Seeds Quality and Quantity of Soybean [Glycine max (L.) Merr.] Cultivars in Response to Cold Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants and Growth Conditions
2.2. Methods and Measurements
2.3. Statistical Analysis
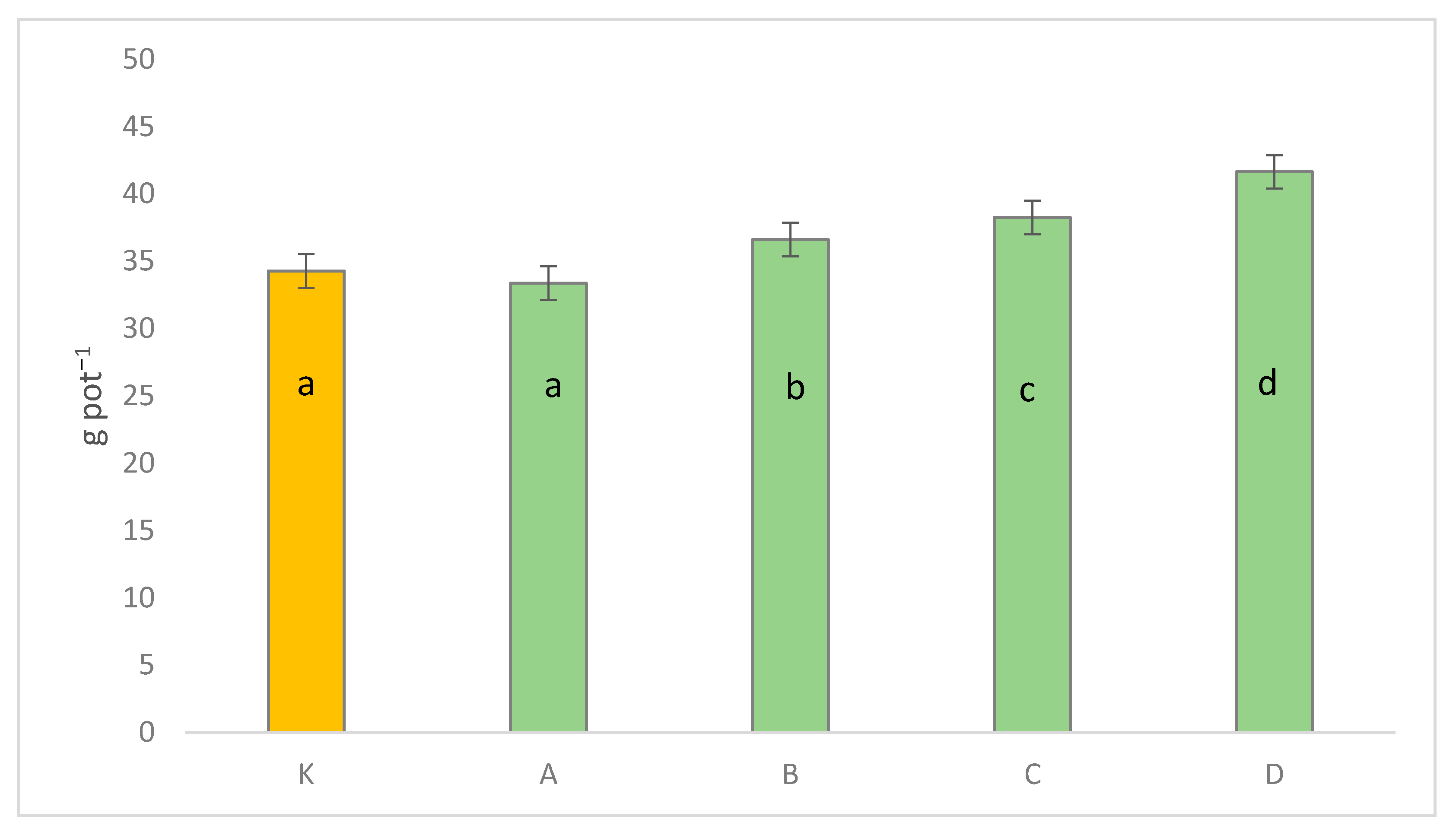
3. Results
3.1. Plant Emergency
3.2. Seed Yield
3.3. Chemical Composition of Seeds
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Factors | Sum of Squares | df | Mean Square | F-Ratio | p-Value |
|---|---|---|---|---|---|
| Stress regime | 2100.69 | 4 | 525.17 | 54.98 | 0.000 |
| Variety | 9887.46 | 15 | 659.16 | 69.00 | 0.000 |
| Interaction | 963.02 | 60 | 16.05 | 1.68 | 0.005 |
References
- Bellaloui, N.; Bruns, H.A.; Abbas, H.K.; Mengistu, A.; Fisher, D.K.; Reddy, K.N. Agricultural practices altered soybean seed protein, oil, fatty acids, sugars, and minerals in the Midsouth USA. Front. Plant Sci. 2015, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- SOYSTATS 2020. Available online: http://soystats.com/ (accessed on 22 January 2021).
