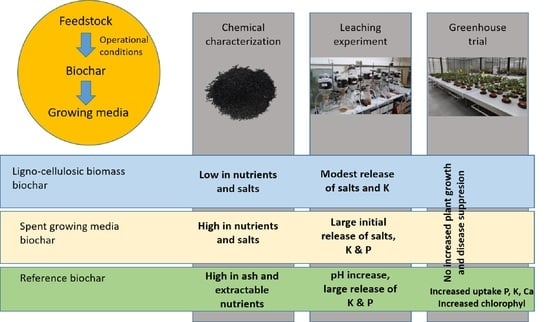
Biochar for Circular Horticulture: Feedstock Related Effects in Soilless Cultivation
Abstract
1. Introduction
1.1. Biochar: Definition and Use
1.2. Nutrients of Biochar
1.3. Feedstock Related Effects
1.4. Biochar Effecting Disease Suppression against Bortrytis cinerea on Strawberry
1.5. Research Questions and Hypothesis
2. Materials and Methods
2.1. Biochar Production and Characterization
2.1.1. Biochar Production
2.1.2. Chemical Characterization and Data Handling
2.2. Leaching Experiment
2.2.1. Input Materials for the Leaching Test
2.2.2. Set-Up
2.2.3. Data Handling
2.3. Greenhouse Strawberry Experiment
2.3.1. Rationale
2.3.2. Experimental Set-Up
2.3.3. Chemical Characterization of the Growing Media, Plant Leaves, and Strawberry Fruits
3. Results
3.1. Chemical Characterization
3.2. Leaching Experiment
3.2.1. Course of the Leaching Experiment
3.2.2. Interaction of Biochar with Percolating Solution
3.3. Greenhouse Strawberry Experiment
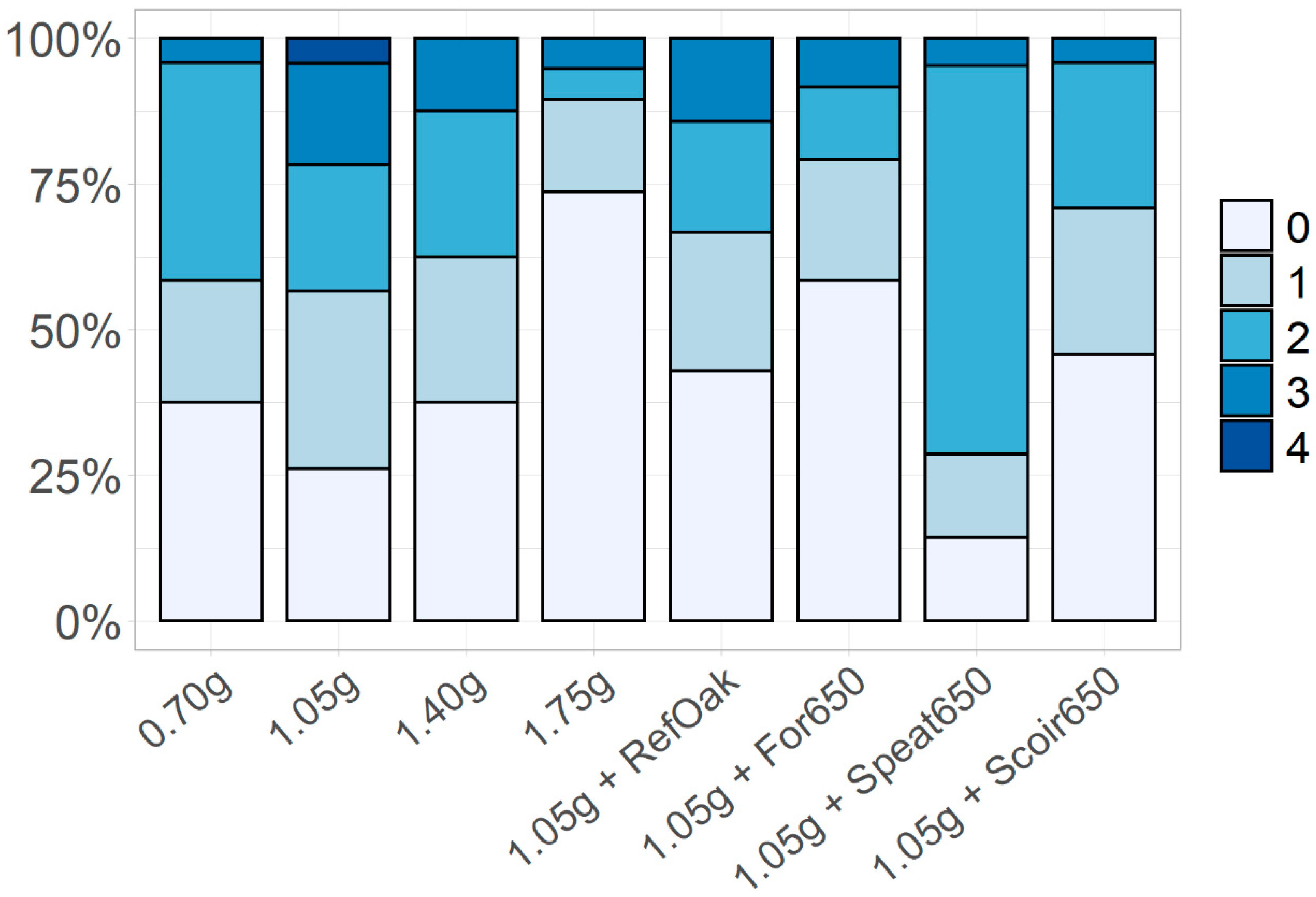
3.3.1. Water Use, Plant Parameters and Disease Resistance
3.3.2. Nutrient Uptake
4. Discussion
4.1. Biochar: Effects on Salts and Nutrients
4.2. Biochar and Plant Growth
4.3. Biochar and Disease Suppression
4.4. Role of Biochar in Growing Media: Bulk Replacement or Additive
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science and Technology; Earthscan: London, UK, 2009. [Google Scholar]
- Jindo, K.; Sanchez-Monedero, M.A.; Mastrolonardo, G.; Audette, Y.; Higashikawa, F.S.; Silva, C.A.; Akashi, K.; Mondini, C. Role of biochar in promoting circular economy in the agriculture sector. Part 2: A review of the biochar roles in growing media, composting and as soil amendment. Chem. Biol. Technol. Agric. 2020, 7, 10. [Google Scholar] [CrossRef]
- Sanchez-Monedero, M.A.; Cayuela, M.L.; Roig, A.; Jindo, K.; Mondini, C.; Bolan, N. Role of biochar as an additive in organic waste composting. Bioresour. Technol. 2018, 247, 1155–1164. [Google Scholar] [CrossRef]
- Nieto, A.; Gasco, G.; Paz-Ferreiro, J.; Fernandez, J.M.; Plaza, C.; Mendez, A. The effect of pruning waste and biochar addition on brown peat based growing media properties. Sci. Hortic. 2016, 199, 142–148. [Google Scholar] [CrossRef]
- Verhagen, J.; van den Akker, J.; Blok, C.; Diemont, H.; Joosten, H.; Schouten, M.; Schrijver, R.; den Uyl, R.; Verweij, P.; Wösten, H. Climate Change. Scientific Assessment and Policy Analysis. Peatlands and Carbon Flows. Outlook and Importance for the Netherlands, Wab 500102 027; Netherlands Environmental Assessment Agency PBL: Bilthoven, The Netherlands, 2009.
- Huang, L.; Gu, M.M. Effects of biochar on container substrate properties and growth of plants-A review. Horticulturae 2019, 5, 14. [Google Scholar] [CrossRef]
- Blok, C.; van der Salm, C.; Hofland-Zijlstra, J.; Streminska, M.; Eveleens, B.; Regelink, I.; Fryda, L.; Visser, R. Biochar for horticultural rooting media improvement: Evaluation of biochar from gasification and slow pyrolysis. Agronomy 2017, 7, 6. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Prasad, M.; Kavanagh, A.; Tzortzakis, N. Biochar type and ratio as a peat additive/partial peat replacement in growing media for cabbage seedling production. Agronomy 2019, 9, 693. [Google Scholar] [CrossRef]
