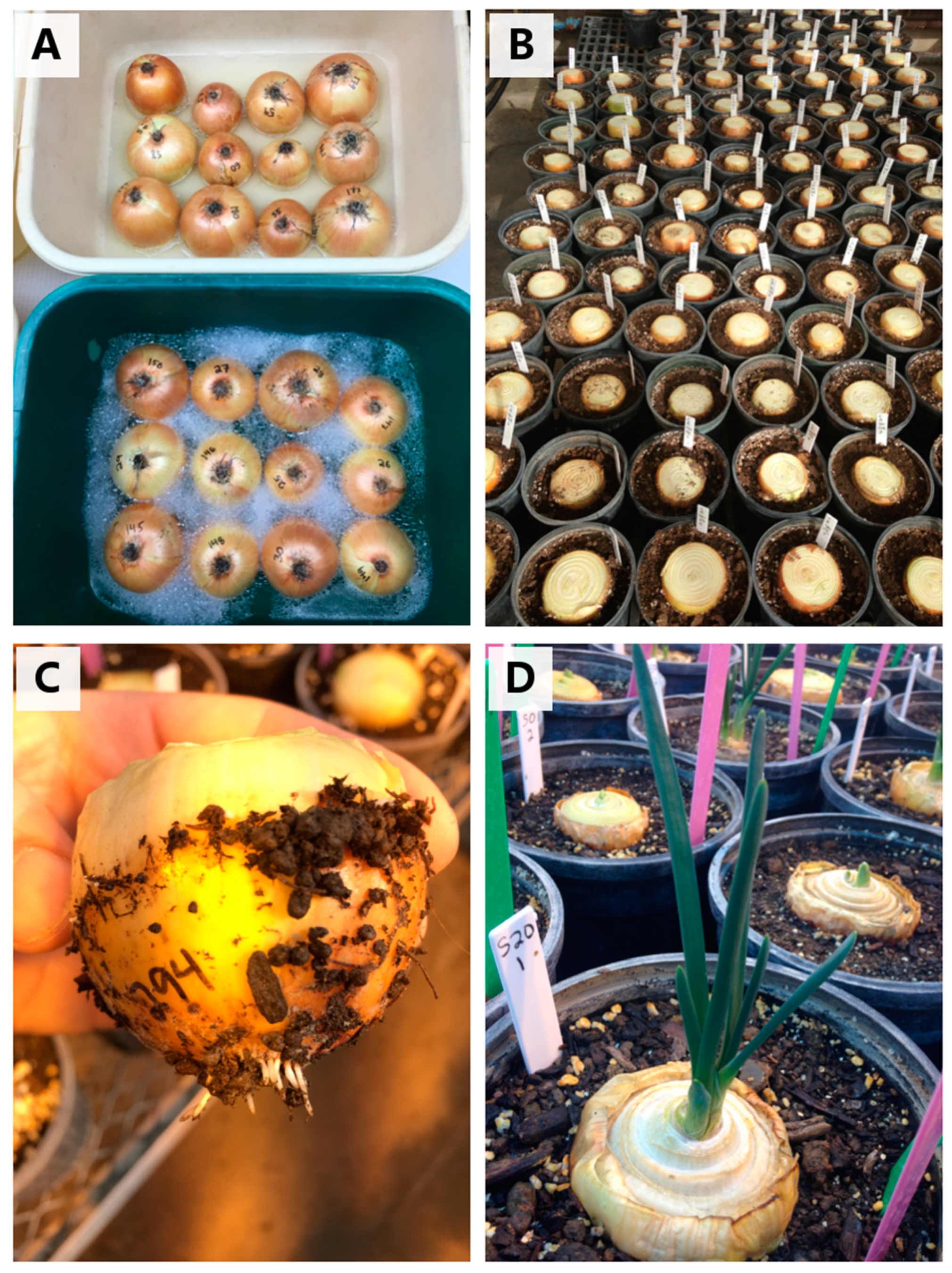
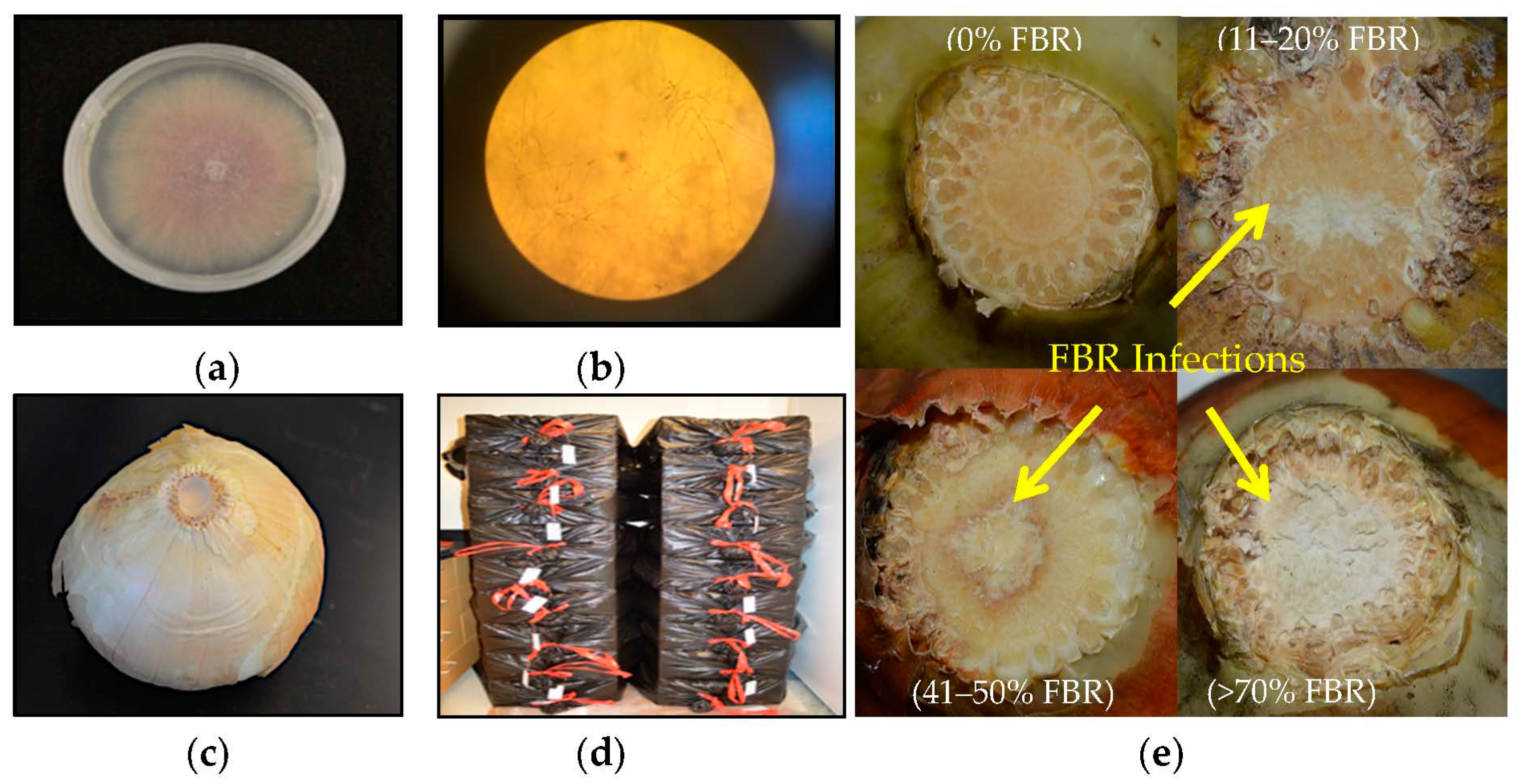
2. Fusarium Basal Rot
3. Iris Yellow Spot and Onion Thrips
4. Pungency
5. Bulb Dormancy and Vernalization
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT. Food and Agriculture Commodity Production Data; Food and Agriculture Organization: Rome, Italy, 2018. [Google Scholar]
- Jones, H.A.; Mann, L.K. Onions and Their Allies; London Leonard Hill [Books] Limited Interscience Publishers, Inc.: New York, NY, USA, 1963; p. 284. [Google Scholar]
- D’Angelo, C.J.; Goldman, I.L. Annualization of the long-day onion breeding cycle through threshold vernalization and dormancy disruption. Crop Breed. Genet. Genom. 2019, 1, e190009. [Google Scholar] [CrossRef]
- Pike, L.M. Onion Breeding. In Breeding Vegetable Crops; AVI Publishing Co.: Westport, CT, USA, 1986; pp. 357–394. [Google Scholar]
- Schwartz, H.F.; Mohan, S.K. Compendium of Onion and Garlic Diseases; APS Press: St. Paul, MN, USA, 1985. [Google Scholar]
- Gent, D.H.; du Toit, L.J.; Fichtner, S.F.; Mohan, S.K.; Pappu, H.R.; Schwartz, H.F. Iris yellow spot virus: An emerging threat to onion bulb and seed production. Plant Dis. 2006, 90, 1468–1480. [Google Scholar] [CrossRef] [PubMed]
- Cramer, C.S. Breeding and genetics of Fusarium basal rot resistance in onion. Euphytica 2000, 115, 159–166. [Google Scholar] [CrossRef]
- Selby, A.D. A brief handbook of the diseases of cultivated plants in Ohio. In Ohio Agricultural Experiment Station Bulletin Number 214; Ohio Agricultural Experiment Station: Columbus, OH, USA, 1910. [Google Scholar]
- Saxena, A.; Cramer, C.S. Screening of onion seedlings for resistance against New Mexico isolates of Fusarium oxysporum f.sp. cepae. J. Plant Pathol. 2009, 91, 197–200. [Google Scholar]
- Rabiei-Motlagh, E.; Falahati-Rastegar, M.; Rouhani, H.; Jafarpour, B.; Jahanbakhsh, V. Root diseases of onion caused by some root colonizing fungi in northeast of Iran. Am. J. Agric. Environ. Sci. 2010, 7, 484–491. [Google Scholar]
- Southwood, M.J.; Viljoen, A.; Mostert, L.; Rose, L.J.; McLeod, A. Phylogenetic and biological characterization of Fusarium oxysporum isolates associated with onion in South Africa. Plant Dis. 2012, 96, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Vagany, V.; Barbara, D.J.; Thomas, B.; Pink, D.A.C.; Jones, J.E.; Clarkson, J.P. Identification of differential resistance to six Fusarium oxysporum f.sp. cepae isolates in commercial onion cultivars through the development of a rapid seedling assay. Plant Pathol. 2013, 62, 103–111. [Google Scholar] [CrossRef]
- Esfahani, M.N. Genetic and virulence variation in Fusarium oxysporum f.sp. cepae causing root and basal rot of common onion in Iran. J. Phytopathol. 2018, 166, 572–580. [Google Scholar] [CrossRef]
- Vivero, G.A.G. Resistance to Fusarium Basal Rot and Response to Arbuscular Mycorrhizal Fungi in Allium. Ph.D. Dissertation, Wageningen University, Wageningen, The Netherlands, 2009. [Google Scholar]
