Enhancement to Salt Stress Tolerance in Strawberry Plants by Iodine Products Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Iodine Based Products Application and Salt Stress Conditions
2.3. Plant Sampling
2.4. Quantification of Plant Growth
- Leaf area
- Fresh weight
- Dry weight
2.5. Determination of Antioxidants
- Phosphate buffer extraction
- Water-acetone extraction
- Ascorbic acid (Asa)
- Total phenols (Phe)
- Superoxide dismutase (SOD)
- Total proteins
- Ascorbate peroxidase (APX)
- Catalase (CAT)
- Glutathione peroxidase (GPX)
- Glutathione (GSH)
- Total anthocyanins
- Chlorophylls (Chlo)
Chlorophyll b (µg·g−1) = 30.38 × A645 − 6.58 × A663
Chlorophyll total (µg·g−1) = 18.8 × A663 + 34.02 × A645
2.6. Determination of Essential Minerals
- Acid digestion of plant tissue
2.7. Quantification of Minerals
2.8. Quantification of Iodine Concentration
2.9. Fruit Quality
- Firmness
- Brix grades
- % Loss of Firmness
2.10. Fruit Production
2.11. Statistical Analysis
3. Results
3.1. Growth Parameters—Anova One Way
3.2. Antioxidants
3.2.1. Fruits
3.2.2. Leaves
3.3. Essential Minerals
3.3.1. Fruits
3.3.2. Leaves
3.4. Production and Fruit Quality
3.5. Multivariate Analysis
Multiple Lineal Regression: Pearson Correlation
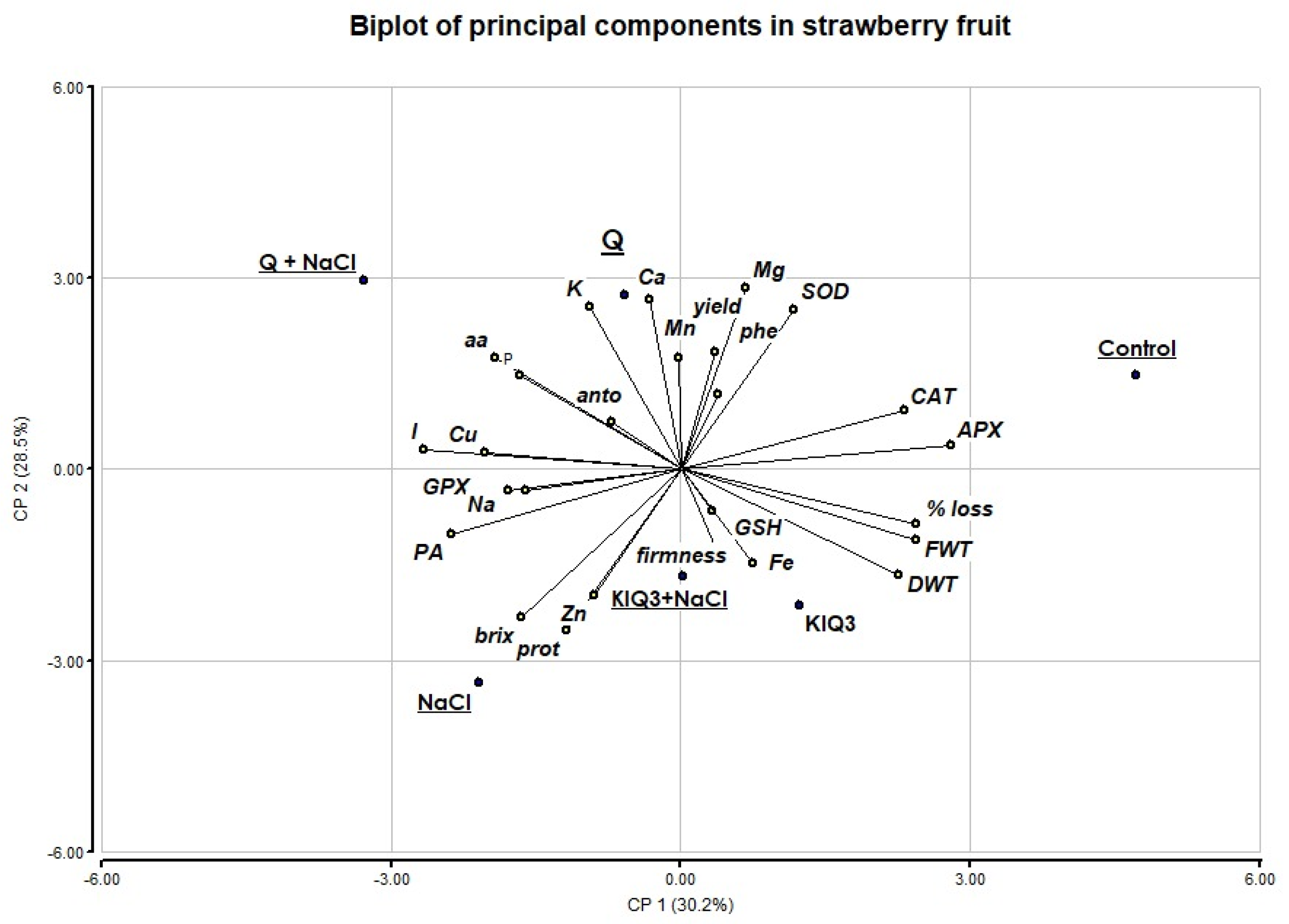
3.6. Principal Components Analysis (PCA)
4. Discussion
5. Conclusions
- The application of iodine-based products did not modify the growth of strawberry plants under normal conditions. However, stress yield and fruit quality were improved with iodine-based products.
- Some enzymatic and non-enzymatic antioxidants were increased with the application of iodine-based products in both leaves and fruits.
- Both under normal conditions and under salinity stress, synergy with essential elements was found in leaves and fruits treated with iodine-based products.
- The accumulation of iodine in leaves was increased with both iodine treatments under salt stress conditions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gonzali, S.; Kiferle, C.; Perata, P. Iodine biofortification of crops: Agronomic biofortification, metabolic engineering and iodine bioavailability. Curr. Opin. Biotechnol. 2017, 44, 16–26. [Google Scholar] [CrossRef]
- Venturi, S. Evolutionary Significance of Iodine. Curr. Chem. Biol. 2011, 5, 155–162. [Google Scholar] [CrossRef]