- Dumroese, R.K.; Pinto, J.R.; Heiskanen, J.; Tervahauta, A.; McBurney, K.G.; Page-Dumroese, D.S.; Englund, K. Biochar can be a suitable replacement for sphagnum peat in nursery production of Pinus ponderosa seedlings. Forests 2018, 9, 232. [Google Scholar] [CrossRef]
- De Tender, C.; Haegeman, A.; Vandecasteele, B.; Ciement, L.; Cremelie, P.; Dawyndt, P.; Maes, M.; Debode, J. Dynamics in the strawberry rhizosphere microbiome in response to biochar and Botrytis cinerea leaf infection. Front. Microbiol. 2016, 7, 2062. [Google Scholar] [CrossRef] [PubMed]
- De Tender, C.A.; Debode, J.; Vandecasteele, B.; D’Hose, T.; Cremelie, P.; Haegeman, A.; Ruttink, T.; Dawyndt, P.; Maes, M. Biological, physicochemical and plant health responses in lettuce and strawberry in soil or peat amended with biochar. Appl. Soil Ecol. 2016, 107, 1–12. [Google Scholar] [CrossRef]
- Altland, J.E.; Locke, J.C. Biochar affects macronutrient leaching from a soilless substrate. Hortscience 2012, 47, 1136–1140. [Google Scholar] [CrossRef]
- Bedussi, F.; Zaccheo, P.; Crippa, L. Pattern of pore water nutrients in planted and non-planted soilless substrates as affected by the addition of biochars from wood gasification. Biol. Fertil. Soils 2015, 51, 625–635. [Google Scholar] [CrossRef]
- Olmo, M.; Villar, R.; Salazar, P.; Alburquerque, J.A. Changes in soil nutrient availability explain biochar’s impact on wheat root development. Plant Soil 2016, 399, 333–343. [Google Scholar] [CrossRef]
- Glaser, B.; Wiedner, K.; Seelig, S.; Schmidt, H.P.; Gerber, H. Biochar organic fertilizers from natural resources as substitute for mineral fertilizers. Agron. Sustain. Dev. 2015, 35, 667–678. [Google Scholar] [CrossRef]
- Levesque, V.; Jeanne, T.; Dorais, M.; Ziadi, N.; Hogue, R.; Antoun, H. Biochars improve tomato and sweet pepper performance and shift bacterial composition in a peat-based growing medium. Appl. Soil Ecol. 2020, 153, 12. [Google Scholar] [CrossRef]
- Daniele, M.; Alessandra, B.; Sonia, C.; Cecilia, F.; Domenico, P.; Lorenza, T.; Raffaella, P. Soilless tomato grown under nutritional stress increases green biomass but not yield or quality in presence of biochar as growing medium. Hortic. Environ. Biotechnol. 2019, 60, 871–881. [Google Scholar] [CrossRef]
- Locke, J.C.; Altland, J.E.; Ford, C.W. Gasified rice full biochar affects nutrition and growth of horticultural crops in container substrates. J. Environ. Hortic. 2013, 31, 195–202. [Google Scholar] [CrossRef]
- Lawrinenko, M.; Laird, D.A. Anion exchange capacity of biochar. Green Chem. 2015, 17, 4628–4636. [Google Scholar] [CrossRef]
- Marshall, J.A.; Morton, B.J.; Muhlack, R.; Chittleborough, D.; Kwong, C.W. Recovery of phosphate from calcium-containing aqueous solution resulting from biochar-induced calcium phosphate precipitation. J. Clean Prod. 2017, 165, 27–35. [Google Scholar] [CrossRef]
- Vandecasteele, B.; Blindeman, L.; Amery, F.; Pieters, C.; Ommeslag, S.; Van Loo, K.; De Tender, C.; Debode, J. Grow–StoreSteamRe-peat: Reuse of spent growing media for circular cultivation of Chrysanthemum. J. Clean Prod. 2020, 276, 124128. [Google Scholar] [CrossRef]
- Veijalainen, A.M.; Juntunen, M.L.; Lilja, A.; Heinonen-Tanski, H.; Tervo, L. Forest nursery waste composting in windrows with or without horse manure or urea—The composting process and nutrient leaching. Silva Fenn. 2007, 41, 13–27. [Google Scholar] [CrossRef][Green Version]