- Kalman, B.; Abraham, D.; Graph, S.; Perl-Treves, R.; Harel, Y.M.; Degani, O. Isolation and identification of Fusarium spp., the causal agents of onion (Allium cepa) basal rot in Northeastern Israel. Biology 2020, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Havey, M.J. Onion and other cultivated Alliums. In Evolution of Crop Plants; Smart, J., Simmonds, N.W., Eds.; Wiley: New York, NY, USA, 1995; pp. 344–350. [Google Scholar]
- Bennett, A.J.; Bending, G.D.; Chandler, D.; Hilton, S.; Mills, P. Meeting the demand for crop production: The challenge of yield decline in crops grown in short rotations. Biol. Rev. 2012, 87, 52–71. [Google Scholar] [CrossRef] [PubMed]
- Thornton, M.K.; Mohan, S.K. Response of sweet spanish onion cultivars to basal rot and pink root. Plant Dis. 1996, 80, 660–663. [Google Scholar] [CrossRef]
- Taylor, A.; Teakle, G.R.; Walley, P.G.; Finch-Savage, W.E.; Jackson, A.C.; Jones, J.E.; Hand, P.; Thomas, B.; Havey, M.J.; Pink, D.A.; et al. Assembly and characterisation of a unique onion diversity set identifies resistance to Fusarium basal rot and improved seedling vigour. Theor. Appl. Genet. 2019, 132, 3245–3264. [Google Scholar] [CrossRef] [PubMed]
- Caligiore-Gei, P.F.; Valdez, J.G.; Piccolo, R.J.; Galmarini, C.R. Influence of Fusarium spp. isolate and inoculum density on resistance screening tests in onion. Trop. Plant Pathol. 2014, 39, 19–27. [Google Scholar] [CrossRef]
- Mandal, S.; Cramer, C.S. An artificial inoculation method to select mature onion bulbs resistant to fusarium basal rot. HortScience 2020, 55, 1840–1847. [Google Scholar] [CrossRef]
- Taylor, A.; Vágány, V.; Jackson, A.C.; Harrison, R.J.; Rainoni, A.; Clarkson, J.P. Identification of pathogenicity-related genes in Fusarium oxysporum f. sp. cepae. Mol. Plant Pathol. 2016, 17, 1032–1047. [Google Scholar] [CrossRef] [PubMed]
- Armitage, A.D.; Taylor, A.; Sobczyk, M.K.; Baxter, L.; Greenfield, B.P.J.; Bates, H.J.; Fiona, W.; Jackson, A.C.; Ott, S.; Harrison, R.J.; et al. Characterisation of pathogen-specific regions and novel effector candidates in Fusarium oxysporum f. sp. cepae. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mahdy, H. Identification of Fusarium Species Causing Onion Basal Rot in Egypt and Their Virulence on seeds, Seedlings and Onion Bulbs. Ann. Agric. Sci. Moshtohor 2018, 56, 79–88. [Google Scholar] [CrossRef]
- Armitage, A.D.; Taylor, A.; Hulin, M.T.; Jackson, A.C.; Harrison, R.J.; Clarkson, J.P. Draft Genome Sequence of an Onion Basal Rot Isolate of Fusarium proliferatum. Microbiol. Resour. Announc. 2019, 8, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Bektast, I.; Kusek, M. Phylogenetic and Morphological Characterization of Fusarium Oxysporum f. sp. cepae the Causal Agent of Basal Rot on Onion Isolated From Turkey. Fresenius. Environ. Bull. 2019, 28, 1733–1742. [Google Scholar]
- Latvala, S.; Haapalainen, M.; Kivijärvi, P.; Suojala-Ahlfors, T.; Iivonen, S.; Hannukkala, A. Sampling and PCR method for detecting pathogenic Fusarium oxysporum strains in onion harvest. Lett. Appl. Microbiol. 2020, 70, 210–220. [Google Scholar] [CrossRef]
- Mandal, S.; Saxena, A.; Cramer, C.S.; Steiner, R.L. Comparing efficiencies of two selection approaches for improving Fusarium basal rot resistance in short-day onion after a single cycle of selection. Horticulturae 2020, 6, 26. [Google Scholar] [CrossRef]