- Krishnan, H.B. Engineering soybean for enhanced sulfur amino acid content. Crop Sci. 2005, 45, 454–461. [Google Scholar] [CrossRef]
- Borawska, J.; Darewicz, M.; Iwaniak, A.; Minkiewicz, P. Biologically active peptides derived from food proteins as prevention factors for selected diet-related diseases. Bromat. Chem. Toksykol. 2014, 47, 230–236. (In Polish) [Google Scholar]
- Ghani, M.; Kulkarni, K.P.; Song, J.T.; Shannon, J.G.; Lee, L.J. Soybean sprouts: A review of nutrient composition, health benefits and genetic variation. Plant Breed. Biotech. 2016, 4, 398–412. [Google Scholar] [CrossRef]
- Kotecki, A. (Ed.) Plant Cultivation, Volume 3; Uniwersytet Przyrodniczy we Wrocławiu: Wrocław, Poland, 2020; pp. 161–206. (In Polish) [Google Scholar]
- Badaruddin, M.; Meyer, D.W. Grain legume effects on soil nitrogen, grain yield, and nitrogen nutrition of wheat. Crop Sci. 1994, 34, 1304–1309. [Google Scholar] [CrossRef]
- Martyniuk, S. Scientific and practical aspects of legume symbiosis with nodule bacteria. Pol. J. Agr. 2012, 9, 17–21. (In Polish) [Google Scholar]
- FAOSTAT 2020. Available online: http://www.fao.org/faostat/en/-data/QC (accessed on 12 January 2021).
- Kozyra, J.; Doroszewski, A.; Nieróbca, A. Climate changes and their expected impact on agriculture in Poland. Studia i Raporty IUNG-PIB 2009, 14, 243–257. (In Polish) [Google Scholar]
- Câmara, G.M.S.; Sediyama, T.; Dourado-Neto, D.; Bernardes, M.S. Influence of photoperiod and air temperature on the growth, flowering and maturation of soybean (Glycine max (L.) Merrill). Sci. Agric. 1997, 54, 149–154. [Google Scholar] [CrossRef]
- Gass, T.; Schori, A.; Fossati, A.; Soldati, A.; Stamp, P. Cold tolerance of soybean (Glycine max (L.) Merr.) during the reproductive phase. Eur. J. Agron. 1996, 5, 71–88. [Google Scholar] [CrossRef]
- Gaynor, L.G.; Lawn, R.J.; James, A.T. Agronomic studies on irrigated soybean in southern New South Wales. I. Phenological adaptation of genotypes to sowing date. Crop Pasture Sci. 2011, 62, 1056–1066. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, L.; Zhang, Y.; Yuan, S.; Su, Q.; Sun, S.; Wu, C.; Yao, W.; Han, T.; Hou, W. Target base editing in soybean using a modified CRISPR/Cas9 system. Plant Biotechnol. J. 2020, 18, 1996–1998. [Google Scholar] [CrossRef]
- Ohnishi, S.; Miyoshi, T.; Shirai, S. Low temperature stress at different flower developmental stages affects pollen development, pollination, and pod set in soybean. Environ. Exp. Bot. 2010, 69, 56–62. [Google Scholar] [CrossRef]
- Łykowski, B. Climatic Conditions for the Development and Yielding of Soybean in Poland; Rozpr. Nauk. i Monogr. SGGW Warszawa: Warszawa, Poland, 1984; pp. 5–84. [Google Scholar]