- Viaene, J.; Reubens, B.; Willekens, K.; Van Waes, C.; De Neve, S.; Vandecasteele, B. Potential of chopped heath biomass and spent growth media to replace wood chips as bulking agent for composting high N-containing residues. J. Environ. Manag. 2017, 197, 338–350. [Google Scholar] [CrossRef]
- Kraska, T.; Kleinschmidt, B.; Weinand, J.; Pude, R. Cascading use of Miscanthus as growing substrate in soilless cultivation of vegetables (tomatoes, cucumbers) and subsequent direct combustion. Sci. Hortic. 2018, 235, 205–213. [Google Scholar] [CrossRef]
- Petrasch, S.; Knapp, S.J.; Van Kan, J.A.L.; Blanco-Ulate, B. Grey mould of strawberry, a devastating disease caused by the ubiquitous necrotrophic fungal pathogen Botrytis cinerea. Mol. Plant Pathol. 2019, 20, 877–892. [Google Scholar] [CrossRef]
- Harel, Y.M.; Elad, Y.; Rav-David, D.; Borenstein, M.; Shulchani, R.; Lew, B.; Graber, E.R. Biochar mediates systemic response of strawberry to foliar fungal pathogens. Plant Soil 2012, 357, 245–257. [Google Scholar] [CrossRef]
- Vandecasteele, B.; Debode, J.; Willekens, K.; Van Delm, T. Recycling of P and K in circular horticulture through compost application in sustainable growing media for fertigated strawberry cultivation. Eur. J. Agron. 2018, 96, 131–145. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Alkan, N.; Elad, Y.; Sela, N.; Philosoph, A.M.; Graber, E.R.; Frenkel, O. Molecular insights into biochar-mediated plant growth promotion and systemic resistance in tomato againstFusarium crown and root rot disease. Sci. Rep. 2020, 10, 15. [Google Scholar] [CrossRef]
- Debode, J.; Ebrahimi, N.; D’Hose, T.; Cremelie, P.; Viaene, N.; Vandecasteele, B. Has compost with biochar added during the process added value over biochar or compost to increase disease suppression? Appl. Soil Ecol. 2020, 153, 8. [Google Scholar] [CrossRef]
- D’Hose, T.; Debode, J.; De Tender, C.; Ruysschaert, G.; Vandecasteele, B. Has compost with biochar applied during the process added value over biochar or compost for increasing soil quality in an arable cropping system? Appl. Soil Ecol. 2020, 156, 9. [Google Scholar] [CrossRef]
- Huang, W.K.; Ji, H.L.; Gheysen, G.; Debode, J.; Kyndt, T. Biochar-amended potting medium reduces the susceptibility of rice to root-knot nematode infections. BMC Plant Biol. 2015, 15, 15. [Google Scholar] [CrossRef]
- Lopez-Cano, I.; Roig, A.; Cayuela, M.L.; Alburquerque, J.A.; Sanchez-Monedero, M.A. Biochar improves N cycling during composting of olive mill wastes and sheep manure. Waste Manag. 2016, 49, 553–559. [Google Scholar] [CrossRef]
- Sanchez-Garcia, M.; Sanchez-Monedero, M.A.; Roig, A.; Lopez-Cano, I.; Moreno, B.; Benitez, E.; Cayuela, M.L. Compost vs biochar amendment: A two-year field study evaluating soil C build-up and N dynamics in an organically managed olive crop. Plant Soil 2016, 408, 1–14. [Google Scholar] [CrossRef]
- Takaya, C.A.; Fletcher, L.A.; Singh, S.; Anyikude, K.U.; Ross, A.B. Phosphate and ammonium sorption capacity of biochar and hydrochar from different wastes. Chemosphere 2016, 145, 518–527. [Google Scholar] [CrossRef]
- Vandecasteele, B.; Sinicco, T.; D’Hose, T.; Vanden Nest, T.; Mondini, C. Biochar amendment before or after composting affects compost quality and N losses, but not P plant uptake. J. Environ. Manag. 2016, 168, 200–209. [Google Scholar] [CrossRef]
