The Effects of Various Doses and Types of Effective Microorganism Applications on Microbial and Enzyme Activity of Medium and the Photosynthetic Activity of Scarlet Sage
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Gas Exchange Parameters, Morphological Parameters, and Chlorophyll Contents of Plants
2.3. Microorganisms Number and Enzyme Activity
2.4. Statistical Analysis
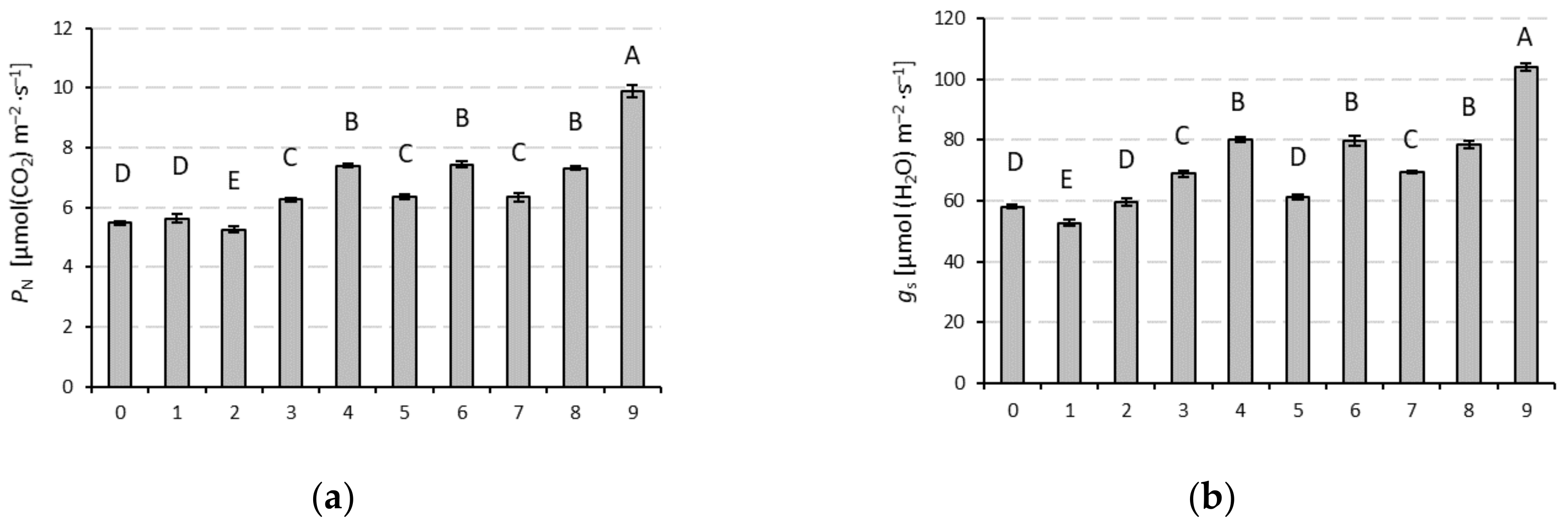
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mikkelsen, R.L.; Bruulsema, T.W. Fertilizer Use for Horticultural Crops in the U.S. during the 20th Century. HortTechnology 2005, 15, 24–30. [Google Scholar] [CrossRef]
- Savci, S. An Agricultural Pollutant: Chemical Fertilizer. Int. J. Environ. Sci. Dev. 2012, 3, 73–80. [Google Scholar] [CrossRef]
- Omwoma, S.; Lalah, J.O.; Ongeri, D.M.K.; Wanyonyi, M.B. Impact of Fertilizers on Heavy Metal Loads in Surface Soils in Nzoia Nucleus Estate Sugarcane Farms in Western Kenya. Bull. Environ. Contam. Toxicol. 2010, 85, 602–608. [Google Scholar] [CrossRef]
- García-Fraile, P.; Menéndez, E.; Rivas, R. Role of bacterial biofertilizers in agriculture and forestry. AIMS Environ. Sci. 2015, 2, 183–205. [Google Scholar] [CrossRef]
- Adeleke, R.A.; Nunthkumar, B.; Roopnarain, A.; Obi, L. Applications of Plant–Microbe Interactions in Agro-Ecosystems. In Microbiome in Plant Health and Disease; Metzler, J.B., Ed.; Springer, Nature Ltd.: Singapore, 2019; pp. 1–34. [Google Scholar]
- Lévai, L.; Veres, S.Z.; Makleit, P.; Marozsán, M.; Szabó, B. New trends in plant nutrition. In Proceedings of the 41st Croatian and 1st International Symposium on Agriculture, Opatija, Croatia, 13–17 February 2006; pp. 435–436, ISBN 953-6331-39-X. [Google Scholar]
- Bishnoi, U. PGPR Interaction. In Advances in Botanical Research; Elsevier BV: New York, NY, USA, 2015; Volume 75, pp. 81–113. [Google Scholar]
- Kumar, R.; Saurabh, K.; Kumawat, N.; Sundaram, P.K.; Mishra, J.S.; Singh, D.K.; Hans, H.; Krishna, B.; Bhatt, B.P. Sustaining Productivity Through Integrated Use of Microbes in Agriculture. In Role of Microbial Communities for Sustainability; Springer International Publishing: Singapore, 2021; pp. 109–145. [Google Scholar]
- Kloepper, J.W.; Leong, J.; Teintze, M.; Schroth, M.N. Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 1980, 286, 885–886. [Google Scholar]
- Muthaura, C.; Musyimi, D.M.; Ogur, J.A.; Okello, S.A. Effective microorganisms and their influence on growth and yield of pigweed (Amaranthus dubians). ARPN J. Agric. Biol. Sci. 2010, 5, 17–22. [Google Scholar]
- Kang, B.G.; Kim, W.T.; Yun, H.S.; Chang, S.C. Use of plant growth-promoting rhizobacteria to control stress responses of plant roots. Plant. Biotechnol. Rep. 2010, 4, 179–183. [Google Scholar] [CrossRef]
- Dodd, I.C.; Pérez-Alfocea, F. Microbial amelioration of crop salinity stress. J. Exp. Bot. 2012, 63, 3415–3428. [Google Scholar] [CrossRef]