- Esfahani, M.N.; Hosseini, M.; Nasehi, A.; Golkhandan, E. Screening of onion seed sets for resistance against new iranian isolates of Fusarium oxysporum f.sp. cepa. Arch. Phytopath. Plant Prot. 2013, 46, 1864–1873. [Google Scholar] [CrossRef]
- Rout, E.; Tripathy, P.; Nanda, S.; Nayak, S.; Joshi, R.K. Evaluation of cultivated and wild Allium accessions for resistance to Fusarium oxysporum f.sp. cepae. Proc. Natl. Acad. Sci. India. Sect. B Biol. Sci. 2016, 86, 643–649. [Google Scholar] [CrossRef]
- Caligiore-Gei, P.F.; Ciotti, M.L.; Valdez, J.G.; Galmarini, C.R. Breeding onion for resistance to Fusarium basal rot: Comparison of field selection and artificial inoculation. Trop. Plant Pathol. 2020, 45, 493–498. [Google Scholar] [CrossRef]
- Saxena, A. Screening of Onion Cultivars for Fusarium Basal Rot and Spatial Distribution of Fusarium oxysporum f.sp. cepae. Master’s Thesis, New Mexico State University, Las Cruces, NM, USA, 2007. [Google Scholar]
- Busby, P.E.; Ridout, M.; Newcombe, G. Fungal endophytes: Modifiers of plant disease. Plant Mol. Biol. 2016, 90, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Abawi, G.S.; Lorbeer, J.W. Populations of Fusarium oxysporum f.sp. cepae in organic soils in New York. Phytopathology 1971, 61, 1042–1048. [Google Scholar] [CrossRef]
- Mandal, S.; Cramer, C.S. Breeding for fusarium basal rot resistance in short-day onions. HortScience 2018, 53, S287. [Google Scholar]
- Mandal, S.; Cramer, C.S. Advancement in breeding for Fusarium basal rot resistance of onion (Allium cepa L.). HortScience 2020, 55, S22. [Google Scholar]
- Mandal, S.; Cramer, C.S. Can onion tolerate a storage rot? In Proceedings of the American Society of Agronomy, the Crop Science Society of America, and the Soil Science Society of America International Annual Meeting, Madison, WI, USA, 9–13 November 2020. [Google Scholar]
- Marzu, J.C. Genetic Analyses of Resistances to Fusarium Basal Rot and Pink Root in Onion. Master’s Thesis, University of Wisconsin-Madison, Madison, WI, USA, 2015. [Google Scholar]
- Teshima, Y.; Ikeda, T.; Imada, K.; Sasaki, K.; El-Sayed, M.A.; Shigyo, M.; Tanaka, S.; Ito, S. Identification and biological activity of antifungal saponins from shallot (Allium cepa L. Aggregatum Group). J. Agric. Food Chem. 2013, 61, 7440–7445. [Google Scholar] [CrossRef]
- Vu, H.Q.; El-Sayed, M.A.; Ito, S.I.; Yamauchi, N.; Shigyo, M. Discovery of a new source of resistance to Fusarium oxysporum, cause of Fusarium wilt in Allium fistulosum, located on chromosome 2 of Allium cepa Aggregatum group. Genome 2012, 55, 797–807. [Google Scholar] [CrossRef]
- Abdelrahman, M.; El-Sayed, M.; Sato, S.; Hirakawa, H.; Ito, S.; Tanaka, K.; Mine, Y.; Sugiyama, N.; Suzuki, M.; Yamauchi, N.; et al. RNA-sequencing-based transcriptome and biochemical analyses of steroidal saponin pathway in a complete set of Allium fistulosum—A cepa monosomic addition lines. PLoS ONE 2017, 12, e0181784. [Google Scholar] [CrossRef]
- Lewis, T. Pest thrips in perspective. In Thrips as Crop Pests; Lewis, T., Ed.; CAB International: New York, NY, USA, 1997; pp. 1–13. [Google Scholar]