- Vandecasteele, B.; Amery, F.; Ommeslag, S.; Vanhoutte, K.; Visser, R.; Robbens, J.; De Tender, C.; Debode, J. Chemically versus thermally processed brown shrimp shells or Chinese mitten crab as a source of chitin, nutrients or salts and as microbial stimulant in soilless strawberry cultivation. Sci. Total Environ. 2021, 771, 145263. [Google Scholar] [CrossRef] [PubMed]
- Rajkovich, S.; Enders, A.; Hanley, K.; Hyland, C.; Zimmerman, A.R.; Lehmann, J. Corn growth and nitrogen nutrition after additions of biochars with varying properties to a temperate soil. Biol. Fertil. Soils 2012, 48, 271–284. [Google Scholar] [CrossRef]
- Amery, F.; Van Loo, K.; Vandecasteele, B. Nutrients in circular horticulture: Blending peat with biochar alters interaction with fertigation solution. In Proceedings of the ISHS Acta Horticulturae: III International Symposium on Growing Media, Composting and Substrate Analysis, Milan, Italy, 24–28 June 2019. in press. [Google Scholar]
- Debode, J.; De Tender, C.; Cremelie, P.; Lee, A.S.; Kyndt, T.; Muylle, H.; De Swaef, T.; Vandecasteele, B. Trichoderma-inoculated miscanthus straw can replace peat in strawberry cultivation, with beneficial effects on disease control. Front. Plant Sci. 2018, 9, 213. [Google Scholar] [CrossRef]
- De Tender, C.; Vandecasteele, B.; Verstraeten, B.; Ommeslag, S.; De Meyer, T.; De Visscher, J.; Dawyndt, P.; Clement, L.; Kyndt, T.; Debode, J. Chitin in strawberry cultivation: Foliar growth and defense response promotion, but reduced fruit yield and disease resistance by nutrient imbalances. Mol. Plant-Microbe Interact. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Debode, J.; Van Hemelrijck, W.; Creemers, P.; Maes, M. Effect of fungicides on epiphytic yeasts associated with strawberry. MicrobiologyOpen 2013, 2, 482–491. [Google Scholar] [CrossRef]
- Reddy, M.V.B.; Belkacemi, K.; Corcuff, R.; Castaigne, F.; Arul, J. Effect of pre-harvest chitosan sprays on post-harvest infection by Botrytis cinerea and quality of strawberry fruit. Postharvest Biol. Technol. 2000, 20, 39–51. [Google Scholar] [CrossRef]
- Campbell, C.L.; Madden, L.V. Introduction to Plant Disease Epidemiology; John Wiley & Sons: New York, NY, USA, 1990; p. 532. [Google Scholar]
- Schandry, N. A practical guide to visualization and statistical analysis of R. solanacearum infection data using R. Front. Plant Sci. 2017, 8, 623. [Google Scholar] [CrossRef]
- Li, W.W.; Amin, F.R.; Fu, Y.S.; Zhang, H.; He, Y.F.; Huang, Y.; Liu, G.Q.; Chen, C. Effects of temperature, heating rate, residence time, reaction atmosphere, and pressure on biochar properties. J. Biobased Mater. Bioenergy 2019, 13, 1–10. [Google Scholar] [CrossRef]
- Prasad, M.; Chrysargyris, A.; McDaniel, N.; Kavanagh, A.; Gruda, N.S.; Tzortzakis, N. Plant nutrient availability and pH of biochars and their fractions, with the possible use as a component in a growing media. Agronomy 2020, 10, 10. [Google Scholar] [CrossRef]
- Altland, J.E.; Locke, J.C. Effect of biochar type on macronutrient retention and release from soilless substrate. Hortscience 2013, 48, 1397–1402. [Google Scholar] [CrossRef]
