Genome-Wide Identification of WRKY Gene Family and Expression Analysis under Abiotic Stress in Barley
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of WRKY Family Members in Barley and Their Chromosomal Locations
2.2. Phylogenetic Analysis of WRKY Family Members and Sequence Alignment
2.3. Structural Analysis of WRKY Genes
2.4. Expression Profile of WRKY Genes at Different Growth Stages and in Different Tissues
2.5. Stress-Related cis-Elements in HvWRKY Promoter Regions
2.6. Abiotic Stress Treatment at the Barley Seedling Stage
2.7. Quantitative RT-PCR Analysis of the HvWRKY Gene Response to Abiotic Stress
3. Results
3.1. Identification and Chromosomal Locations of HvWRKYs
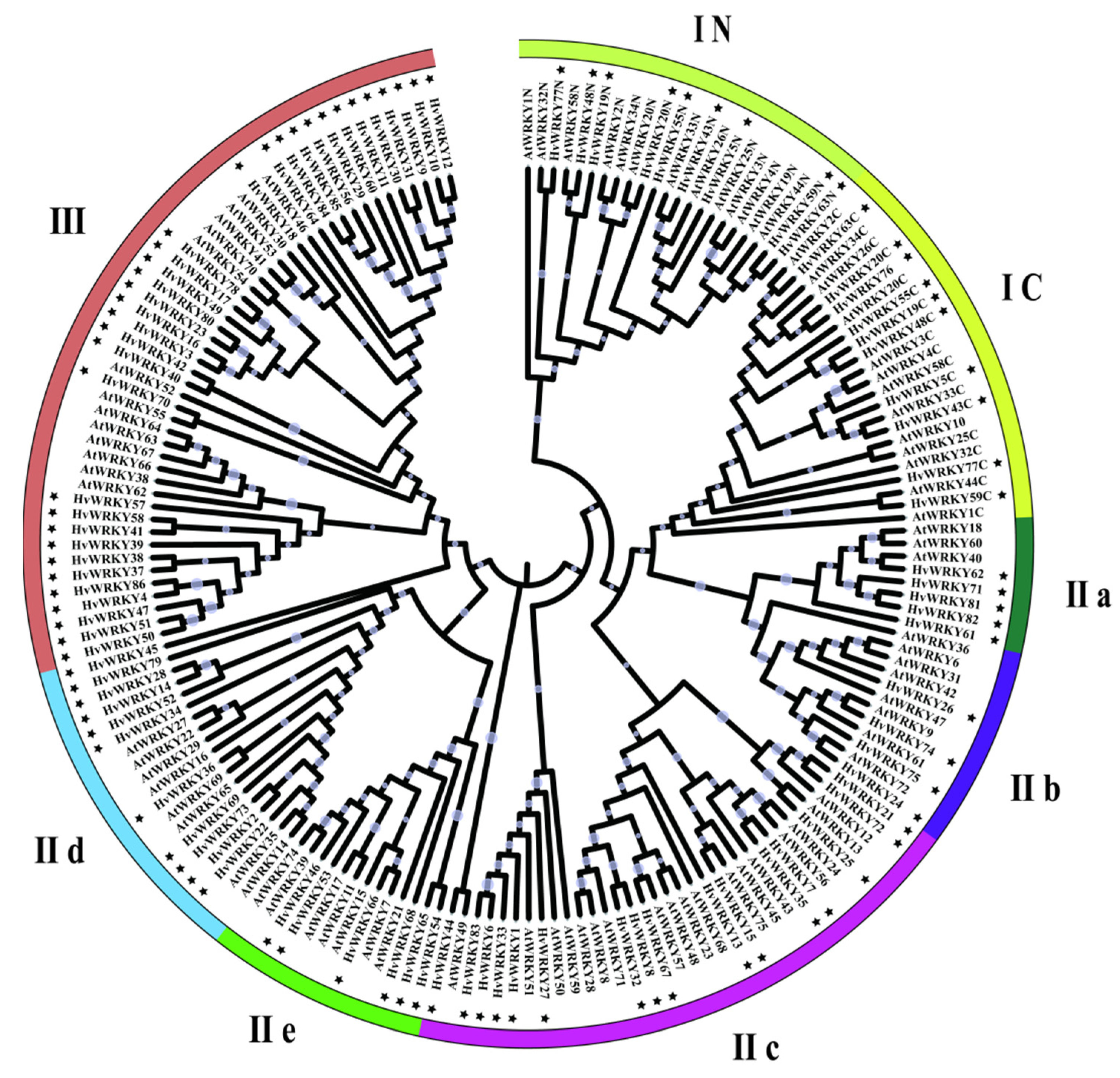
3.2. Phylogenetic Classification of HvWRKY Genes
3.3. Multiple Sequence Alignment of WRKY Domains
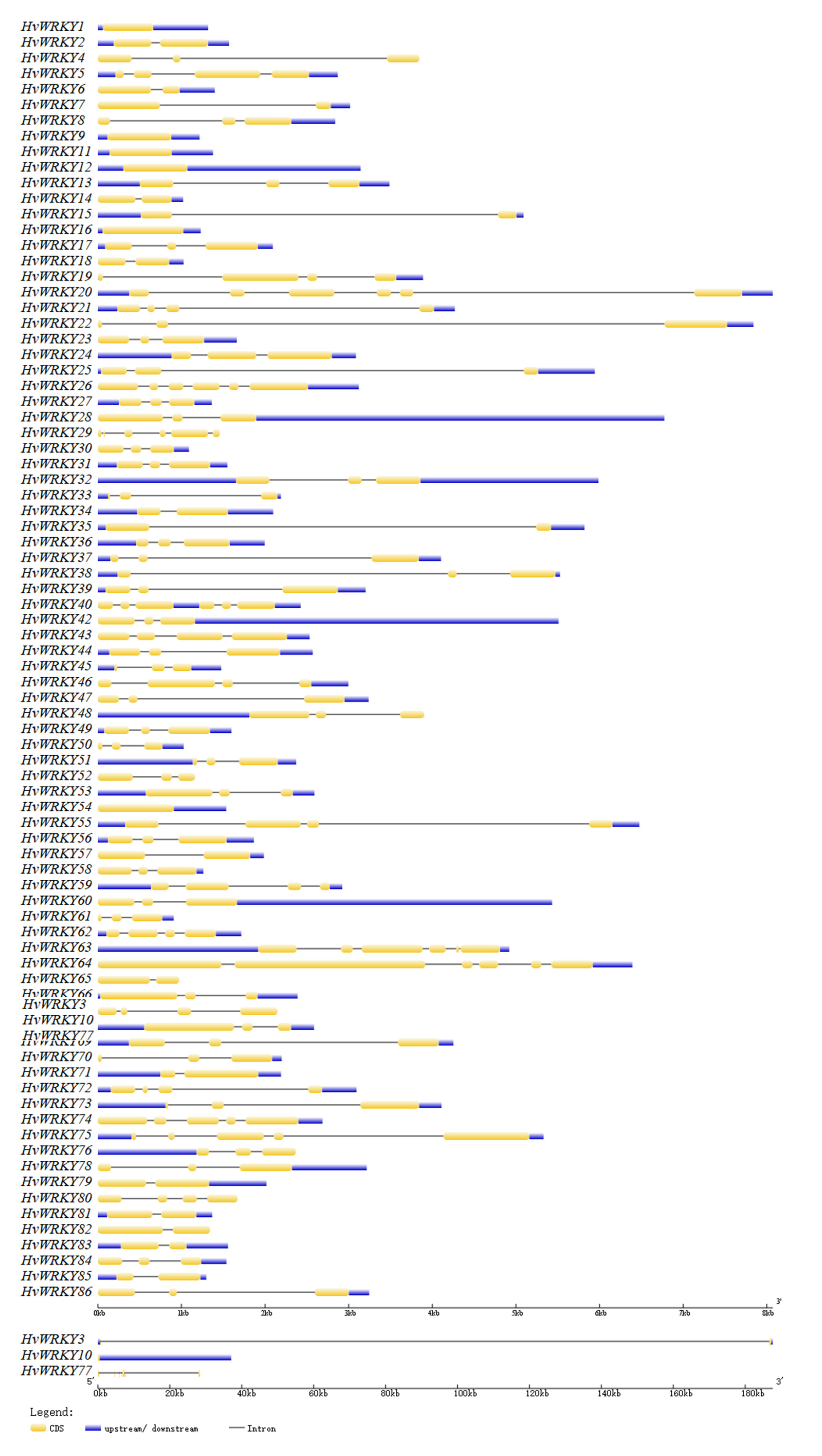
3.4. Conserved Motifs of HvWRKY and the Structure of Their Genes
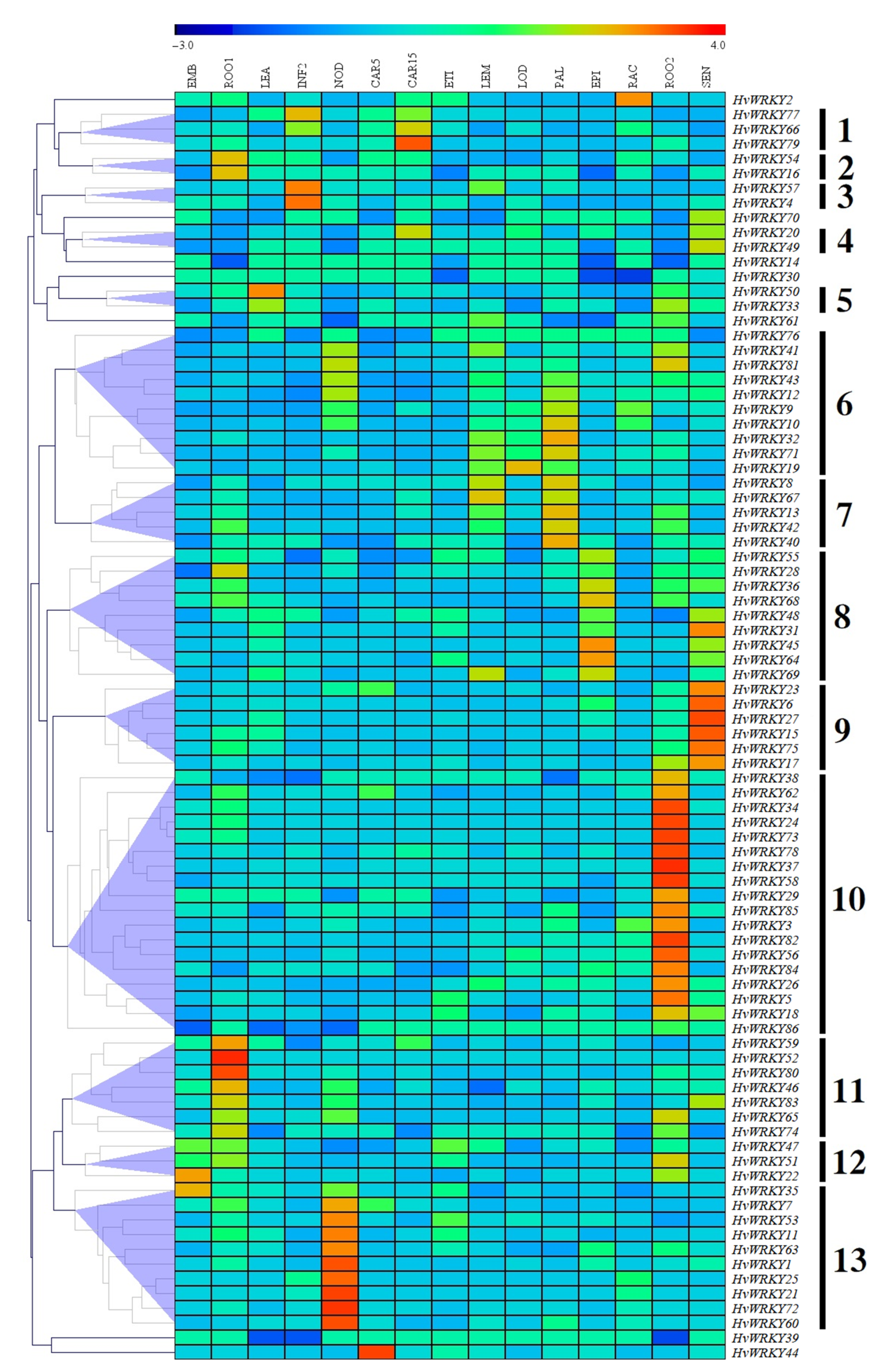
3.5. Analysis of Expression Levels at Different Growth Stages and in Different Tissues
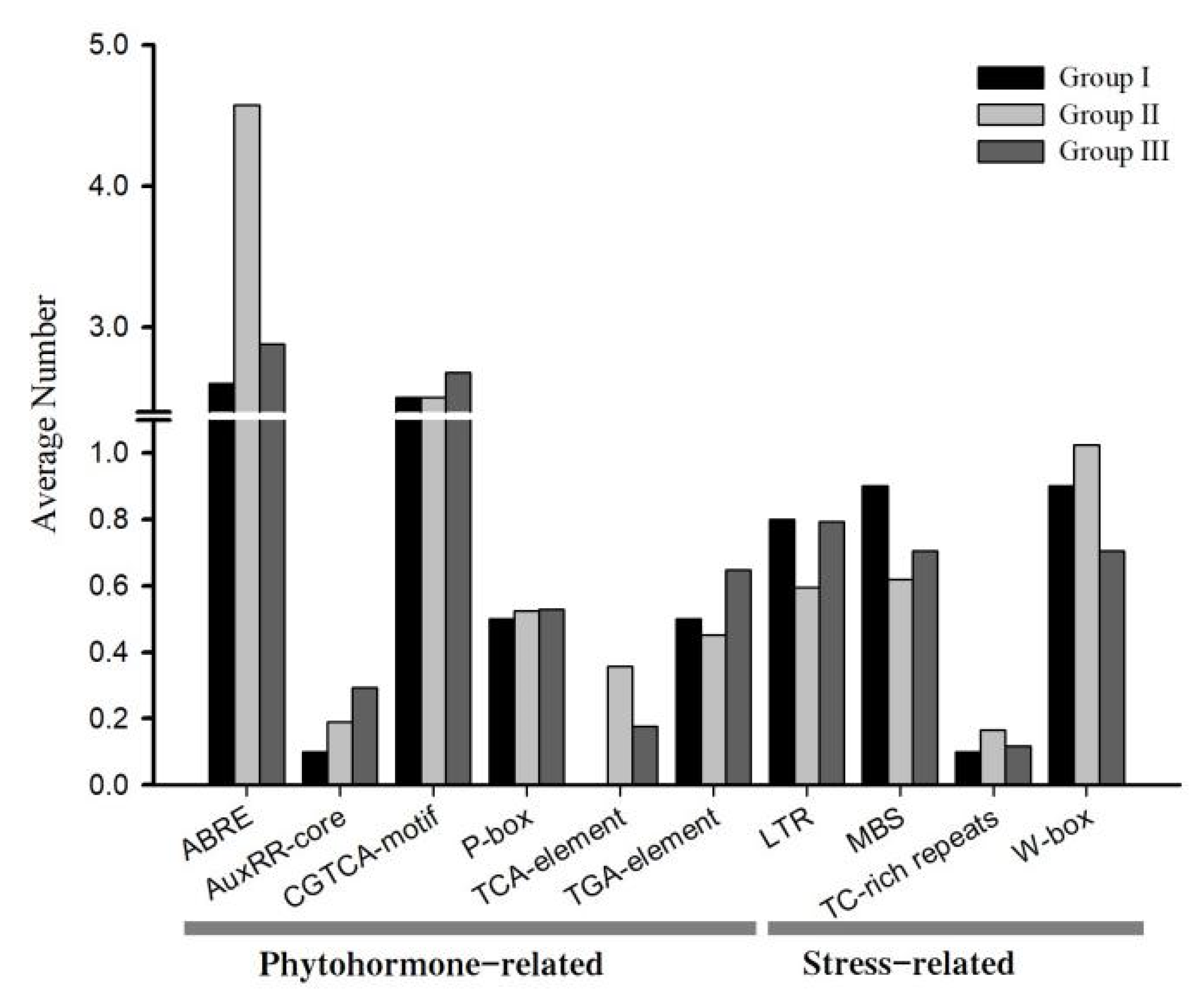
3.6. Analysis of cis-Elements
3.7. Transcriptional Responses of HvWRKY Genes to Abiotic Stresses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eulgem, T.; Rushton, P.J.; Robatzek, S.; Somssich, I.E. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000, 5, 199–206. [Google Scholar] [CrossRef]
- Rushton, P.J.; Somssich, I.E.; Ringler, P.; Shen, Q.J. WRKY transcription factors. Trends Plant Sci. 2010, 15, 247–258. [Google Scholar] [CrossRef]