- Cranshaw, W.S. Thrips. In Compendium of Onion and Garlic Diseases and Pests, 2nd ed.; Schwartz, H.F., Mohan, S.K., Eds.; APS Press: Minneapolis, MN, USA, 2008; pp. 89–91. [Google Scholar]
- Diaz-Montano, J.; Fuchs, M.; Nault, B.A.; Fail, J.; Shelton, A.M. Onion thrips (Thysanoptera: Thripidae): A global pest of increasing concern in onion. J. Econ. Entomol. 2011, 104, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fournier, F.; Boivin, G.; Stewart, R. Effect of Thrips tabaci (Thysanoptera: Thripidae) on yellow onion yields and economic thresholds for its management. J. Econ. Entomol. 1995, 88, 1401–1407. [Google Scholar] [CrossRef]
- Leach, A.; Fuchs, M.; Harding, R.; Nault, B.A. Iris Yellow Spot Virus Prolongs the Adult Lifespan of Its Primary Vector, Onion Thrips (Thrips tabaci) (Thysanoptera: Thripidae). J. Insect Sci. 2019, 19, 3. [Google Scholar] [CrossRef] [PubMed]
- Kritzman, A.; Lampel, M.; Raccah, B.; Gera, A. Distribution and transmission of Iris yellow spot virus. Plant Dis. 2001, 85, 838–842. [Google Scholar] [CrossRef] [PubMed]
- Nagata, T.; Almeida, A.C.L.; Resende, R.D.O.; de Ávila, A.C. The identification of the vector species of iris yellow spot tospovirus occurring on onion in Brazil. Plant Dis. 1999, 83, 399. [Google Scholar] [CrossRef] [PubMed]
- Abdelkhalek, A.; ElMorsi, A.; AlShehaby, O.; Sanan-Mishra, N.; Hafez, E. Identification of genes differentially expressed in Iris yellow spot virus infected onion. Phytopathol. Mediterr. 2018, 257, 334–340. [Google Scholar]
- Abdelkhalek, A.; Quari, S.H.; Hafez, E. Iris yellow spot virus -induced chloroplast malformation results in male sterility. J. Biosci. 2019, 44, 142. [Google Scholar] [CrossRef]
- Cramer, C.S.; Singh, N.; Kamal, N.; Pappu, H.R. Screening onion plant introduction accessions for tolerance to onion thrips and Iris yellow spot. HortScience 2014, 49, 1253–1261. [Google Scholar] [CrossRef]
- Shelton, A.M.; Nault, B.A.; Plate, J.; Zhao, J.Z. Regional and temporal variation in susceptibility to lambda-cyhalothrin in onion thrips, Thrips tabaci (Thysanoptera: Thripidae), in onion fields in NY. J. Econ. Entomol. 2003, 96, 1843–1848. [Google Scholar] [CrossRef] [PubMed]
- Shelton, A.M.; Zhao, J.Z.; Nault, B.A.; Plate, J.; Musser, F.R.; Larentzaki, E. Patterns of insecticide resistance in onion thrips (Thysanoptera: Thripidae) in onion fields in NY. J. Econ Entomol. 2006, 99, 1798–1804. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.K.M.; Scott-Dupree, C.D.; Tolman, J.H.; Harris, C.R. Resistance of Thrips tabaci to pyrethroid and organophosphate insecticides in Ontario. Can. Pest Manag. Sci. 2005, 61, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Mohseni-Moghadam, M.M.; Cramer, C.S.; Steiner, R.L.; Creamer, R. Evaluating winter-sown onion entries for Iris yellow spot virus susceptibility. HortScience 2011, 46, 1224–1229. [Google Scholar] [CrossRef]
- Singh, N.; Cramer, C.S. Improved tolerance for onion thrips and Iris yellow spot in onion plant introductions after two selection cycles. Horticulturae 2019, 5, 18. [Google Scholar] [CrossRef]