- Higa, T. Effective Microorganisms: A Biotechnology for Mankind. In Proceedings of the first International Conference on Kyusei Nature Farming, Washington, DC, USA, 17–21 October 1991; pp. 8–14. [Google Scholar]
- Yamada, K.; Xu, H.-L. Properties and Applications of an Organic Fertilizer Inoculated with Effective Microorganisms. J. Crop. Prod. 2001, 3, 255–268. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.A.; Bardgett, R.D.; Van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The Role of Soil Microorganisms in Plant Mineral Nutrition—Current Knowledge and Future Directions. Front. Plant. Sci. 2017, 8, 1617. [Google Scholar] [CrossRef]
- Wang, Z.; Li, T.; Wen, X.; Liu, Y.; Han, J.; Liao, Y.; Debruyn, J.M. Fungal Communities in Rhizosphere Soil under Conservation Tillage Shift in Response to Plant Growth. Front. Microbiol. 2017, 8, 1301. [Google Scholar] [CrossRef]
- Rao, C.S.; Grover, M.; Kundu, S.; Desai, S. Soil Enzymes; Informa UK Limited: London, UK, 2017; pp. 2100–2107. [Google Scholar]
- Nannipieri, P.; Giagnoni, L.; Landi, L.; Renella, G. Role of phosphatase enzymes in soil. In Phosphorus in Action; Bünemann, E., Oberson, A., Frossard, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 26, pp. 215–243. [Google Scholar]
- Bielińska, E. Methods of determination of photosphatase activity. ACTA Agroph. 2005, 3, 63–74. [Google Scholar]
- Krämer, S. Acid and alkaline phosphatase dynamics and their relationship to soil microclimate in a semiarid woodland. Soil Biol. Biochem. 2000, 32, 179–188. [Google Scholar] [CrossRef]
- Richardson, A.E.; Simpson, R.J. Soil Microorganisms Mediating Phosphorus Availability Update on Microbial Phosphorus. Plant. Physiol. 2011, 156, 989–996. [Google Scholar] [CrossRef]
- Quilchano, C.; Marañón, T. Dehydrogenase activity in Mediterranean forest soils. Biol. Fertil. Soils 2002, 35, 102–107. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, P.; Kong, C. Urease, invertase, dehydrogenase and polyphenoloxidase activities in paddy soil influenced by allelopathic rice variety. Eur. J. Soil Biol. 2009, 45, 436–441. [Google Scholar] [CrossRef]
- Salazar, S.; Sánchez, L.; Alvarez, J.; Valverde, A.; Galindo, P.; Igual, J.; Peix, A.; Santa-Regina, I. Correlation among soil enzyme activities under different forest system management practices. Ecol. Eng. 2011, 37, 1123–1131. [Google Scholar] [CrossRef]
- Moeskops, B.; Buchan, D.; Sleutel, S.; Herawaty, L.; Husen, E.; Saraswati, R.; Setyorini, D.; De Neve, S. Soil microbial communities and activities under intensive organic and conventional vegetable farming in West Java, Indonesia. Appl. Soil Ecol. 2010, 45, 112–120. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, J.; Zhang, J.; Qin, S. Soil microbial biomass and activity response to repeated drying–rewetting cycles along a soil fertility gradient modified by long-term fertilization management practices. Geoderma 2010, 160, 218–224. [Google Scholar] [CrossRef]