- Küpper, F.C.; Carrano, C.J. Key aspects of the iodine metabolism in brown algae: A brief critical review. Metallomic 2019, 11, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Medrano-Macías, J.; Leija-Martínez, P.; Juárez-Maldonado, A.; Rocha-Estrada, A.; Benavides-Mendoza, A. Efecto de la aplicación de yodo sobre antioxidantes en plántulas de jitomate. Rev. Chapingo Ser. Hortic. 2016, 22, 133–143. [Google Scholar] [CrossRef]
- Smoleń, S.; Kowalska, I.; Sady, W. Assessment of biofortification with iodine and selenium of lettuce cultivated in the NFT hydroponic system. Sci. Hortic. 2014, 166, 9–16. [Google Scholar] [CrossRef]
- Kiferle, C.; Ascrizzi, R.; Martinelli, M.; Gonzali, S.; Mariotti, L.; Pistelli, L.; Flamini, G.; Perata, P. Correction: Effect of Iodine treatments on Ocimum basilicum L.: Biofortification, phenolics production and essential oil composition. PLoS ONE 2020, 15, e0229016. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, D.-W.; Liu, H.-P.; Hong, C.-L.; Song, M.-Y.; Dai, Z.-X.; Liu, J.-W.; Zhou, J.; Weng, H.-X. Enhancing iodine content and fruit quality of pepper (Capsicum annuum L.) through biofortification. Sci. Hortic. 2017, 214, 165–173. [Google Scholar] [CrossRef]
- Cakmak, I.; Prom-U.-Thai, C.; Guilherme, L.R.G.; Rashid, A.; Hora, K.H.; Yazici, A.; Savasli, E.; Kalayci, M.; Tutus, Y.; Phuphong, P.; et al. Iodine biofortification of wheat, rice and maize through fertilizer strategy. Plant. Soil 2017, 418, 319–335. [Google Scholar] [CrossRef]
- Halka, M.; Smoleń, S.; Ledwożyw-Smoleń, I.; Sady, W. Iodosalicylates and iodobenzoates supplied to tomato plants affect the antioxidative and sugar metabolism differently than potassium iodide. Folia Hortic. 2019, 31, 385–400. [Google Scholar] [CrossRef]
- Gupta, N.; Bajpai, M.; Majumdar, R.; Mishra, P. Response of iodine on antioxidant levels of Glycine max L. Grown under Cd2+ stress. Adv. Biol. Res. 2015, 9, 40–48. [Google Scholar] [CrossRef]
- Wu, F.; Guan, Z.; Coronado, J.J.A.; Garcia-Nazariega, M. An Overview of Strawberry Production in Mexico. EDIS 2018, 2018, 5. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant Responses to Salt Stress: Adaptive Mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef]
- Crizel, R.; Perin, E.; Siebeneichler, T.; Borowski, J.; Messias, R.; Rombaldi, C.; Galli, V. Abscisic acid and stress induced by salt: Effect on the phenylpropanoid, L-ascorbic acid and abscisic acid metabolism of strawberry fruits. Plant. Physiol. Biochem. 2020, 152, 211–220. [Google Scholar] [CrossRef]
- D’Anna, F.; Incalcaterra, G.; Moncada, A.; Miceli, A. Effects of different electrical conductivity levels on strawberry grown in soilless culture. ACTA Hortic. 2003, 609, 355–360. [Google Scholar] [CrossRef]