- Gao, C. The future of CRISPR technologies agriculture. Nat. Rev. Mol. Cell Biol. 2018, 19, 275–276. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, W.; Tsegaw, M.; Xu, X.; Qi, Y.; Sapey, E.; Liu, L.; Wu, T.; Sun, S.; Han, T. Principles and practices of the photo-thermal adaptability improvement in soybean. J. Integr. Agric. 2020, 19, 295–310. [Google Scholar] [CrossRef]
- Hou, G.; Ablett, G.R.; Pauls, K.P.; Rajcan, I. Environmental effects on fatty acid levels in soybean seed oil. J. Am. Oil Chem. Soc. 2006, 83, 759–763. [Google Scholar] [CrossRef]
- Thomashow, M.F. Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 571–599. [Google Scholar] [CrossRef]
- Livingston, D.P.; Hincha, D.K.; Heyer, A.G. Fructan and its relationship to abiotic stress tolerance in plants. Cell. Mol. Life Sci. 2009, 66, 2007–2023. [Google Scholar] [CrossRef] [PubMed]
- Yuanyuan, M.; Yali, Z.; Jiang, L.; Hongbo, S. Roles of plant soluble sugars and their responses to plant cold stress. Afr. J. Biotechnol. 2009, 8, 2004–2010. [Google Scholar] [CrossRef]
- Michałek, S.; Borowski, E. Yielding, oil, fatty acids and protein content in the seeds of polish soybean cultivars under drought conditions. Acta Agroph. 2006, 8, 459–471. [Google Scholar]
- Kołodziej, J.; Pisulewska, E. Influence of meteorological factors on the yield of seeds and fat as well as fat content in seeds of two soybean cultivars. Rośliny Oleiste 2000, XXI, 759–773. (In Polish) [Google Scholar]
- Anioł, A.; Bielecki, S.; Twardowski, T. Genetically modified organisms—Opportunities and threats for Poland. Nauka 2008, 1, 63–84. (In Polish) [Google Scholar]
- EU Common Catalogue of Varieties of Agricultural Plant Species. 2020. Consolidated Version, 20 September 2019. Available online: https://ec.europa.eu/food/sites/food/files/plant/docs/plant-variety-catalogues_agricultural-plant-species.pdf (accessed on 22 January 2021).
- Kjeldahl, J.A. New Method for the Determination of Nitrogen in Organic Matter. Z. Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef]
- Soxhlet, F. The weight analytic determination of milk fat. Polytech. J. 1879, 232, 461–465. [Google Scholar]
- Official Methods of Analysis of the AOAC. Wyd. XX; Association of Official Analytical Chemists: Rockville, MD, USA, 2016.
- Nogala-Kałucka, M. (Ed.) Analiza Żywności. Wybrane Metody Oznaczeń Jakościowych i Ilościowych Składników Żywności; Wyd. UP: Poznań, Poland, 2016. (In Polish) [Google Scholar]
- Jones, D.B. Factors for Converting Percentages of Nitrogen in Foods and Feed into Percentages of Proteins; Circular, 1941, No. 183 (Original Version, 1931); United States Department of Agriculture: Washington, DC, USA, 1931.