- Yuan, B.-C.; Yue, D.-X. Soil Microbial and Enzymatic Activities Across a Chronosequence of Chinese Pine Plantation Development on the Loess Plateau of China. Pedosphere 2012, 22, 1–12. [Google Scholar] [CrossRef]
- Wolińska, A.; Stępniewska, Z. Dehydrogenase Activity in the Soil Environment. In Dehydrogenases; Canuto, R.A., Ed.; Intech: Rijeka, Croatia, 2020; Chapter 8; pp. 183–210. [Google Scholar]
- Yuan, L.; Huang, J.; Yu, S. Responses of nitrogen and related enzyme activities to fertilization in rhizosphere of wheat. Pedosphere 1997, 7, 141–148. [Google Scholar]
- Liang, Y.; Yang, Y.; Yang, C.; Shen, Q.; Zhou, J.; Yang, L. Soil enzymatic activity and growth of rice and barley as influenced by organic manure in an anthropogenic soil. Geoderma 2003, 115, 149–160. [Google Scholar] [CrossRef]
- Okorski, A.; Olszewski, J.; Pszczółkowska, A.; Kulik, T. Effect of Fungal Infection and the Application of the Biological Agent EM 1™ on the Rate of Photosynthesis and Transpiration in Pea (Pisum sativum L.) Leaves. Pol. J. Nat. Sci. 2008, 23, 35–47. [Google Scholar] [CrossRef]
- El-Shatnawi, M.K.J.; Makhadmeh, I.M. Ecophysiology of the Plant-Rhizosphere System. J. Agron. Crop. Sci. 2001, 187, 1–9. [Google Scholar] [CrossRef]
- Davelos, A.L.; Kinkel, L.L.; Samac, D.A. Spatial Variation in Frequency and Intensity of Antibiotic Interactions among Streptomycetes from Prairie Soil. Appl. Environ. Microbiol. 2004, 70, 1051–1058. [Google Scholar] [CrossRef]
- Valarini, P.J.; Alvarez, M.C.D.; Gascó, J.M.; Guerrero, F.; Tokeshi, H. Assessment of soil properties by organic matter and EM-microorganism incorporation. Rev. Bras. Ciência Solo 2003, 27, 519–525. [Google Scholar] [CrossRef]
- Fravel, D.; Deahl, K.; Stommel, J. Compatibility of the biocontrol fungus Fusarium oxysporum strain CS-20 with selected fungicides. Biol. Control. 2005, 34, 165–169. [Google Scholar] [CrossRef]
- Errampalli, D.; Brubacher, N.R. Biological and integrated control of postharvest blue mold (Penicillium expansum) of apples by Pseudomonas syringae and cyprodinil. Biol. Control. 2006, 36, 49–56. [Google Scholar] [CrossRef]
- Iriti, M.; Scarafoni, A.; Pierce, S.; Castorina, G.; Vitalini, S. Soil Application of Effective Microorganisms (EM) Maintains Leaf Photosynthetic Efficiency, Increases Seed Yield and Quality Traits of Bean (Phaseolus vulgaris L.) Plants Grown on Different Substrates. Int. J. Mol. Sci. 2019, 20, 2327. [Google Scholar] [CrossRef]
- Olle, M.; Williams, I.H. Effective microorganisms and their influence on vegetable production-a review. J. Hortic. Sci. Biotechnol. 2013, 88, 380–386. [Google Scholar] [CrossRef]
- Shoaf, W.T.; Lium, B.W. Improved extraction of chlorophyll a and b from algae using dimethyl sulfoxide. Limnol. Oceanogr. 1976, 21, 926–928. [Google Scholar] [CrossRef]
- Hiscox, J.D.; Israelstam, G.F. Erratum: A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1980, 58, 403. [Google Scholar] [CrossRef]