- Boateng, C.O.; Schwartz, H.F.; Havey, M.J.; Otto, K. Evaluation of onion germplasm for resistance to Iris yellow spot (Iris yellow spot virus) and onion thrips, Thrips tabaci. Southwest. Entomol. 2014, 39, 237–260. [Google Scholar] [CrossRef]
- Jones, H.A.; Bailey, S.F.; Emsweller, S.L. Thrips resistance in onion. Hilgardia 1934, 8, 215–252. [Google Scholar] [CrossRef]
- Kamal, N.; Shahabeddin Nourbakhsh, S.; Cramer, C.S. Reduced Iris Yellow Spot Symptoms through Selection within Onion Breeding Lines. Horticulturae 2021, 7, 12. [Google Scholar] [CrossRef]
- Njau, G.M.; Nyomora, A.M.S.; Dinssa, F.F. Evaluation of onion (Allium cepa) germplasm entries for resistance to onion thrips, Thrips tabaci (Lindeman) in Tanzania. Int. J. Trop. Insect Sci. 2017, 37, 98–113. [Google Scholar] [CrossRef]
- De Oliveira, F.G.; Santos, C.A.F.; Oliveira, V.R.; de Alencar, J.A.; da Silva, D.O.M. Evaluation of onion accessions for resistance to thrips in Brazilian semi-arid regions. J. Hortic. Sci. Biotechnol. 2017, 92, 550–558. [Google Scholar] [CrossRef]
- Raut, A.M.; Pal, S.; Wahengbam, J.; Najitha Banu, A. Population dynamics of onion thrips (Thrips tabaci Lind., Thysanoptera; Thripidae) and varietal response of onion cultivars against onion thrips. J. Entomol. Res. 2020, 44, 547–554. [Google Scholar] [CrossRef]
- Coudriet, D.L.; Kishaba, A.N.; McCreight, J.D.; Bohn, W.G. Varietal resistance in onions to thrips (Thysanoptera, Thripidae). J. Econ. Entomol. 1979, 72, 614–615. [Google Scholar] [CrossRef]
- Damon, S.J.; Groves, R.L.; Havey, M.J. Variation for epicuticular waxes on onion foliage and impacts on numbers of onion thrips. J. Am. Soc. Hortic. Sci. 2014, 139, 495–501. [Google Scholar] [CrossRef]
- Alimousavi, S.A.; Hassandokht, M.R.; Moharramipour, S. Evaluation of Iranian onion germplasms for resistance to thrips. Int. J. Agric. Biol. 2007, 9, 897–900. [Google Scholar]
- Molenaar, N. Genetics, Thrips (Thrips tabaci L.) Resistance and Epicuticular Wax Characteristics of Nonglossy and Glossy Onions (Allium cepa L.). Ph.D. Thesis, University of Wisconsin, Madison, WI, USA, 1984. [Google Scholar]
- Mote, U.N.; Sonone, H.N. Relative susceptibility of different varieties of onion (Allium cepa) to thrips (Thrips tabaci Lind.). J. Maharashtra Agric. Univ. 1977, 2, 152–155. [Google Scholar]
- Pawar, B.B.; Patil, A.V.; Sonone, H.N. A thrips resistant glossy selection in white onions. Res. J. Mahatma Phule Agric. Univ. 1975, 6, 152–153. [Google Scholar]
- Jones, H.A.; Clarke, A.E.; Stevenson, F.J. Studies in the genetics of the onion (Allium cepa L.). Proc. Am. Soc. Hort. Sci. 1944, 44, 479–484. [Google Scholar]
- Munaiz, E.D.; Havey, M.J. Genetic analyses of epicuticular waxes associated with the glossy phenotype of ‘White Persian’ onion. J. Am. Soc. Hort. Sci. 2020, 145, 67–72. [Google Scholar] [CrossRef]
- Munaiz, E.D.; Townsend, P.A.; Havey, M.J. Reflectance spectroscopy for non-destructive measurement and genetic analysis of amounts and types of epicuticular waxes on onion leaves. Molecules 2020, 25, 3454. [Google Scholar] [CrossRef] [PubMed]