- Hinson, K.; Hartwig, E.E. Soybean Production in the Tropics; FAO Plant Production and Protection Paper; FAO: Roma, Italy, 1982; Volume 4, pp. 2–12. [Google Scholar]
- Mourtzinis, S.; Gaspar, A.P.; Naeve, S.L.; Conley, S.P. Planting date, maturity, and temperature effects on soybean seed yield and composition. Agron. J. 2017, 109, 2040–2049. [Google Scholar] [CrossRef]
- Vollmann, J.; Wagentristl, H.; Hartl, W. The effects of simulated weed pressure on early maturity soybeans. Eur. J. Agron. 2010, 32, 243–248. [Google Scholar] [CrossRef]
- Gawęda, D.; Haliniarz, M.; Bronowicka-Mielniczuk, U.; Łukasz, J. Weed infestation and health of the soybean crop depending on cropping system and tillage system. Agriculture 2020, 10, 208. [Google Scholar] [CrossRef]
- Biszczak, W.; Różyło, K.; Kraska, P. Yielding parameters, nutritional value of soybean seed and weed infestation in relay-strip intercropping system with buckwheat. Acta Agric. Scand. B Soil Plant Sci. 2020, 70, 640–647. [Google Scholar] [CrossRef]
- Egli, D.B.; Cornelius, P.L. A regional analysis of the response of soybean yield to planting date. J. Agron. 2009, 101, 330–335. [Google Scholar] [CrossRef]
- Pedersen, P.; Lauer, J.G. Response of soybean yield components to management system and planting date. J. Agron. 2004, 96, 1372–1381. [Google Scholar] [CrossRef]
- Egli, D.B.; Bruening, W.P. Potential of early-maturing soybean cultivars in late plantings. J. Agron. 2000, 92, 532–537. [Google Scholar] [CrossRef]
- De Bruin, J.L.; Pedersen, P. Soybean seed yield response to planting date and seeding rate in the upper Midwest. J. Agron. 2008, 100, 696–703. [Google Scholar] [CrossRef]
- Gaspar, A.P.; Conley, S.P. Responses of canopy reflectance, light interception, and soybean seed yield to replanting suboptimal stands. Crop Sci. 2015, 55, 377–385. [Google Scholar] [CrossRef]
- Marburger, D.A.; Smith, D.L.; Conley, S.P. Revisiting planting date and cultivar effects on soybean sudden death syndrome development and yield loss. Plant Dis. 2016, 100, 2152–2157. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.W.; Badaruddin, M. Frost tolerance of ten seedling legume species at four growth stages. Crop Sci. 2001, 41, 1838–1842. [Google Scholar] [CrossRef]
- Murillo-Williams, A.; Pedersen, P. Early incidence of soybean seedling pathogens in Iowa. J. Agron. 2008, 100, 1481–1487. [Google Scholar] [CrossRef]
- Helms, T.C.; Deckard, E.; Goos, R.J.; Enz, J.W. Soybean seedling emergence influenced by days of soil water stress and soil temperature. J. Agron. 1996, 88, 657–661. [Google Scholar] [CrossRef]