- Kunicki-Goldfinger, W.J.H. Life of Bact; PWN: Warsaw, Poland, 2001; p. 616. [Google Scholar]
- Martin, J.P. USE OF ACID, ROSE Bengal, and streptomycin in the plate method for estimating soil fungi. Soil Sci. 1950, 69, 215–232. [Google Scholar] [CrossRef]
- Kańska, Z.; Grabińska-Łoniewska, A.; Łebkowska, M.; Żechowska, E. Laboratory Exercises in Sanitary Biology; Warsaw Technology University: Warsaw, Poland, 2001. [Google Scholar]
- Thalmann, A. Zur methodik der bestimmung der dehydrogenase aktivität in boden mittels triphenyltetrazoliumchlorid (TTC). Landwirtsch Forsch 1968, 21, 249–258. [Google Scholar]
- Tabatabai, M.; Bremner, J. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Hoffmann, G.; Teicher, K. Ein kolorimetrisches Verfahren zur Bestimmung der Ureaseaktivität in Böden. J. Plant. Nutr. Soil Sci. 1961, 95, 55–63. [Google Scholar] [CrossRef]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The Role Of Root Exudates In Rhizosphere Interactions with Plants and Other Organisms. Annu. Rev. Plant. Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef]
- Biedrzycki, M.L.; Jilany, T.A.; Dudley, S.A.; Bais, H.P. Root exudates mediate kin recognition in plants. Commun. Integr. Biol. 2010, 3, 28–35. [Google Scholar] [CrossRef]
- Lamont, J.R.; Wilkins, O.; Bywater-Ekegärd, M.; Smith, D.L. From yogurt to yield: Potential applications of lactic acid bacteria in plant production. Soil Biol. Biochem. 2017, 111, 1–9. [Google Scholar] [CrossRef]
- Wolna-Maruwka, A.; Schroeter-Zakrzewska, A.; Borowiak, K. Wpływ preparatu EM na stan mikrobiologiczny podłoża przeznaczonego do uprawy pelargonii (Pelargonium × hortorum)/ Effect of EM inoculum on the microbiological state of substrate designed for pelargonium (Pelargonium × hortorum) (in Polish). Nauka Przyr. Technol. 2010, 4, 1–12. [Google Scholar]
- Kucharski, J.; Jastrzębska, E. Rola mikroorganizmów efektywnych (EM) i glebowych w kształtowaniu właściwości mikrobiologicznych gleb / Role of effective microorganisms in development of proper soil quality (in Polish). Zesz. Probl. Postępów Nauk Rol. 2005, 506, 315–322. [Google Scholar]
- Kaczmarek, Z.; Wolna-Maruwka, A.; Jakubus, M. Changes in the number of selected groups of soil microorganisms and enzymatic activity in the soil inoculated with Effective Microorganisms (EM). J. Res. Appl. Agric. Eng. 2008, 53, 122–127. [Google Scholar]
- Kowalska, j. Impact of fertilizers on soil properties in the case of solanum tuberosum l. During conversion to organic farming. Appl. Ecol. Environ. Res. 2017, 15, 369–383. [Google Scholar] [CrossRef]
- Shrestha, A.; Kim, B.S.; Park, D.H. Biological control of bacterial spot disease and plant growth-promoting effects of lactic acid bacteria on pepper. Biocontrol Sci. Technol. 2014, 24, 763–779. [Google Scholar] [CrossRef]
- Giassi, V.; Kiritani, C.; Kupper, K.C. Bacteria as growth-promoting agents for citrus rootstocks. Microbiol. Res. 2016, 190, 46–54. [Google Scholar] [CrossRef]