- Sheng, Y.B.; Yan, X.X.; Huang, Y.; Han, Y.Y.; Zhang, C.; Ren, Y.B.; Fan, T.T.; Xiao, F.M.; Liu, Y.S.; Cao, S.Q. The WRKY transcription factor, WRKY13, activates PDR8 expression to positively regulate cadmium tolerance in Arabidopsis. Plant Cell Environ. 2019, 42, 891–903. [Google Scholar] [CrossRef]
- Xu, Z.L.; Raza, Q.; Xu, L.; He, X.L.; Huang, Y.H.; Yi, J.X.; Zhang, D.Y.; Shao, H.B.; Ma, H.X.; Ali, Z. GmWRKY49, a Salt-Responsive Nuclear Protein, Improved Root Length and Governed Better Salinity Tolerance in Transgenic Arabidopsis. Front. Plant Sci. 2018, 9, 809. [Google Scholar] [CrossRef]
- Lee, H.; Cha, J.; Choi, C.; Choi, N.; Ji, H.S.; Park, S.R.; Lee, S.; Hwang, D.J. Rice WRKY11 Plays a Role in Pathogen Defense and Drought Tolerance. Rice 2018, 11, 5. [Google Scholar] [CrossRef]
- Wang, C.T.; Ru, J.N.; Liu, Y.W.; Li, M.; Zhao, D.; Yang, J.F.; Fu, J.D.; Xu, Z.S. Maize WRKY Transcription Factor ZmWRKY106 Confers Drought and Heat Tolerance in Transgenic Plants. Int. J. Mol. Sci. 2018, 19, 3046. [Google Scholar] [CrossRef]
- Ishiguro, S.; Nakamura, K. Characterization of a cDNA encoding a novel DNA.binding protein, SPF1, that recognizes SP8 sequences in the 5’ upstream regions of genes coding for sporamin and β-amylase from sweet potato. Mol. Gen. Genet. 1994, 244, 563–571. [Google Scholar] [CrossRef]
- Wang, Q.S.; Wang, M.H.; Zhang, X.Z.; Hao, B.J.; Kaushik, S.K.; Pan, Y.C. WRKY gene family evolution in Arabidopsis thaliana. Genetica 2011, 139, 973. [Google Scholar] [CrossRef]