- Steiner, A.A. A universal method for preparing nutrient solutions of a certain desired composition. Plant. Soil 1961, 15, 134–154. [Google Scholar] [CrossRef]
- Ramos, S.; Faquin, V.; Guilherme, L.; Castro, E.; Ávila, F.; Carvalho, G.; Bastos, C.; Oliveira, C. Selenium biofortification and antioxidant activity in lettuce plants fed with selenate and selenite. Plant. Soil Environ. 2010, 56, 584–588. [Google Scholar] [CrossRef]
- Nour, V.; Trandafir, I.; Ionica, M.E. HPLC organic acid analysis in different citrus juices under reversed phase conditions. Not. Bot. Horti Agrobot. Cluj-Napoca 2010, 38, 44–48. [Google Scholar] [CrossRef]
- Yu, Z.; Dahlgren, R.A. Evaluation of Methods for Measuring Polyphenols in Conifer Foliage. J. Chem. Ecol. 2000, 26, 2119–2140. [Google Scholar] [CrossRef]
- Cheng, Y.; Wei, H.; Sun, R.; Tian, Z.; Zheng, X. Rapid method for protein quantitation by Bradford assay after elimination of the interference of polysorbate 80. Anal. Biochem. 2016, 494, 37–39. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scarvenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Cansev, A.; Gulen, H.; Eris, A. The activities of catalase and ascorbate peroxidase in olive (Olea europaea L. cv. Gemlik) under low temperature stress. Hortic. Environ. Biotechnol. 2011, 52, 113–120. [Google Scholar] [CrossRef]
- Flohé, L.; Günzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–120. [Google Scholar] [CrossRef]
- Xue, T.; Hartikainen, H.; Piironen, V. Antioxidative and growth-promoting effect of selenium on senescing lettuce. Plant. Soil 2001, 237, 55–61. [Google Scholar] [CrossRef]
- Sahoo, S.; Prakash-Awasthi, J.; Sunkar, R.; Kumar Panda, S. Methods Molecular Biology. In Determining Glutathione Levels in Plants; Sunkar, R., Ed.; Humana Press: New York, NY, USA, 2017; pp. 273–277. [Google Scholar]
- Atanacković, M.; Cvejić, J.; Gojković-Bukarica, L.; Veljović, M.; Despotović, S.; Pecić, S.; Gaćeša, B.; Leskošek-Čukalović, I. Quantitative determination of total anthocyanins and flavonoids in natural products obtained from grapes and malt. In Proceedings of the 6th Central European Congress on Food (CEFood 2012), Novi Sad, Serbia, 23–26 May 2012; pp. 183–188. [Google Scholar]
- Munira, S.; Hossain, M.M.; Zakaria, M.; Ahmed, J.U.; Islam, M.M. Evaluation of Potato Varieties against Salinity Stress in Bangladesh. Int. J. Plant. Soil Sci. 2015, 6, 73–81. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; Association of official Analytical Chemist : Washington, DC, USA; 1990.