- Salmeron, M.; Gbur, E.E.; Bourland, F.M.; Buehring, N.W.; Earnest, L.; Fritschi, F.B.; Golden, B.R.; Hathcoat, D.; Lofton, J.; Miller, T.D.; et al. Soybean maturity group choices for early and late plantings in the Midsouth. J. Agron. 2014, 106, 1893–1901. [Google Scholar] [CrossRef]
- Kane, M.V.; Steele, C.C.; Grabau, L.J.; MacKown, C.T.; Hildebrand, D.F. Early-maturing soybean cropping system: III. Protein and oil contents and oil composition. J. Agron. 1997, 89, 464–469. [Google Scholar] [CrossRef]
- Nakagawa, A.C.S.; Ario, N.; Tomita, Y.; Tanaka, S.; Murayama, N.; Mizuta, C.; Iwaya-Inoue, M.; Ishibashi, Y. High temperature during soybean seed development differentially alters lipid and protein metabolism. Plant Prod. Sci. 2020, 23, 504–512. [Google Scholar] [CrossRef]
- Rotundo, J.L.; Miller-Garvin, J.E.; Naeve, S.L. Regional and temporal variation in soybean seed protein and oil across the United States. Crop Sci. 2016, 56, 797–808. [Google Scholar] [CrossRef]
- Piper, E.L.; Boote, K.J. Temperature and cultivar effects on soybean seed oil and protein concentrations. J. Am. Oil Chem. Soc. 1999, 76, 1233–1241. [Google Scholar] [CrossRef]
- Kozak, M.; Malarz, W.; Kotecki, A.; Černý, I.; Serafin-Andrzejewska, M. Influence of different amounts of sowing and Asahi SL biostimulator on the chemical composition of seeds and post-harvest residues of soybean. Rośliny Oleiste 2008, 29, 217–230. (In Polish) [Google Scholar]
- Biel, W.; Gawęda, D.; Łysoń, E.; Hury, G. The influence of genetic and agrotechnical factors on the nutritional value of soybean. Acta Agroph. 2017, 24, 395–404. (In Polish) [Google Scholar]
- Nascimento, M.; Finoto, E.L.; Sediyama, T.; Cruz, C.D. Adaptability and stability of soybean in terms of oil and protein content. Crop Breed. Appl. Biotechnol. 2010, 10, 48–54. [Google Scholar] [CrossRef]
- Alsajri, F.A.; Wijewardana, C.; Irby, J.T.; Bellaloui, N.; Krutz, L.J.; Golden, B.; Gao, W.; Reddy, K.R. Developing functional relationships between temperature and soybean yield and seed quality. J. Agron. 2020, 112, 194–204. [Google Scholar] [CrossRef]

| Cultivar | Earliness Group | Stress Regime | ||||
|---|---|---|---|---|---|---|
| K | A | B | C | D | ||
| Augusta | EC | 94.4 | 95.8 | 93.1 | 88.9 | 0.0 |
| Annushka | EC | 83.3 | 65.3 | 62.5 | 62.5 | 0.0 |
| Aldana | EC | 94.4 | 88.9 | 83.3 | 79.2 | 0.0 |
| Erica | EC | 73.6 | 65.3 | 66.7 | 66.7 | 0.0 |
| Paradis | EC | 93.1 | 88.9 | 94.4 | 91.7 | 0.0 |
| Oressa | EC | 84.7 | 62.5 | 75.0 | 62.5 | 0.0 |
| Merlin | SC | 91.7 | 75.0 | 76.4 | 84.7 | 0.0 |
| Lissabon | LC | 80.6 | 66.7 | 76.4 | 76.4 | 0.0 |