- Wu, K.L.; Guo, Z.J.; Wang, H.H.; Li, J. The WRKY Family of Transcription Factors in Rice and Arabidopsis and Their Origins. DNA Res. 2005, 12, 9–26. [Google Scholar] [CrossRef]
- Bencke-Malato, M.; Cabreira, C.; Wiebke-Strohm, B.; Bucker-Neto, L.; Mancini, E.; Osorio, M.B.; Homrich, M.S.; Turchetto-Zolet, A.C.; Carvalho, M.C.D.; Stolf, R. Genome-wide annotation of the soybean WRKY family and functional characterization of genes involved in response to Phakopsora pachyrhizi infection. BMC Plant Biol. 2014, 14, 236. [Google Scholar] [CrossRef]
- Xie, T.; Chen, C.J.; Li, C.H.; Liu, J.R.; Liu, C.Y.; He, Y.H. Genome-wide investigation of WRKY gene family in pineapple: Evolution and expression profiles during development and stress. BMC Genom. 2018, 19, 490. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.X.; Gao, Y.F.; Liu, J.K.; Peng, X.L.; Niu, X.L.; Fei, Z.J.; Cao, S.Q.; Liu, Y.S. Genome-wide analysis of WRKY transcription factors in Solanum lycopersicum. Mol. Genet. Genom. 495–513. [CrossRef]
- Dou, L.L.; Zhang, X.H.; Pang, C.Y.; Song, M.Z.; Wei, H.L.; Fan, S.L.; Yu, S.X. Genome-wide analysis of the WRKY gene family in cotton. Mol. Genet. Genom. 2014, 289, 1103–1121. [Google Scholar] [CrossRef] [PubMed]
- Wen, F.; Zhu, H.; Li, P.; Jiang, M.; Mao, W.Q.; Ong, C.; Chu, Z.Q. Genome-wide evolutionary characterization and expression analyses of WRKY family genes in Brachypodium distachyon. DNA Res. 2014, 21, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Fang, Y.X.; Zhang, Z.L.; Zheng, J.J.; Zhang, X.; Li, J.; Niu, C.Y.; Xue, D.W.; Zhang, X.Q. Genome-wide identification and expression pattern analysis of the KCS gene family in barley. Plant Growth Regul. 2021, 93, 89–103. [Google Scholar] [CrossRef]