- Patel, S.P.; Patel, M.A.; Modi, H.R.; Katyare, S.S. Improved method for estimation of inorganic phosphate: Implications for its application in enzyme assays. Indian J. Biochem. Biophys. 2007, 44, 88–93. [Google Scholar] [PubMed]
- Ujowundu, C.O.; Ukoha, A.I.; Agha, N.C.; Nwachukwu, N.; Igwe, K.O. Iodine Biofortification of Selected Plants Using Potassium Iodide. Niger. J. Biochem. Mol. Biol. 2009, 24, 17–21. [Google Scholar]
- Eggink, L.L.; LoBrutto, R.; Brune, D.C.; Brusslan, J.; Yamasato, A.; Tanaka, A.; Hoober, J.K. Synthesis of chlorophyll b: Localization of chlorophyllide a oxygenase and discovery of a stable radical in the catalytic subunit. BMC Plant. Biol. 2004, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Puccinelli, M.; Pezzarossa, B.; Rosellini, I.; Malorgio, F. Selenium Enrichment Enhances the Quality and Shelf Life of Basil Leaves. Plants 2020, 9, 801. [Google Scholar] [CrossRef] [PubMed]
- Blasco, B.; Rios, J.J.; Leyva, R.; Cervilla, L.M.; Sánchez-Rodríguez, E.; Rubio-Wilhelmi, M.M.; Rosales, M. Ángel; Ruiz, J.M.; Romero, L. Does Iodine Biofortification Affect Oxidative Metabolism in Lettuce Plants? Biol. Trace Elem. Res. 2010, 142, 831–842. [Google Scholar] [CrossRef]
- Blasco, B.; Rios, J.J.; Cervilla, L.M.; Sánchez-Rodríguez, E.; Rubio-Wilhelmi, M.M.; Rosales, M.A.; Ruiz, J.M.; Romero, L. Photorespiration Process and Nitrogen Metabolism in Lettuce Plants (Lactuca sativa L.): Induced Changes in Response to Iodine Biofortification. J. Plant. Growth Regul. 2010, 29, 477–486. [Google Scholar] [CrossRef]
- Krzepiłko, A.; Prażak, R.; Skwaryło-Bednarz, B.; Molas, J. Agronomic biofortification as a means of enriching plant foodstuffs with iodine. ACTA Agrobot. 2019, 72, 1–9. [Google Scholar] [CrossRef]
- Li, Z.; Liang, D.; Peng, Q.; Cui, Z.; Huang, J.; Lin, Z. Interaction between selenium and soil organic matter and its impact on soil selenium bioavailability: A review. Geoderma 2017, 295, 69–79. [Google Scholar] [CrossRef]
- Weng, H.-X.; Hong, C.-L.; Yan, A.-L.; Pan, L.-H.; Qin, Y.-C.; Bao, L.-T.; Xie, L.-L. Mechanism of Iodine Uptake by Cabbage: Effects of Iodine Species and Where It is Stored. Biol. Trace Elem. Res. 2008, 125, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.-X.; Weng, J.-K.; Yan, A.-L.; Hong, C.-L.; Yong, W.-B.; Qin, Y.-C. Increment of Iodine Content in Vegetable Plants by Applying Iodized Fertilizer and the Residual Characteristics of Iodine in Soil. Biol. Trace Elem. Res. 2008, 123, 218–228. [Google Scholar] [CrossRef]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.-K.; Bohnert, H.J. Plantcellular and molecularresponses tohighsalinity. Annu. Rev. Plant. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef]