- Vanden Nest, T.; Amery, F.; Fryda, L.; Boogaerts, C.; Bilbao, J.; Vandecasteele, B. Renewable P sources: P use efficiency of digestate, processed animal manure, compost, biochar and struvite. Sci. Total Environ. 2021, 750, 141699. [Google Scholar] [CrossRef]
- Fan, R.M.; Chen, C.L.; Lin, J.Y.; Tzeng, J.H.; Huang, C.P.; Dong, C.D.; Huang, C.P. Adsorption characteristics of ammonium ion onto hydrous biochars in dilute aqueous solutions. Bioresour. Technol. 2019, 272, 465–472. [Google Scholar] [CrossRef]
- Yang, H.I.; Lou, K.; Rajapaksha, A.U.; Ok, Y.S.; Anyia, A.O.; Chang, S.X. Adsorption of ammonium in aqueous solutions by pine sawdust and wheat straw biochars. Environ. Sci. Pollut. Res. 2018, 25, 25638–25647. [Google Scholar] [CrossRef]
- Kharel, G.; Sacko, O.; Feng, X.; Morris, J.R.; Phillips, C.L.; Trippe, K.; Kumar, S.; Lee, J.W. Biochar surface oxygenation by ozonization for super high cation exchange capacity. ACS Sustain. Chem. Eng. 2019, 7, 16410–16418. [Google Scholar] [CrossRef]
- Nobile, C.; Denier, J.; Houben, D. Linking biochar properties to biomass of basil, lettuce and pansy cultivated in growing media. Sci. Hortic. 2020, 261, 5. [Google Scholar] [CrossRef]
- Alvarez, J.M.; Pasian, C.; Lal, R.; Lopez, R.; Fernandez, M. Vermicompost and biochar substrates can reduce nutrients leachates on containerized ornamental plant production. Hortic. Bras. 2019, 37, 47–53. [Google Scholar] [CrossRef]
- Prasad, M.; Tzortzakis, N.; McDaniel, N. Chemical characterization of biochar and assessment of the nutrient dynamics by means of preliminary plant growth tests. J. Environ. Manag. 2018, 216, 89–95. [Google Scholar] [CrossRef]
- Fidel, R.B.; Laird, D.A.; Spokas, K.A. Sorption of ammonium and nitrate to biochars is electrostatic and pH-dependent. Sci. Rep. 2018, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Lecompte, F.; Abro, M.A.; Nicot, P.C. Contrasted responses of Botrytis cinerea isolates developing on tomato plants grown under different nitrogen nutrition regimes. Plant Pathol. 2010, 59, 891–899. [Google Scholar] [CrossRef]
- Nam, M.H.; Jeong, S.K.; Lee, Y.S.; Choi, J.M.; Kim, H.G. Effects of nitrogen, phosphorus, potassium and calcium nutrition on strawberry anthracnose. Plant Pathol. 2006, 55, 246–249. [Google Scholar] [CrossRef]
- Xu, X.M.; Robinson, J.; Else, M.A. Effects of nitrogen input and deficit irrigation within the commercial acceptable range on susceptibility of strawberry leaves to powdery mildew. Eur. J. Plant Pathol. 2013, 135, 695–701. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Elad, Y.; Graber, E.R.; Frenkel, O. Rhizoctonia solani suppression and plant growth promotion in cucumber as affected by biochar pyrolysis temperature, feedstock and concentration. Soil Biol. Biochem. 2014, 69, 110–118. [Google Scholar] [CrossRef]
- Karasov, T.L.; Chae, E.; Herman, J.J.; Bergelson, J. Mechanisms to mitigate the trade-off between growth and defense. Plant Cell 2017, 29, 666–680. [Google Scholar] [CrossRef]
- Vanden Nest, T.; Vandecasteele, B.; Ruysschaert, G.; Cougnon, M.; Merckx, R. Effect of organic and mineral fertilizers on soil P and C levels, crop yield and P leaching in a long term trial on a silt loam soil. Agric. Ecosyst. Environ. 2014, 197, 309–317. [Google Scholar] [CrossRef]

| Code | Matrix | Feedstock | pH-H2O | EC | C/N | IC | OC | N | Ash | Dry Bulk Density | Pore Volume | CEC | P | K | Mg | Ca | Na | Al | Mn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| - | µS cm−1 | - | %/DM | g DM L−1 | vol% | cmolc (kg DM)−1 | g (kg DM)−1 | ||||||||||||