- Tong, T.; Fang, Y.X.; Zhang, Z.L.; Zheng, J.J.; Lu, X.L.; Zhang, X.Q.; Xue, D.W. Genome-wide identification, phylogenetic and expression analysis of SBP-box gene family in barley (Hordeum vulgare L.). Plant Growth Regul. 2020, 90, 137–149. [Google Scholar] [CrossRef]
- Finn, R.D.; Clements, J.; Eddy, S.R. HMMER Web Server: Interactive Sequence Similarity Searching. Nucleic Acids Res. 2011, 39, W29–W37. [Google Scholar] [CrossRef]
- Huang, Y.; Niu, B.F.; Gao, Y.; Fu, L.M.; Li, W.Z. CD-HIT Suite: A web server for clustering and comparing biological sequences. Bioinformatics 2010, 26, 680–682. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. 20 years of the SMART protein domain annotation resource. Nucleic Acids Res. 2018, 46, 493–496. [Google Scholar] [CrossRef]
- Chao, J.T.; Kong, Y.Z.; Wang, Q.; Sun, Y.H.; Gong, D.P.; Lv, J.; Liu, G.S. MapGene2Chrom, a tool to draw gene physical map based on Perl and SVG languages. Hereditas 2015, 37, 91–97. [Google Scholar] [CrossRef]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, 256–259. [Google Scholar] [CrossRef]
- Guo, A.Y.; Zhu, Q.H.; Chen, X.; Luo, J.C. GSDS: A gene structure display server. Hereditas 2007, 29, 1023–1026. [Google Scholar] [CrossRef]