- Choudhury, F.; Rivero, R.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination–Choudhury. Plant J. 2017. [Google Scholar] [CrossRef]
- Leyva, R.; Sánchez-Rodríguez, E.; Ríos, J.J.; Rubio-Wilhelmi, M.M.; Romero, L.; Ruiz, J.M.; Blasco, B. Beneficial effects of exogenous iodine in lettuce plants subjected to salinity stress. Plant. Sci. 2011, 181, 195–202. [Google Scholar] [CrossRef]
- Zhou, Y.; Wen, Z.; Zhang, J.; Chen, X.; Cui, J.; Xu, W.; Liu, H.-Y. Exogenous glutathione alleviates salt-induced oxidative stress in tomato seedlings by regulating glutathione metabolism, redox status, and the antioxidant system. Sci. Hortic. 2017, 220, 90–101. [Google Scholar] [CrossRef]
- Li, R.; Liu, H.-P.; Hong, C.-L.; Dai, Z.-X.; Liu, J.-W.; Zhou, J.; Hu, C.-Q.; Weng, H.-X. Iodide and iodate effects on the growth and fruit quality of strawberry. J. Sci. Food Agric. 2016, 97, 230–235. [Google Scholar] [CrossRef]
- Smoleń, S.; Rożek, S.; Strzetelski, P.; Ledwożyw-Smoleń, I. Preliminary evaluation of the influence of soil fertilization and foliar nutrition with iodine on the efficiency of iodine biofortification and chemical composition of lettuce. J. Elem. 2011, 16, 613–622. [Google Scholar] [CrossRef]
- Smoleń, S.; Sady, W.; Rożek, S.; Strzetelski, P.; Ledwożyw-Smoleń, I. Preliminary evaluation of the influence of iodine and nitrogen fertilization on the effectiveness of iodine biofortification and mineral composition of carrot storage roots. J. Elem. 2011, 16, 275–285. [Google Scholar] [CrossRef]
- García Osuna, H.T.; Mendoza, A.B.; Morales, C.R.; Rubio, E.M.; Star, J.V.; Ruvalcaba, R.M.; Pakistan, L. Iodine application increased ascorbic acid content and modified the vascular tissue in Opuntia Ficus-Indica. J. Bot. 2014, 46, 127–134. [Google Scholar]
- Smoleń, S.; Skoczylas, Ł.; Rakoczy, R.; Ledwożyw-Smolen, I.; Kopec, A.; Piatkowska, E.; Bieżanowska-Kopec, R.; Pysz, M.; Koronowicz, A.; Kapusta-Duch, J.; et al. Mineral composition of field-grown lettuce (Lactuca sativa L.) depending on the diversified fertilization with iodine and selenium compounds. ACTA Sci. Pol. Hortorum Cultus 2015, 14, 97–114. [Google Scholar]
- Terzano, R.; Cesco, S.; Mimmo, T. Dynamics, thermodynamics and kinetics of exudates: Crucial issues in understanding rhizosphere processes. Plant. Soil 2015, 386, 399–406. [Google Scholar] [CrossRef]
- Venturi, S.; Venturi, M. Evolution of Dietary Antioxidants: Role of Iodine. Tutto Sulla Nutr. 2007, 1–25. [Google Scholar]