| Abelina | SC | 83.3 | 69.4 | 72.2 | 72.2 | 0.0 |
| Maja | SC | 93.1 | 77.8 | 72.2 | 79.2 | 0.0 |
| Mavka | SC | 47.2 | 13.9 | 16.7 | 18.1 | 0.0 |
| Sculptor | SC | 70.8 | 59.7 | 55.6 | 68.1 | 0.0 |
| Aligator | LC | 86.1 | 73.6 | 77.8 | 70.8 | 0.0 |
| GL Melanie | LC | 62.5 | 45.8 | 52.8 | 45.8 | 0.0 |
| Madlen | LC | 88.9 | 76.4 | 72.2 | 81.9 | 0.0 |
| Petrina | LC | 93.1 | 88.9 | 79.2 | 79.2 | 0.0 |
| Average | 82.6 | 69.6 | 70.4 | 70.5 | 0.0 | |
| Cultivar | Earliness Group | Stress Regime | ||||
|---|---|---|---|---|---|---|
| K | A | B | C | D | ||
| Augusta | EC | 95.8 | 97.2 | 97.2 | 95.8 | 95.8 |
| Annushka | EC | 88.9 | 72.2 | 70.8 | 77.8 | 83.3 |
| Aldana | EC | 97.2 | 95.8 | 95.8 | 91.7 | 91.7 |
| Erica | EC | 97.2 | 84.7 | 86.1 | 77.8 | 79.2 |
| Paradis | EC | 95.8 | 94.4 | 97.2 | 97.2 | 98.6 |
| Oressa | EC | 93.1 | 72.2 | 84.7 | 75.0 | 87.5 |
| Merlin | SC | 94.4 | 86.1 | 90.3 | 94.4 | 95.8 |
| Lissabon | LC | 91.7 | 80.6 | 91.7 | 87.5 | 90.3 |
| Abelina | SC | 93.1 | 88.9 | 93.1 | 87.5 | 90.3 |
| Maja | SC | 95.8 | 86.1 | 80.6 | 91.7 | 90.3 |
| Mavka | SC | 61.1 | 29.2 | 44.4 | 48.6 | 31.9 |
| Sculptor | SC | 84.7 | 70.8 | 70.8 | 79.2 | 80.6 |
| Aligator | LC | 88.9 | 90.3 | 91.7 | 86.1 | 97.2 |
| GL Melanie | LC | 73.6 | 62.5 | 66.7 | 65.3 | 68.1 |
| Madlen | LC | 90.3 | 86.1 | 83.3 | 97.2 | 90.3 |
| Petrina | LC | 95.8 | 88.9 | 87.5 | 91.7 | 86.1 |
| Average | 89.8 | 80.4 | 83.2 | 84.0 | 84.8 | |
| Cultivar (II) | Earliness Group | Regime Stress (I) | ||||
|---|---|---|---|---|---|---|
| K | A | B | C | D | ||
| Madlen | LC | 20.7 ± 1.4 | 25.3 ± 2.8 | 30.3 ± 1.9 * | 25.3 ± 1.6 | 27.8 ± 2.9 * |
| Annushka | EC | 24.2 ± 1.2 | 24.4 ± 1.6 | 23.5 ± 4.2 | 28.1 ± 2.4 | 34.3 ± 4.0 * |
| Augusta | EC | 28.2 ± 1.4 | 26.7 ± 3.1 | 25.4 ± 3.6 | 30.4 ± 4.3 | 35.2 ± 2.7 * |
| Paradis | EC | 27.9 ± 4.2 | 28.2 ± 6.4 | 30.9 ± 1.5 | 33.9 ± 1.7 * | 32.5 ± 4.7 |
| Maja | SC | 30.5 ± 4.2 | 29.8 ± 1.4 | 32.9 ± 2.1 | 35.1 ± 1.3 | 36.3 ± 3.8 * |
| Oressa | EC | 29.6 ± 2.8 | 29.0 ± 6.0 | 35.4 ± 5.2 * | 36.4 ± 2.6 * | 34.2 ± 3.4 |
| Aldana | EC | 30.4 ± 0.7 | 32.0 ± 1.0 | 33.1 ± 3.6 | 33.5 ± 1.7 | 41.9 ± 4.2 * |
| Sculptor | SC | 33.1 ± 1.5 | 32.2 ± 1.1 | 37.2 ± 2.4 | 37.6 ± 2.9 | 40.6 ± 0.6 * |
| Mavka | SC | 36.1 ± 1.3 | 36.3 ± 2.1 | 36.6 ± 2.9 | 38.6 ± 0.4 | 46.6 ± 0.5 * |
| Lissabon | LC | 38.0 ± 1.8 | 38.8 ± 2.8 | 40.5 ± 1.5 | 43.5 ± 3.0 * | 42.0 ± 2.9 |
| Merlin | SC | 40.8 ± 1.3 | 36.1 ± 3.2 | 38.7 ± 1.7 | 45.1 ± 3.0 | 45.5 ± 4.4 |
| Erica | EC | 38.0 ± 0.6 | 39.4 ± 0.5 | 38.7 ± 4.9 | 41.8 ± 2.0 | 48.4 ± 3.1 * |
| Aligator | LC | 40.1 ± 1.0 | 42.4 ± 1.6 | 42.0 ± 4.0 | 42.2 ± 6.3 | 45.5 ± 3.3 * |