- Saeed, A.I.; Sharov, V.; White, J.; Li, J.; Liang, W.; Bhagabati, N.; Braisted, J.; Klapa, M.; Currier, T.; Thiagarajan, M. TM4: A free, open-source system for microarray data management and analysis. Biotechniques 2003, 34, 374–378. [Google Scholar] [CrossRef]
- Rombauts, S.; Déhais, P.; Montagu, M.V.; Rouzé, P. PlantCARE, a plant cis-acting regulatory element database. Nucleic Acids Res. 1999, 27, 295–296. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Tong, T.; Fang, Y.X.; Zheng, J.J.; Zhang, X.; Niu, C.Y.; Li, J.; Zhang, X.Q.; Xue, D.W. Genome-wide identification of barley ABC genes and their expression in response to abiotic stress treatment. Plants 2020, 9, 1281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.T.; Wang, S.Y.; Chen, Y.Y.; Dong, M.Y.; Fang, Y.X.; Zhang, X.; Tong, T.; Zhang, Z.L.; Zheng, J.J.; Xue, D.W. Genome-wide identification of the F-box gene family and expression analysis under drought and salt stress in barley. Phyton-International J. Exp. Bot. 2020, 89, 229–251. [Google Scholar] [CrossRef]
- Huang, X.S.; Li, K.Q.; Xu, X.Y.; Yao, Z.H.; Jin, C.; Zhang, S.L. Genome-wide analysis of WRKY transcription factors in white pear (Pyrus bretschneideri) reveals evolution and patterns under drought stress. BMC Genom. 2015, 16, 1104. [Google Scholar] [CrossRef]
- Guo, C.L.; Guo, R.R.; Xu, X.Z.; Gao, M.; Li, X.Q.; Song, J.Y.; Zheng, Y.; Wang, X.P. Evolution and expression analysis of the grape (Vitis vinifera L.) WRKY gene family. J. Exp. Bot. 2014, 65, 1513–1528. [Google Scholar] [CrossRef]