- Czencz, K. The role of coloured traps in collecting thrips fauna. In Population Structure, Genetics and Taxonomy of Aphids and Thysanoptera; Holman, J., Pelikan, J., Dixon, A.F.G., Weisman, L., Eds.; SPB Academic Publishing: The Hague, The Netherlands, 1987; pp. 426–435. [Google Scholar]
- Kirk, W.D.J. Ecologically selective coloured traps. Ecol. Entomol. 1984, 9, 35–41. [Google Scholar] [CrossRef]
- Diaz-Montano, J.; Fail, J.; Deutschlander, M.; Nault, B.A.; Shelton, A.M. Characterization of resistance, evaluation of the attractiveness of plant odors, and effect of leaf color on different onion cultivars to onion thrips (Thysanoptera: Thripidae). J. Econ. Entomol. 2012, 105, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Muvea, A.M.; Meyhöfer, R.; Maniania, N.K.; Poehling, H.-M.; Ekesi, S.; Subramanian, S. Behavioral responses of Thrips tabaci Lindemann to endophyte inoculated onion plants. J. Pest Sci. 2015, 88, 555–562. [Google Scholar] [CrossRef]
- Muvea, A.M.; Meyhöfer, R.; Subramanian, S.; Poehling, H.-M.; Ekesi, S.; Maniania, N.K. Colonization of onions by endophytic fungi and their impacts on the biology of Thrips tabaci. PLoS ONE 2014, 9, e108242. [Google Scholar] [CrossRef]
- Muvea, A.M.; Subramanian, S.; Maniania, N.K.; Poehling, H.-M.; Ekesi, S.; Meyhöfer, R. Endophytic colonization of onions induces resistance to viruliferous thrips and virus replication. Front. Plant Sci. 2018, 9, 1785. [Google Scholar] [CrossRef]
- Bag, S.; Schwartz, H.F.; Cramer, C.S.; Havey, M.J.; Pappu, H.R. Iris yellow spot virus (Tospovirus: Bunyaviridae): From obscurity to priority. Mol. Plant Pathol. 2015, 16, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Multani, P.S.; Cramer, C.S.; Steiner, R.L.; Creamer, R. Screening winter-sown onion entries for Iris yellow spot virus tolerance. HortScience 2009, 44, 627–632. [Google Scholar] [CrossRef]
- Kamal, N. Selection Progress and Cost Benefit Analysis of Iris Yellow Spot Resistance in Onions. Ph.D. Thesis, New Mexico State University, Las Cruces, NM, USA, 2016. [Google Scholar]
- Kamal, N.; Cramer, C.S. Screening progress for resistance to Iris yellow spot in onions. HortScience 2018, 53, 1088–1094. [Google Scholar] [CrossRef]
- Cramer, C.S.; Mohseni-Moghadam, M.; Creamer, R.J.; Steiner, R.L. Screening winter-sown entries for Iris yellow spot disease susceptibility. In Proceedings of the 2012 National Allium Research Conference, Las Cruces, NM, USA, 12–14 December 2012; Walker, S., Cramer, C.S., Eds.; New Mexico State University: Las Cruces, NM, USA, 2012; pp. 80–99. [Google Scholar]
- Diaz-Montano, J.; Fuchs, M.; Nault, B.A.; Shelton, A.M. Evaluation of onion cultivars for resistance to onion thrips (Thysanoptera: Thripidae) and Iris yellow spot virus. J. Econ. Entomol. 2010, 103, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, L.J.; Pelter, G.Q.; Pappu, H.R. IYSV challenges to the onion seed industry in Washington. In Proceedings of the National Allium Research Conference, Grand Junction, CO, USA, 8–10 December 2004; Swift, C., Ed.; Colorado State University: Ft. Collins, CO, USA, 2004; pp. 213–217. [Google Scholar]