- Hupe, A.; Schulz, H.; Bruns, C.; Haase, T.; Heß, J.; Joergensen, R.G.; Wichern, F. Even flow? Changes of carbon and nitrogen release from pea roots over time. Plant. Soil 2018, 431, 143–157. [Google Scholar] [CrossRef]
- Shokouhian, A.A.; Davarynejad, G.H.; Tehranifar, A.; Imani, A.; Rasoulzadeh, A. Investigation of effective microorganisms (EM) impact in water stress condition on growth of almond (Prunus dulcis Mill) seedling. J. Basic Appl. Sci. Res. 2013, 3, 86–92. [Google Scholar]
- Arafa, A.A.; Farouk, S.; Mohamed, H.S. Effect of potassium fertilizer, biostimulants and effective microorganisms as well as their interactions on potato growth, photosynthetic pigments and stem anatomy. J. Plant. Prod. 2011, 2, 1017–1035. [Google Scholar] [CrossRef]
- Salama, A.S.; Sayed, O.M.E.-; El Gammal, O.H. Effect of Effective Microorganisms (EM) and Potassium Sulphate on Productivity and Fruit Quality of “Hayany” Date Palm Grown Under Salinity Stress. IOSR J. Agric. Veter. Sci. 2014, 7, 90–99. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Cetner, M.D.; Samborska, I.A.; Lukasik, I.; Oukarroum, A.; Rusinowski, S.; Pietkiewicz, S.; Świątek, M.; Dąbrowski, P. Effective microorganisms impact on photosynthetic activity of Arabidopsis plant grown under salinity stress conditions. Ann. Wars. Univ. Life Sci. SGGW. Land Reclam. 2016, 48, 153–163. [Google Scholar] [CrossRef]
- O’Hara, G.W.; Davey, M.R.; Lucas, J.A. Effect of inoculation of Zea mays with Azospirillum brasilense strains under temperate conditions. Can. J. Microbiol. 1981, 27, 871–877. [Google Scholar] [CrossRef]
- Bashan, Y.; Holguin, G. Azospirillum plant relationships: Environmental and physiological advances (1990–1996). Can. J. Microbiol. 1997, 43, 103–121. [Google Scholar] [CrossRef]
- Omay, S.H.; Schmidt, W.A.; Martin, P.; Bangerth, F. Indoleacetic acid production by the rhizosphere bacterium Azospirillum brasilense Cd under in vitro conditions. Can. J. Microbiol. 1993, 39, 187–192. [Google Scholar] [CrossRef]
- Daiziel, J.; Lawrence, D.K. Biochemical and biological effects of kaurene oxidase inhibitors, such as paclobutrazol. In BIO-Chemical Aspects of Synthetic and Naturally Occurring; Menhenett, R., Lawerence, D.K., Eds.; Springer: London, UK, 1984. [Google Scholar]
- Okorski, A.; Olszewski, J.; Głowacka, K.; Okorska, S.; Pszczółkowska, A. The effect of the application of the biological control agent EM1 on gas exchange parameters and productivity of Pisum sativum L. infected with Fusarium oxysporum Schlecht. ACTA Agrobot. 2012, 63, 105–115. [Google Scholar] [CrossRef]
- Xu, H.-L.; Wang, R.; Mridha, A.U. Effects of Organic Fertilizers and a Microbial Inoculant on Leaf Photosynthesis and Fruit Yield and Quality of Tomato Plants. J. Crop. Prod. 2001, 3, 173–182. [Google Scholar] [CrossRef]
- Mayer, J.; Scheid, S.; Widmer, F.; Fließbach, A.; Oberholzer, H.-R. How effective are ‘Effective microorganisms® Results from a field study in temperate climate. Appl. Soil Ecol. 2010, 46, 230–239. [Google Scholar] [CrossRef]