- He, H.S.; Dong, Q.; Shao, Y.H.; Jiang, H.Y.; Zhu, S.W.; Cheng, B.J.; Xiang, Y. Genome-wide survey and characterization of the WRKY gene family in Populus trichocarpa. Plant Cell Rep. 2012, 31, 1199–1217. [Google Scholar] [CrossRef]
- Verk, M.C.; Pappaioannou, D.; Neeleman, L.; Bol, J.F.; Linthorst, H.J.M. A novel WRKY transcription factor is required for induction of PR-1a gene expression by salicylic acid and bacterial elicitors. Plant Physiol. 2008, 146, 1983–1995. [Google Scholar] [CrossRef]
- Zhou, Q.Y.; Tian, A.G.; Zou, H.F.; Xie, Z.M.; Lei, G.; Huang, J.; Wang, C.M.; Wang, H.W.; Zhang, J.S.; Chen, S.Y. Soybean WRKY-type transcription factor genes, GmWRKY13, GmWRKY21, and GmWRKY54, confer differential tolerance to abiotic stresses in transgenic Arabidopsis plants. Plant Biotechnol. J. 2008, 6, 486–503. [Google Scholar] [CrossRef] [PubMed]
- Brand, L.H.; Fischer, N.M.; Harter, K.; Kohlbacher, O.; Wanke, D. Elucidating the evolutionary conserved DNA-binding specificities of WRKY transcription factors by molecular dynamics and in vitro binding assays. Nucleic Acids Res. 2013, 41, 9764–9778. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.F.; Chen, J.; Chen, Y.F.; Wu, L.J.; Xie, D.X. Molecular phylogenetic and expression analysis of the complete WRKY transcription factor family in maize. DNA Res. 2012, 19, 153–164. [Google Scholar] [CrossRef]
- Ross, C.A.; Liu, Y.; Shen, Q.J. The WRKY gene family in rice (Oryza sativa). J. Integr. Plant Biol. 2007, 49, 827–842. [Google Scholar] [CrossRef]