| Petrina | LC | 44.6 ± 2.0 | 35.8 ± 0.4 | 45.9 ± 4.4 | 47.3 ± 1.0 | 50.9 ± 3.9 * |
| GL Melanie | LC | 42.4 ± 3.0 | 37.4 ± 6.1 | 48.1 ± 2.6 * | 44.8 ± 3.1 | 53.5 ± 4.4 * |
| Abelina | SC | 43.7 ± 2.2 | 40.0 ± 1.8 | 46.6 ± 3.6 | 48.4 ± 5.5 | 50.8 ± 2.2 * |
| LSD (p ≤ 0.05) | I × II—4.98 | |||||
| Stress Regime | CP | CFa | WSC | CF | CA |
|---|---|---|---|---|---|
| K | 380.1 | 214.8 | 119.6 | 58.3 | 53.2 |
| A | 381.8 | 214.2 | 119.2 | 58.6 | 52.8 |
| B | 384.5 | 215.4 | 120.2 | 57.8 | 52.1 |
| C | 386.4 | 215.2 | 116.5 | 56.6 | 52.9 |
| D | 386.7 | 213.9 | 117.4 | 56.4 | 52.5 |
| LSD (p ≤ 0.05) | ns | ns | ns | ns | ns |
| Cultivar (II) | CP | CFa | WSC | CF | CA |
|---|---|---|---|---|---|
| Madlen | 391.3 | 192.2 | 120.3 | 54.4 | 54.0 |
| Annushka | 370.5 | 217.7 | 125.0 | 59.8 | 55.0 |
| Augusta | 402.3 | 192.2 | 108.4 | 63.3 | 54.1 |
| Paradis | 392.6 | 210.0 | 122.0 | 61.8 | 53.3 |
| Maja | 427.1 | 210.6 | 115.3 | 50.5 | 50.9 |
| Oressa | 379.7 | 202.8 | 132.7 | 65.8 | 54.5 |
| Aldana | 380.8 | 208.5 | 124.0 | 56.5 | 54.5 |
| Sculptor | 403.6 | 209.7 | 119.5 | 46.4 | 52.6 |
| Mavka | 372.2 | 229.7 | 123.5 | 47.7 | 52.2 |
| Lissabon | 366.9 | 229.2 | 131.5 | 61.1 | 51.5 |
| Merlin | 377.4 | 225.3 | 113.6 | 68.5 | 50.1 |
| Erica | 399.8 | 206.0 | 112.5 | 61.4 | 53.6 |
| Aligator | 348.4 | 230.1 | 110.7 | 58.7 | 52.7 |
| Petrina | 364.0 | 227.7 | 111.3 | 56.8 | 52.2 |
| GL Melanie | 400.5 | 215.8 | 106.1 | 48.9 | 54.6 |
| Abelina | 365.1 | 227.5 | 121.1 | 59.0 | 47.5 |
| LSD (p ≤ 0.05) | 10.83 | 5.13 | 6.36 | 6.00 | 1.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staniak, M.; Stępień-Warda, A.; Czopek, K.; Kocira, A.; Baca, E. Seeds Quality and Quantity of Soybean [Glycine max (L.) Merr.] Cultivars in Response to Cold Stress. Agronomy 2021, 11, 520. https://doi.org/10.3390/agronomy11030520
Staniak M, Stępień-Warda A, Czopek K, Kocira A, Baca E. Seeds Quality and Quantity of Soybean [Glycine max (L.) Merr.] Cultivars in Response to Cold Stress. Agronomy. 2021; 11(3):520. https://doi.org/10.3390/agronomy11030520
Chicago/Turabian StyleStaniak, Mariola, Anna Stępień-Warda, Katarzyna Czopek, Anna Kocira, and Edyta Baca. 2021. "Seeds Quality and Quantity of Soybean [Glycine max (L.) Merr.] Cultivars in Response to Cold Stress" Agronomy 11, no. 3: 520. https://doi.org/10.3390/agronomy11030520
APA StyleStaniak, M., Stępień-Warda, A., Czopek, K., Kocira, A., & Baca, E. (2021). Seeds Quality and Quantity of Soybean [Glycine max (L.) Merr.] Cultivars in Response to Cold Stress. Agronomy, 11(3), 520. https://doi.org/10.3390/agronomy11030520