| Ref biochar | biochar | Holm oak | 9.1 | 384 | 80 | 1.38 | 63 | 0.8 | 20 | 290 | 83 | 41.9 | 1.9 | 8.7 | 4.3 | 85 | 0.3 | 1.6 | 0.9 |
| Flax650 | biochar, 650 °C | Flax shives | 9.8 | 392 | 136 | <0.08 | 94 | 0.7 | 12 | 57 | 97 | 36.4 | 3.6 | 13.8 | 3.3 | 17 | 0.9 | 1.8 | 0.1 |
| Flax400 | biochar, 400 °C | Flax shives | 9.5 | 160 | 91 | <0.08 | 69 | 0.2 | 8 | 71 | 96 | 95.0 | 2.2 | 9.2 | 2.1 | 11 | 0.5 | 0.8 | 0.1 |
| Misc650 | biochar, 650 °C | Miscanthus straw | 9.1 | 191 | 418 | <0.08 | 84 | 0.6 | 8 | 65 | 96 | 44.1 | 2.1 | 7.9 | 1.2 | 5 | 0.2 | 0.1 | 0.5 |
| Misc400 | biochar, 400 °C | Miscanthus straw | 8.7 | 42 | 157 | <0.08 | 71 | 0.3 | 6 | 54 | 97 | 50.5 | 1.2 | 7.3 | 1.2 | 6 | 0.1 | 0.1 | 1.0 |
| For650 | biochar, 650 °C | Wood forestry management | 9.2 | 170 | 249 | <0.08 | 85 | 0.8 | 7 | 81 | 95 | 45.9 | 1.8 | 9.3 | 2.2 | 15 | 0.6 | 0.6 | 0.2 |
| For400 | biochar, 400 °C | Wood forestry management | 8.7 | 168 | 150 | 0.22 | 86 | 0.5 | 5 | 96 | 94 | 56.4 | 1.2 | 5.9 | 1.5 | 10 | 0.4 | 0.1 | 0.1 |
| Speat650 | biochar, 650 °C | Spent peat, batch1 | 8.7 | 718 | 66 | <0.08 | 78 | 1.2 | 27 | 90 | 95 | 17.8 | 2.4 | 8.3 | 9.4 | 60 | 2.1 | 3.4 | 0.4 |
| Speat400 | biochar, 400 °C | Spent peat, batch1 | 9.8 | 1231 | 43 | 0.50 | 56 | 1.2 | 23 | 189 | 89 | 57.9 | 2.2 | 6.6 | 7.6 | 49 | 1.7 | 3.0 | 0.3 |
| Scoir650 | biochar, 650 °C | Spent coir, batch2 | 9.7 | 556 | 73 | <0.08 | 85 | 1.3 | 22 | 64 | 96 | 36.7 | 3.5 | 13.1 | 1.4 | 37 | 2.1 | 1.9 | 0.1 |
| Speat3-650 | biochar, 650 °C | Spent peat, batch3 | 9.6 | 747 | 60 | 0.72 | 71 | 1.2 | 23 | 171 | 90 | 14.3 | 2.1 | 9.7 | 7.2 | 53 | 1.4 | 3.7 | 0.4 |
| Scoir4-650 | biochar, 650 °C | Spent coir, batch4 | 9.3 | 479 | 68 | 0.53 | 68 | 1.0 | 26 | 98 | 94 | 19.7 | 2.6 | 7.3 | 5.5 | 53 | 3.6 | 2.7 | 0.7 |
| SGM | Spent peat, batch1 | 6.3 | 599 | 37 | 0.06 | 46 | 1.2 | 11 | 71 | 96 | 112.0 | 0.8 | 3.0 | 3.5 | 23 | 0.8 | 1.0 | 0.1 | |
| SGM | Spent peat, batch3 | 5.7 | 912 | 35 | <0.08 | 43 | 1.2 | 23 | 185 | 93 | 83.8 | 0.8 | 3.2 | 3.2 | 22 | 0.6 | 0.9 | 0.1 | |
| SGM | Spent coir, batch2 | 4.2 | 431 | 39 | <0.01 | 46 | 1.2 | 8 | 41 | 97 | 109.3 | 1.7 | 3.7 | 0.4 | 13 | 0.5 | 0.3 | 0.0 | |
| SGM | Spent coir, batch4 | 5.7 | 882 | 31 | <0.08 | 45 | 1.4 | 20 | 126 | 95 | 101.0 | 1.1 | 2.5 | 2.3 | 22 | 1.4 | 0.7 | 0.2 | |
| Fe | Al | Mn | Mg | Ca | K | Na | P | NO3-N | NH4-N | SO4 | Cl | pH | EC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg L−1 | - | µS cm−1 | ||||||||||||
| Fertigation solution | 0.5 | 0.00 | 0.49 | 15 | 0 | 96 | 1.2 | 24.1 | 75 | 123 | 304 | 2.1 | 5.0 | 1593 |
| 100% peat | 1.2 (0.0) | 0.13 (0.03) | 0.06 (0) | 25 (1) | 67 (2) | 52 (3) | 5.1 (0.7) | 21.5 (0.2) | 66 (1) | 75 (0) | 274 (9) | 3.7 (0.5) | 4.4 (0) | 1383 (22) |
| Ref biochar | 0.7 (0.0) *** | 0.08 (0.01) | 0.23 (0.00) *** | 25 (0) | 83 (0) ** | 137 (1) *** | 9.9 (1.1) | 25.3 (0.3) *** | 59 (1) | 41 (6) ** | 267 (4) | 5.7 (0.9) | 6.4 (0.0) *** | 1340 (44) |
| Flax650 | 1.2 (0.0) | 0.11 (0.01) | 0.06 (0.00) | 21 (2) | 53 (2) * | 83 (0) *** | 6.9 (0.9) | 25.5 (0.4) *** | 55 (7) * | 68 (6) | 271 (8) | 4.2 (0.1) | 4.6 (0.0) | 1330 (48) |