- Kaliyappan, R.; Viswanathan, S.; Suthanthiram, B.; Subbaraya, U.; Somasundram, S.M.; Muthu, M. Evolutionary expansion of WRKY gene family in banana and its expression profile during the infection of root lesion nematode, Pratylenchus coffeae. PLoS ONE 2016, 11, e0162013. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Ma, B.J.; Wu, Z.G.; Li, N.N.; Zheng, L.L.; Wang, Y.C. Reaumuria trigyna transcription factor RtWRKY23 enhances salt stress tolerance and delays flowering in plants. J. Plant Physiol. 2019, 239, 38–51. [Google Scholar] [CrossRef]
- Phukan, U.J.; Jeena, G.S.; Shukla, R.K. WRKY transcription factors: Molecular regulation and stress responses in plants. Front. Plant Sci. 2016, 7, 760. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, W.; Smet, I.D.; Rybel, B.D.; Robert, H.S.; Cotte, B.V.D.; Willemsen, V.; Gheysen, G.; Weijers, D.; Friml, J.; Beecjman, T. Tightly controlled WRKY23 expression mediates Arabidopsis embryo development. EMBO Rep. 2013, 14, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Z.; Avci, U.; Nakashima, J.; Hahn, M.G.; Chen, F.; Dixon, R.A. Mutation of WRKY transcription factors initiates pith secondary wall formation and increases stem biomass in dicotyledonous plants. Proc. Natl. Acad. Sci. USA 2010, 107, 22338–22343. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Feng, L.; Zhu, Y.X.; Li, Y.; Yan, H.W.; Xiang, Y. Comparative genomic analysis of the WRKY III gene family in populus, grape, arabidopsis and rice. Biol. Direct 2015, 10, 48. [Google Scholar] [CrossRef]