- Ashworth, D.; Shaw, G. Effects of moisture content and redox potential on in situ Kd values for radioiodine in soil. Sci. Total Environ. 2006, 359, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Medrano-Macías, J.; Leija-Martínez, P.; González-Morales, S.; Juárez-Maldonado, A.; Benavides-Mendoza, A. Use of Iodine to Biofortify and Promote Growth and Stress Tolerance in Crops. Front. Plant. Sci. 2016, 7, 1146. [Google Scholar] [CrossRef]
- Shiroyama, K.; Kawasaki, Y.; Unno, Y.; Amachi, S. A putative multicopper oxidase, IoxA, is involved in iodide oxidation by Roseovarius sp. strain A-2. Biosci. Biotechnol. Biochem. 2015, 79, 1898–1905. [Google Scholar] [CrossRef]


| Round 1. First application (Tuesday) | Q 2000 Plus® 7.5 mL L−1, Q Algy® 7.5 mL L−1, Q Riego® 0.5 mL L−1 |
| Second application (Thursday) | Q 2000 Plus® 7.5 mL L−1 |
| Round 2. First application (next Tuesday) | Q 2000 Plus® 7.5 mL L−1, Q Energy® 7.5 mL L−1, Q Riego® 0.5 mL L−1 |
| Second application (next Thursday) | Q 2000 Plus® 7.5 mL L−1. |
| Control | Q Products | KIO3 |
|---|---|---|
| Normal conditions EC 1.5 dS m−1 | ||
| NaCl 10 mM | Q products + NaCl | KIO3 + NaCl |
| Salinity stress conditions EC 2.5 dS m−1 | ||
| Treatment | Number of Leaves | Numbers of Crowns | Leaf Area (LA) | Fresh Weight of Leaves (FWL)(g) | Fresh Weight of Stem (FWS)(g) | Fresh Weight of Root (FWR) (g) | Fresh Weight Total FWT (g) |
|---|---|---|---|---|---|---|---|
| Q | 43.6 a | 7.4 b | 13.47 a | 46 a | 38 a | 44.3 b | 128.68 b |
| KIO3 | 62 a | 13 a | 14.91 a | 54 a | 44 a | 58.5 b | 157.76 b |
| KIO3 + NaCl | 55 a | 8.4 a | 12.9 a | 46 a | 36 a | 54.4 b | 137.71 ab |
| Q + NaCl | 46.4 a | 8.2 a | 13 a | 40 a | 35 a | 34.2 b | 109.93 b |
| control | 63.6 a | 11.2 ab | 11.3 a | 69 a | 52 a | 71.33 a | 192.9 a |
| NaCl | 66.4 a | 11 ab | 13.6 a | 58 a | 63 a | 58.1 b | 160.23 b |
| Stress | Asa g kg−1 | Prot g kg−1 | Phe g kg−1 | Superoxide Dismutase (SOD) (UI g−1) | Ascorbate Peroxidase (APX) (UI) | Catalase CAT [UI] | Glutathione Peroxidase (GPX) (UI) | Glutathione (GSH) (mM) | Anthocy (mg 100 g−1) | Antioxid Capacity (mM Teac) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q | Without NaCl | 2.26 a | 3.38 b | 2.28 a | 6.23 a | 0.11 b | 33.9 b | 75.7 b | 0.38 ab | 48.8 a | 3 a |
| 1.79 a | 2.18 b | 1.68 b | 7.8 a | 0.84 a | 66.4 a | 55.5 b | 0.54 b | 57.8 a | 2.1 a | ||
| KIO3 | Without NaCl | 1.91 a | 4.82 a | 0.94 b | 3.93 a | 0.31 b | 14.98 b | 46.1 b | 0.39 ab | 30.1 b | 2.91 a |
| 2.21 a | 3.07 b | 1.11 b | 6.3 a | 0.07 b | 12.8 b | 73.7 b | 0.45 ab | 58.3 a | 2.97 a | ||
| control | Without NaCl | 1.75 a | 4.11 a | 1.06 b | 4 a | 0.24 b | 19.66 b | 50.1 b | 1.46 a | 48.7 a | 2.94 a |
| 1.99 a | 4.8 a | 1.59 a | 4.1 a | 0.05 c | 24.5 b | 107 a | 0.0.34 c | 61.7 a | 3.04 a |