| Name of Combination | Types of Application |
|---|---|
| 0 | Control–peat substrate + plant |
| 1 | Peat substrate + plants + watering with EM at concentration 1:10 |
| 2 | Peat substrate + plants + watering with EM at concentration 1:50 |
| 3 | Peat substrate + plants + watering with EM at concentration 1:100 |
| 4 | Peat substrate + plants + spraying with EM at concentration 1:10 |
| 5 | Peat substrate + plants + spraying with EM at concentration 1:50 |
| 6 | Peat substrate + plants + spraying with EM at concentration 1:100 |
| 7 | Peat substrate + plants + watering and spraying with EM at concentration 1:10 |
| 8 | Peat substrate + plants + watering and spraying with EM at concentration 1:50 |
| 9 | Peat substrate + plants + watering and spraying with EM at concentration 1:100 |
| Parameter | PN | gs | Ci | E | Chl. a | Chl. b | Chl. a + b | Chl. b/a Ratio |
|---|---|---|---|---|---|---|---|---|
| F statistics | 139.3 | 176.7 | 776.1 | 12.7 | 12.7 | 22.8 | 22.2 | 17.15 |
| Significance | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 |
| Parameter | Bacteria | Fungi | Actinobacteria | Dehydrogenases | Phosphatases | Ureases |
|---|---|---|---|---|---|---|
| Term | 5.3 B | 377.3 A | 40.6 A | 191.3 A | 646.6 A | 15.0 A |
| Application | 3.6 B | 1.2 ns | 9.2 A | 1.7 ns | 24.6 A | 3.0 B |
| Interaction | 2.2 C | 1.4 ns | 5.5 A | 2.9 C | 6.1 A | 3.4A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borowiak, K.; Wolna-Maruwka, A.; Niewiadomska, A.; Budka, A.; Schroeter-Zakrzewska, A.; Stasik, R. The Effects of Various Doses and Types of Effective Microorganism Applications on Microbial and Enzyme Activity of Medium and the Photosynthetic Activity of Scarlet Sage. Agronomy 2021, 11, 603. https://doi.org/10.3390/agronomy11030603
Borowiak K, Wolna-Maruwka A, Niewiadomska A, Budka A, Schroeter-Zakrzewska A, Stasik R. The Effects of Various Doses and Types of Effective Microorganism Applications on Microbial and Enzyme Activity of Medium and the Photosynthetic Activity of Scarlet Sage. Agronomy. 2021; 11(3):603. https://doi.org/10.3390/agronomy11030603
Chicago/Turabian StyleBorowiak, Klaudia, Agnieszka Wolna-Maruwka, Alicja Niewiadomska, Anna Budka, Anita Schroeter-Zakrzewska, and Rafał Stasik. 2021. "The Effects of Various Doses and Types of Effective Microorganism Applications on Microbial and Enzyme Activity of Medium and the Photosynthetic Activity of Scarlet Sage" Agronomy 11, no. 3: 603. https://doi.org/10.3390/agronomy11030603
APA StyleBorowiak, K., Wolna-Maruwka, A., Niewiadomska, A., Budka, A., Schroeter-Zakrzewska, A., & Stasik, R. (2021). The Effects of Various Doses and Types of Effective Microorganism Applications on Microbial and Enzyme Activity of Medium and the Photosynthetic Activity of Scarlet Sage. Agronomy, 11(3), 603. https://doi.org/10.3390/agronomy11030603