- Wang, C.; Deng, P.Y.; Chen, L.L.; Wang, X.T.; Ma, H.; Hu, W.; Yao, N.C.; Feng, Y.; Chai, R.H.; Yang, G.X. A Wheat WRKY Transcription Factor TaWRKY10 Confers Tolerance to Multiple Abiotic Stresses in Transgenic Tobacco. PLoS ONE 2013, 8, e65120. [Google Scholar] [CrossRef]
- Wu, M.; Liu, H.L.; Han, G.M.; Cai, R.H.; Pan, F.; Xiang, Y. A moso bamboo WRKY gene PeWRKY83 confers salinity tolerance in transgenic Arabidopsis plants. Sci. Rep. 2017, 7, 11721. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, J.; Zhang, Z.; Tong, T.; Fang, Y.; Zhang, X.; Niu, C.; Li, J.; Wu, Y.; Xue, D.; Zhang, X. Genome-Wide Identification of WRKY Gene Family and Expression Analysis under Abiotic Stress in Barley. Agronomy 2021, 11, 521. https://doi.org/10.3390/agronomy11030521
Zheng J, Zhang Z, Tong T, Fang Y, Zhang X, Niu C, Li J, Wu Y, Xue D, Zhang X. Genome-Wide Identification of WRKY Gene Family and Expression Analysis under Abiotic Stress in Barley. Agronomy. 2021; 11(3):521. https://doi.org/10.3390/agronomy11030521
Chicago/Turabian StyleZheng, Junjun, Ziling Zhang, Tao Tong, Yunxia Fang, Xian Zhang, Chunyu Niu, Jia Li, Yuhuan Wu, Dawei Xue, and Xiaoqin Zhang. 2021. "Genome-Wide Identification of WRKY Gene Family and Expression Analysis under Abiotic Stress in Barley" Agronomy 11, no. 3: 521. https://doi.org/10.3390/agronomy11030521
APA StyleZheng, J., Zhang, Z., Tong, T., Fang, Y., Zhang, X., Niu, C., Li, J., Wu, Y., Xue, D., & Zhang, X. (2021). Genome-Wide Identification of WRKY Gene Family and Expression Analysis under Abiotic Stress in Barley. Agronomy, 11(3), 521. https://doi.org/10.3390/agronomy11030521