| Stress | Asa g kg−1 | Prot g kg−1 | Phe g kg−1 | Superoxide Dismutase (SOD) (UI g−1) | Ascorbate Peroxidasse (APX) (UI) | Catalase (CAT) (UI) | Glutathione Peroxidase (GPX ) (UI) | Glutathione (GSH) (mM) | Chlo t (μg·g−1) | Chlo a (μg·g−1) | Chlo b (μg·g−1) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q | Without NaCl | 1.41 b | 3.1 a | 7.7 ab | 7.6 a | 0.28 ab | 40.7 a | 94.7 a | 1.11 ab | 127.44 a | 77 a | 45.2 a |
| 1.48 b | 4.1 a | 9.05 a | 6 a | 0.88 a | 41.6 a | 63.44 a | 0.73 b | 115.73 b | 76.1 b | 37.8 b | ||
| KIO3 | WithoutNaCl | 0.47 b | 4.6 a | 5.8 c | 4 a | 0.69 ab | 15.5 a | 50.12 a | 1.06 ab | 114.9 b | 75.9 b | 35.9 b |
| 1.6 a | 5.1 a | 6.2 c | 3.5 a | 0.18 a | 15.9 a | 39.8 | 1.09 ab | 113.3 b | 75.5 b | 33.08 b | ||
| control | WithoutNaCl | 1.16 b | 4.3 a | 9.2 a | 4.3 a | 0.78 a | 10.5 a | 70.7 a | 1.36 a | 100.89 b | 74.7 b | 26.36 b |
| 1.2 b | 4.2 a | 7.2 ab | 6 a | 0.66 ab | 20.35 a | 57.7 a | 1.35 a | 149.3 a | 79.1 a | 62.88 a |
| Macroelements (%) | Microelements (ppm) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stress | P | K | Na | Mg | Ca | Zn | Cu | Fe | Mn | I | |
| Q | Without NaCl | 0.20 b | 1.56 a | 0.02 c | 0.15 a | 0.13 a | 6.0 b | 8.4 a | 25 a | 14 a | 4.08 a |
| 0.3 b | 1.73 a | 0.06 a | 0.15 a | 0.12 a | 8.3 ab | 5.6 a | 0 a | 13 ab | 5.45 a | ||
| KIO3 | Without NaCl | 0.24 b | 1.17 b | 0.02 c | 0.13 a | 0.09 bc | 9.4 ab | 2.9 a | 19 a | 13.5 a | 3.5 a |
| 0.7 a | 1.49 a | 0.07 a | 0.14 ab | 0.08 c | 7.2 ab | 2.5 a | 64 a | 12.5 ab | 4.39 a | ||
| control | Without NaCl | 0.13 b | 1.43 a | 0.02 c | 0.16 a | 0.09 bc | 8.7 ab | 1.8 a | 53 a | 12 bc | 3.32 a |
| 0.11 b | 1.22 b | 0.04 b | 0.12 b | 0.05 c | 24 a | 8.4 a | 0 a | 11 b | 4.9 a | ||
| Macroelements (%) | Microelements (ppm) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stress | P | K | Na | Mg | Ca | Zn | Cu | Fe | Mn | I | |
| Q | Without NaCl | 0.26 bc | 1.34 a | 0.02 b | 0.35 a | 0.9 bc | 20 a | 11 a | 200 a | 45 bc | 17.58 a |
| 0.59 a | 1.43 a | 0.05 a | 0.44 a | 1 ab | 20 a | 12.5 a | 100 a | 100 a | 17.95 a | ||
| KIO3 | Without NaCl | 0.46 ab | 1.32 a | 0.01 b | 0.33 a | 0.78 c | 8.4 a | 12 a | 29 a | 36 c | 14.10 b |
| 0.40 bc | 1.31 a | 0.04 a | 0.37 a | 0.86 bc | 15 a | 13 a | 100 a | 47 ab | 17.65 a | ||
| control | Without NaCl | 0.25 bc | 1.45 a | 0.03 b | 0.42 a | 1.13 a | 11 a | 14 a | 100 a | 42 bc | 6.28 b |
| 0.1 c | 1.37 a | 0.04 a | 0.36 a | 0.83 c | 11 a | 10 a | 22 a | 40 bc | 14.6 b | ||
| Stress | Yield (g) | Brix | Firmness (kg cm−1) | % loss Firmness | |
|---|---|---|---|---|---|
| Q | Without NaCl | 260.6 a | 5.41 b | 6.78 ab | 80.11 a |
| 227.35 a | 5.78 ab | 4.52 c | 58.64 b | ||
| KIO3 | Without NaCl | 262.3 a | 5.92 ab | 5.7 bc | 81.46 a |
| 140.37 b | 5.1 ab | 8.0 a | 85.89 a | ||