| Misc650 | 1.2 (0.0) | 0.17 (0.07) | 0.14 (0.02) * | 26 (3) | 65 (6) | 66 (4) ** | 7.5 (1.7) | 21.1 (1.2) | 60 (4) | 59 (11) | 273 (1) | 6.0 (1.4) | 4.4 (0.0) | 1283 (74) |
| For400 | 1.2 (0.0) | 0.13 (0.02) | 0.08 (0.02) | 23 (1) | 61 (3) | 70 (2) *** | 6.8 (0.5) | 22.0 (0.0) | 68 (3) | 71 (2) | 278 (7) | 4.5 (0.1) | 4.4 (0.0) | 1371 (8) |
| For650 | 1.2 (0.0) | 0.10 (0.01) | 0.07 (0.01) | 22 (1) | 56 (4) | 72 (2) *** | 5.7 (1.0) | 21.6 (0.4) | 63 (0) | 73 (6) | 272 (0) | 4.5 (0.8) | 4.6 (0.1) | 1373 (42) |
| Speat650 | 1.1 (0.0) * | 0.12 (0.02) | 0.11 (0.01) | 22 (0) | 61 (4) | 79 (4) *** | 11.7 (2.7) | 22.0 (0.4) | 52 (2) * | 70 (5) | 317 (27) * | 9.5 (2.2) ** | 5.0 (0.0) *** | 1374 (7) |
| Scoir650 | 1.2 (0.0) | 0.11 (0.01) | 0.07 (0.04) | 20 (0) * | 52 (1) ** | 86 (0) *** | 11.2 (2.0) * | 24.1 (0.4) ** | 58 (0) | 73 (3) | 300 (3) | 9.0 (1.6) ** | 4.7 (0.1) ** | 1380 (41) |
| p (ANOVA) | <0.0001 | 0.29 | 0.0002 | 0.031 | 0.0002 | <0.0001 | 0.0135 | <0.0001 | 0.0118 | 0.05 | 0.01 | 0.0065 | <0.0001 | 0.33 |
| Fe | Al | Mn | Mg | Ca | K | Na | P | NO3-N | NH4-N | SO4 | Cl | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 100% peat | 2.1 (0.3) | 0.33 (0.02) | −0.84 (0.19) | 26 (1) | 149 (22) | −46 (11) | 8.7 (0.2) | 6.1 (0.7) | 13 (5) | −45 (19) | 67 (30) | 5.2 (0.2) |
| Ref biochar | 0.7 (0.0) ** | 0.27 (0.07) | −0.26 (0.08) * | 26 (4) | 162 (27) | 134 (11) *** | 14.1 (0.9) ** | 19.8 (1.7) *** | 19 (7) | −57 (11) | 100 (16) | 7.4 (0.0) |
| Flax650 | 2.3 (0.3) | 0.37 (0.00) | −0.94 (0.14) | 21 (3) | 136 (9) | 25 (5) *** | 14.0 (0.3) ** | 16.6 (0.9) | −1 (22) | −56 (3) | 70 (28) | 6.9 (0.2) |
| Misc650 | 1.8 (0.2) | 0.38 (0.09) | −0.50 (0.21) | 26 (2) | 123 (17) | −3 (5) *** | 10.9 (0.2) | 8.3 (0.4) | 13 (0) | −43 (7) | 94 (14) | 7.6 (0.4) * |
| For400 | 2.0 (0.2) | 0.34 (0.01) | −0.79 (0.00) | 23 (3) | 141 (14) | −11 (0) ** | 12.8 (1.3) | 7.6 (1.6) | 20 (12) | −52 (0) | 82 (29) | 6.1 (0.8) |
| For650 | 2.2 (0.4) | 0.29 (0.05) | −0.89 (0.26) | 22 (1) | 138 (22) | −5 (4) ** | 10.9 (0.1) | 6.7 (0.2) | 9 (4) | −47 (11) | 68 (14) | 6.6 (0.4) |
| Speat650 | 2.2 (0.2) | 0.40 (0.02) | −0.84 (0.17) | 24 (1) | 161 (15) | 17 (6) *** | 25.7 (1.9) *** | 9.7 (1.1) *** | −3 (4) | −52 (1) | 173 (48) * | 18.1 (1.3) *** |
| Scoir650 | 2.3 (0.4) | 0.36 (0.09) | −0.96 (0.07) | 18 (2) | 141 (16) | 35 (3) *** | 24.8 (1.0) *** | 14.1 (1.7) *** | 10 (2) | −48 (5) | 142 (13) | 17.1 (1.2) *** |
| p (ANOVA) | 0.006 | 0.34 | 0.024 | 0.56 | 0.50 | <0.001 | <0.001 | <0.001 | 0.27 | 0.75 | 0.03 | <0.001 |
| Fe (%) | Mn (%) | K (%) | NO3-N (%) | NH4-N (%) | |
|---|---|---|---|---|---|
| 100% peat | −201 (15) | 86 (1) | 24 (0) | −10 (5) | 18 (4) |
| Ref biochar | −100 (20) ** | 39 (2) *** | −104 (18) *** | −20 (12) | 34 (2) *** |
| Flax650 | −194 (2) | 85 (2) | −12 (4) ** | 0 (13) | 20 (2) |
| Misc650 | −229 (48) | 64 (8) ** | 1 (3) * | −12 (3) | 23 (3) |
| For400 | −186 (5) | 81 (5) | 6 (0) | −13 (7) | 21 (1) |
| For650 | −193 (13) | 84 (3) | 2 (1) | −6 (4) | 18 (0) |
| Speat650 | −178 (14) | 74 (1) | −8 (4) ** | 2 (3) | 19 (4) |
| Scoir650 | −183 (2) | 83 (7) | −16 (1) ** | −6 (0) | 17 (1) |
| p (ANOVA) | 0.0077 | <0.0001 | <0.0001 | 0.1848 | 0.0018 |
| Treatment | Above Green Parts | Strawberry Yield | Total Fresh Biomass | Root Score(0–3) | TLA | CCI | × 1000 = Total Plant CCI | AUDPC | ||
|---|---|---|---|---|---|---|---|---|---|---|