- Reitz, S.R.; Cramer, C.S.; Shock, C.C.; Feibert, E.B.G.; Rivera, A.; Saunders, L.D. Evaluation of New Onion Lines for Resistance to Onion Thrips and Iris Yellow Spot Virus—Malheur Experiment Station Annual Report 2015; Oregon State University Agricultural Experiment Station Ext/CrS 156; Oregon State University: Corvallis, OR, USA, 2016; pp. 170–174. [Google Scholar]
- Schwartz, H.F.; Gent, D.H.; Fichtner, S.F.; Hammon, R.W.; Khosla, R. Integrated management of Iris yellow spot virus in onion. In Proceedings of the 2004 National Allium Research Conference, Grand Junction, CO, USA, 8–10 December 2004; Swift, C., Ed.; Colorado State University: Ft. Collins, CO, USA, 2004; pp. 207–212. [Google Scholar]
- Creamer, R.; Sanogo, S.; Moya, A.; Romero, J.; Molina-Bravo, R.; Cramer, C.S. Iris yellow spot virus on onion in New Mexico. Plant Dis. 2004, 88, 1049. [Google Scholar] [CrossRef] [PubMed]
- Schwimmer, S.; Carson, J.F.; Makower, R.U.; Mazelis, M.; Wong, F.F. Demonstration of alliinase in a protein preparation from onion. Experientia 1960, 16, 449–450. [Google Scholar] [CrossRef]
- Brodnitz, M.H.; Pascale, J.V. Thiopropanal s-oxide: A lachrymatory factor in onions. J. Agric. Food Chem. 1971, 19, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.; Tsuge, N.; Tomotake, M.; Nagatome, Y.; Sawada, H.; Nagata, T.; Kumagai, H. An onion enzyme that makes the eyes water. Nature 2002, 419, 685. [Google Scholar] [CrossRef] [PubMed]
- Randle, W.M.; Bussard, M.L. Pungency and sugars of short-day onions as affected by sulfur nutrition. J. Am. Soc. Hortic. Sci. 1993, 118, 766–770. [Google Scholar] [CrossRef]
- Randle, W.M.; Lancaster, J.E.; Shaw, M.L.; Sutton, K.H.; Hay, R.L.; Bussard, M.L. Quantifying onion flavor compounds responding to sulfur fertility-sulfur increases levels of alk(en)yl cysteine sulfoxides and biosynthetic intermediates. J. Am. Soc. Hortic. Sci. 1995, 120, 1075–1081. [Google Scholar] [CrossRef]
- Randle, W.M. Onion flavor chemistry and factors influencing flavor intensity. In Spices: Flavor Chemistry and Antioxidant Properties; Risch, S.J., Ho, C.T., Eds.; American Chemical Society: Washington, DC, USA, 1997; Volume 119, pp. 41–52. [Google Scholar] [CrossRef]
- Kopsell, D.E.; Randle, W.M.; Eiteman, M.A. Changes in the s-alk(en)yl cysteine sulfoxides and their biosynthetic intermediates during onion storage. J. Am. Soc. Hortic. Sci. 1999, 124, 177–183. [Google Scholar] [CrossRef]
- Yoo, K.S.; Pike, L.M.; Patil, B.S.; Lee, E.J. Developing sweet onions by recurrent selection in a short-day onion breeding program. Sci. Hortic. 2020, 266, 109269. [Google Scholar] [CrossRef]
- Schwimmer, S.; Weston, W.J. Onion flavor and odor, enzymatic development of pyruvic acid in onion as a measure of pungency. J. Agric. Food Chem. 1961, 9, 301–304. [Google Scholar] [CrossRef]
- Eady, C.C.; Kamoi, T.; Kato, M.; Porter, N.G.; Davis, S.; Shaw, M.K.; Kamoi, A.; Imai, S. Silencing onion lachrymatory factor synthase causes a significant change in the sulfur secondary metabolite profile. Plant Physiol. 2008, 147, 2096–2106. [Google Scholar] [CrossRef] [PubMed]