| control | Without NaCl | 233.26 a | 5.3 b | 5.74 bc | 88.11 a |
| 194.9 b | 6.5 a | 6.4 bc | 73.31 a |
| Prot | SOD | CAT | APX | Anthocy | GSH | PA | P | K | Ca | Mg | Na | Fe | Mn | I | FWT | DWT | Yield | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| asa | 0.4 | 0.8 | 0.60 | 0.1 | 0.9 | 0.5 | 0.6 | 0.5 | 0.5 | 0.1 | 0.4 | 0.3 | 0.3 | 0.5 | 0.2 | 0.2 | 0.3 | 0.2 |
| prot | 1 | 0.1 | 0.1 | 0.1 | 0.6 | 0.9 | 0.5 | 0.3 | 0.1 | 0.4 | 0.1 | 0.9 | 0.7 | 0.1 | 0.3 | 0.6 | 0.6 | 0.7 |
| phe | 1 | 0.3 | 0.1 | 0.9 | 0.3 | 0.3 | 0.5 | 0.3 | 0.1 | 0.3 | 0.1 | 0.1 | 0.1 | 0.9 | 0.9 | 0.8 | 0.9 | 0.7 |
| SOD | −0.8 | 1 | 0.1 | 0.1 | 0.8 | 0.4 | 0.9 | 0.6 | 0.4 | 0.4 | 0.1 | 0.6 | 0.7 | 0.2 | 0.9 | 0.8 | 0.9 | 0.6 |
| APX | −0.4 | 0.5 | 0.1 | 1 | 0.9 | 0.9 | 0.2 | 0.2 | 0.5 | 0.5 | 0.2 | 0.1 | 0.6 | 0.9 | 0.1 | 0.1 | 0.1 | 0.7 |
| CAT | −0.5 | 0.7 | 1 | 0.5 | 0.9 | 0.8 | 0.2 | 0.2 | 0.7 | 0.9 | 0.1 | 0.1 | 0.3 | 0.6 | 0.6 | 0.1 | 0.1 | 0.7 |
| GPX | −0.2 | 0.2 | 0.1 | −0.3 | 0.3 | 0.2 | 0.8 | 0.7 | 0.9 | 0.8 | 0.9 | 0.8 | 0.8 | 0.8 | 0.9 | 0.5 | 0.2 | 0.3 |
| GSH | −0.1 | −0.2 | −0.1 | 0.1 | 0.9 | 1 | 0.7 | 0.7 | 0.6 | 0.6 | 0.9 | 0.1 | 0.9 | 0.9 | 0.9 | 1 | 0.7 | 0.1 |
| Antoc | −0.1 | 0.1 | 0.1 | −0.1 | 1 | 0.1 | 0.5 | 0.3 | 0.5 | 0.4 | 0.4 | 0.8 | 0.2 | 0.5 | 0.9 | 0.2 | 0.6 | 0.40 |
| PA | 0.1 | 0.1 | −0.2 | −0.1 | −0.1 | −0.1 | 1 | 0.5 | 0.8 | 0.8 | 0.5 | 0.3 | 0.3 | 0.8 | 0.9 | 0.8 | 0.8 | 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medrano Macías, J.; López Caltzontzit, M.G.; Rivas Martínez, E.N.; Narváez Ortiz, W.A.; Benavides Mendoza, A.; Martínez Lagunes, P. Enhancement to Salt Stress Tolerance in Strawberry Plants by Iodine Products Application. Agronomy 2021, 11, 602. https://doi.org/10.3390/agronomy11030602
Medrano Macías J, López Caltzontzit MG, Rivas Martínez EN, Narváez Ortiz WA, Benavides Mendoza A, Martínez Lagunes P. Enhancement to Salt Stress Tolerance in Strawberry Plants by Iodine Products Application. Agronomy. 2021; 11(3):602. https://doi.org/10.3390/agronomy11030602
Chicago/Turabian StyleMedrano Macías, Julia, María Guadalupe López Caltzontzit, Erika Nohemi Rivas Martínez, Willian Alfredo Narváez Ortiz, Adalberto Benavides Mendoza, and Paulino Martínez Lagunes. 2021. "Enhancement to Salt Stress Tolerance in Strawberry Plants by Iodine Products Application" Agronomy 11, no. 3: 602. https://doi.org/10.3390/agronomy11030602
APA StyleMedrano Macías, J., López Caltzontzit, M. G., Rivas Martínez, E. N., Narváez Ortiz, W. A., Benavides Mendoza, A., & Martínez Lagunes, P. (2021). Enhancement to Salt Stress Tolerance in Strawberry Plants by Iodine Products Application. Agronomy, 11(3), 602. https://doi.org/10.3390/agronomy11030602