| FW | DW | Number of Fruits/Plant | Fruit Weight/Plant | |||||||
| g | g | - | g | g FW | - | cm2 | - | - | - | |
| 0.70 g PGMix L−1 | 49.3 (4.3) | 11.1 (0.6) | 16.7 (1.6) | 77.0 (11.6) | 126.3 (14.0) | 2.0 (0.0) | 675.3 (63.1) | 35.9 (3.8) | 23.6 (2.5) ** | 2.4 (0.2) |
| 1.05 g PGMix L−1 | 55.5 (8.8) | 13.0 (0.5) | 15.2 (2.1) | 96.8 (13.7) | 152.3 (15.7) | 1.8 (0.2) | 912.4 (66.2) | 39.6 (2.9) | 34.0 (4.1) | 2.3 (0.3) |
| 1.40 g PGMix L−1 | 66.5 (10.4) | 14.7 (1.0) | 15.2 (1.0) | 90.0 (5.3) | 156.5 (12.8) | 2.3 (0.3) | 1003.1 (82.4) | 39.3 (4.1) | 40.2 (5.0) | 2.4 (0.3) |
| 1.75 g PGMix L−1 | 55.2 (5.0) | 14.8 (0.8) | 14.5 (1.4) | 83.8 (9.7) | 139.1 (13.6) | 1.8 (0.3) | 1001.8 (103.7) | 41.0 (6.8) | 46.5 (1.2) | 2.5 (0.2) |
| 1.05 g PGMix L−1 + Ref biochar (2 g L−1) | 51.5(9.6) | 12.9 (1.0) | 11.2 (2.2) | 58.3 (2.2) | 109.9 (24.2) | 2.0 (0.4) | 699.5 (101.7) | 56.5 (5.2) * | 38.4 (3.0) | 2.2 (0.3) |
| 1.05 g PGMix L−1 + For650 (2 g L−1) | 50.8 (12.6) | 10.4 (1.2) * | 11.5 (3.0) | 67.2 (3.0) | 118.0 (27.3) | 1.5 (0.2) | 679.5 (94.4) | 34.6 (3.9) | 23.1 (3.0) | 2.4 (0.2) |
| 1.05 g PGMix L−1 + Speat650 (2 g L−1) | 43.6 (2.4) | 10.9 (0.8) | 11.0 (2.9) | 64.2 (2.9) | 107.8 (21.8) | 1.5 (0.2) | 681.6 (93.2) * | 53.5 (7.8) | 36.9 (1.8) | 2.5 (0.2) |
| 1.05 g PGMix L−1 + Scoir650 (2 g L−1) | 53.9 (6.8) | 12.1 (0.8) | 17.6 (1.1) | 96.2 (1.1) | 150.0 (10.2) | 1.7 (0.2) | 862.8 (43.4) | 38.6 (4.0) | 33.7 (4.5) | 2.0 (0.2) |
| Treatment: Limed Peat + | N | P | K | Mg | Ca | Na |
|---|---|---|---|---|---|---|
| 0.70 g PGMix L−1 | 108 (13) | 21 (2) | 74 (16) | 43 (5) | 109 (9) | 0.33 (0.09) |
| 1.05 g PGMix L−1 | 135 (10) | 28 (5) | 99 (16) | 47 (5) | 118 (14) | 0.35 (0.08) |
| 1.40 g PGMix L−1 | 141 (11) | 28 (3) | 130 (10) | 47 (8) | 122 (21) | 0.36 (0.1) |
| 1.75 g PGMix L−1 | 157 (23) | 36 (6) | 147 (20) * | 48 (8) | 116 (20) | 0.43 (0.11) |
| 1.05 g PGMix L−1 + Ref biochar (2 g L−1) | 159 (23) | 39 (8) * | 153 (31) ** | 53 (8) | 156 (22) *** | 0.39 (0.1) |
| 1.05 g PGMix L−1 + For650 (2 g L−1) | 127 (32) | 31 (8) | 97 (42) | 40 (9) | 109 (27) | 0.33 (0.09) |
| 1.05 g PGMix L−1 + Speat650 (2 g L−1) | 132 (27) | 32 (7) | 122 (38) | 43 (7) | 121 (17) | 0.38 (0.07) |
| 1.05 g PGMix L−1 + Scoir650 (2 g L−1) | 119 (21) | 26 (5) | 98 (21) | 44 (6) | 125 (19) | 0.36 (0.07) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amery, F.; Debode, J.; Ommeslag, S.; Visser, R.; De Tender, C.; Vandecasteele, B. Biochar for Circular Horticulture: Feedstock Related Effects in Soilless Cultivation. Agronomy 2021, 11, 629. https://doi.org/10.3390/agronomy11040629
Amery F, Debode J, Ommeslag S, Visser R, De Tender C, Vandecasteele B. Biochar for Circular Horticulture: Feedstock Related Effects in Soilless Cultivation. Agronomy. 2021; 11(4):629. https://doi.org/10.3390/agronomy11040629
Chicago/Turabian StyleAmery, Fien, Jane Debode, Sarah Ommeslag, Rian Visser, Caroline De Tender, and Bart Vandecasteele. 2021. "Biochar for Circular Horticulture: Feedstock Related Effects in Soilless Cultivation" Agronomy 11, no. 4: 629. https://doi.org/10.3390/agronomy11040629
APA StyleAmery, F., Debode, J., Ommeslag, S., Visser, R., De Tender, C., & Vandecasteele, B. (2021). Biochar for Circular Horticulture: Feedstock Related Effects in Soilless Cultivation. Agronomy, 11(4), 629. https://doi.org/10.3390/agronomy11040629