- Randle, W.M.; Block, E.; Littlejohn, M.H.; Putnman, D.; Bussard, M.L. Onion (Allium cepa L.) thiosulfinates respond to increasing sulfur fertility. J. Agric. Food Chem. 1994, 42, 2085–2088. [Google Scholar] [CrossRef]
- Yoo, K.S.; Pike, L.M.; Hamilton, B.K. A simplified pyruvic acid analysis suitable for onion breeding programs. HortScience 1995, 30, 1306. [Google Scholar] [CrossRef]
- Kato, M.; Masamura, N.; Shono, J.; Okamoto, D.; Abe, T.; Imai, S. Production and characterization of tearless and non-pungent onion. Sci. Rep. 2016, 6, 23779. [Google Scholar] [CrossRef] [PubMed]
- Vegetable Growers News: Sunion, the ‘Tearless’ Onions, Hit Select Store Shelves. Available online: https://vegetablegrowersnews.com/news/sunions-tearless-onions-hit-select-store-shelves/ (accessed on 18 November 2020).
- Scully, N.J.; Parker, M.W.; Borthwick, H.A. Interaction of nitrogen nutrition and photoperiod as expressed in bulbing and flower-stalk development of onion. Bot. Gaz. 1945, 107, 52–61. [Google Scholar] [CrossRef]
- Van Kampen, I.J. Shortening the breeding cycle in onions (Allium cepa L.). Meded. Proefstn. Voor Groentet. Vollegrond Ned. 1970, 51, 1–65. [Google Scholar]
- D’Angelo, C.J.; Goldman, I.L. Breaking onion bulb endodormancy with hydrogen peroxide. Accepted upon acceptable revision. HortScience 2019, 54, 1694–1702. [Google Scholar] [CrossRef]
- D’Angelo, C.J.; Goldman, I.L. Temporal aspects of vernalization and flowering in long-day storage onion. J. Am. Soc. Hortic. Sci. 2018, 143, 446–453. [Google Scholar] [CrossRef]
- Baldwin, S.; Revanna, R.; Pither-Joyce, M.; Shaw, M.; Wright, K.; Thomson, S.; Moya, L.; Lee, R.; Macknight, R.C.; McCallum, J.A. Genetic analyses of bolting in bulb onion (Allium cepa L.). Theor. Appl. Genet. 2014, 127, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Bertaud, D.S. Effects of chilling duration, photoperiod and temperature on floral initiation and development in sprouted and unsprouted onion bulbs. In Proceedings of the 4th EUCARPIA Allium Symposium, Wellesbourne, UK, 6–9 September 1988; pp. 254–261. [Google Scholar]
- Shishido, Y.; Saito, T. Studies on the flower bud formation in onion plants III. Effects of physiologial conditions on the low temperature induction of flower buds in bulbs. J. Jpn. Soc. Hortic. Sci. 1977, 46, 310–316. [Google Scholar] [CrossRef][Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cramer, C.S.; Mandal, S.; Sharma, S.; Nourbakhsh, S.S.; Goldman, I.; Guzman, I. Recent Advances in Onion Genetic Improvement. Agronomy 2021, 11, 482. https://doi.org/10.3390/agronomy11030482
Cramer CS, Mandal S, Sharma S, Nourbakhsh SS, Goldman I, Guzman I. Recent Advances in Onion Genetic Improvement. Agronomy. 2021; 11(3):482. https://doi.org/10.3390/agronomy11030482
Chicago/Turabian StyleCramer, Christopher S., Subhankar Mandal, Suman Sharma, Seyed Shahabedddin Nourbakhsh, Irwin Goldman, and Ivette Guzman. 2021. "Recent Advances in Onion Genetic Improvement" Agronomy 11, no. 3: 482. https://doi.org/10.3390/agronomy11030482
APA StyleCramer, C. S., Mandal, S., Sharma, S., Nourbakhsh, S. S., Goldman, I., & Guzman, I. (2021). Recent Advances in Onion Genetic Improvement. Agronomy, 11(3), 482. https://doi.org/10.3390/agronomy11030482






