Genetic Mapping of Grain Shape Associated QTL Utilizing Recombinant Inbred Sister Lines in High Yielding Rice (Oryza sativa L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Cultivation
2.2. Phenotypic Analysis
2.3. QTLs Mapping and Statistical Analysis
2.4. Bulk Segregant Analysis and Re-Sequencing
3. Results
3.1. Phenotypes of Parents and RISLs
3.2. QTLs Analysis for GL, GW, and LWR
| QTLs of This Study | QTLs of Previous Study | |||
|---|---|---|---|---|
| QTLs | Physical (Mb) | Published_Symbol | Maker | Reference |
| qGL-1b/qGW-1c | 34.25–40.65 | qGL-1 | R210-C955 | [47] |
| qGL-2b | 4.95–6.55 | qGL-2b | GL1327-C421 | [48] |
| qGL-2d/qLWR-2b | 26.05–28.85 | qGL-2 | C601-R3393 | [47] |
| qGL-5 | 26.25–28.95 | qGL5 | RM3321-RM274 | [28] |
| qGL-6b/qGW-6 | 32.05–32.25 | qTGW-6c | Bin1883-Bin1884 | [36] |
| qGL-7a | 1.55–2.05 | - | - | - |
| qGL-7b/qGW-7 | 18.85–23.65 | qGS7.1 | RM500-RM21772 | [49] |
| GLW7 | - | [12] | ||
| qGL-8 | 22.70–25.05 | - | - | - |
| qGL-10b | 20.55–23.35 | kl10.1 | G2155-RG134 | [50] |
| qGL10 | RM27588-RM590 | [49] | ||
| qGL-11a | 0.15–1.85 | - | - | - |
| qGL-12b/qLWR-12a | 3.75–6.45 | TGW12 | RM7003-RM247 | [51] |
| qGW-1a/qLWR-1a/qLWR-1b | 0.05–4.25 | qGW-1 | C112-Xnpb113 | [47] |
| qGW-2b/qLWR-2c | 31.05–36.25 | qGW-2 | Tw35293-RM266 | [28] |
| TGW2 | - | |||
| qGW-12/qLWR-12c | 23.15–26.55 | GL12.2 | RM5479–RM28597 | [52] |
| qTGW-12 | Bin3246-Bin3266 | [36] | ||
| qLWR-9a | 7.75–18.35 | qLWR9-1 | RM23969-RM24002 | [49] |
| GS9 | - | [11] | ||
3.3. Verification of qLWR-12c/qGW-12
3.4. Fine Mapping of qLWR-12c/qGW-12 in RHLs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xia, D.; Zhou, H.; Qiu, L.; Jiang, H.; Zhang, Q.; Gao, G.; He, Y. Mapping and verification of grain shape QTLs based on an advanced backcross population in rice. PLoS ONE 2017, 12, e187553. [Google Scholar] [CrossRef]
- Fan, C.; Xing, Y.; Mao, H.; Lu, T.; Han, B.; Xu, C.; Li, X.; Zhang, Q. GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. Theor. Appl. Genet. 2006, 112, 1164–1171. [Google Scholar] [CrossRef]
- Song, X.J.; Huang, W.; Shi, M.; Zhu, M.Z.; Lin, H.X. A QTL for rice grain width and weight encodes a previously unknown RING-type E3 ubiquitin ligase. Nat. Genet. 2007, 39, 623–630. [Google Scholar] [CrossRef]
- Liu, J.; Chen, J.; Zheng, X.; Wu, F.; Lin, Q.; Heng, Y.; Tian, P.; Cheng, Z.; Yu, X.; Zhou, K.; et al. GW5 acts in the brassinosteroid signalling pathway to regulate grain width and weight in rice. Nat. Plants 2017, 3, 17043. [Google Scholar] [CrossRef] [PubMed]
- Ruan, B.; Shang, L.; Zhang, B.; Hu, J.; Wang, Y.; Lin, H.; Zhang, A.; Liu, C.; Peng, Y.; Zhu, L.; et al. Natural variation in the promoter of TGW2 determines grain width and weight in rice. N. Phytol. 2020, 227, 629–640. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Huang, J.; Lan, H.; Wang, C.; Yin, C.; Wu, Y.; Tang, H.; Qian, Q.; Li, J.; et al. Rare allele of OsPPKL1 associated with grain length causes extra-large grain and a significant yield increase in rice. Proc. Natl. Acad. Sci. USA 2012, 109, 21534–21539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishimaru, K.; Hirotsu, N.; Madoka, Y.; Murakami, N.; Hara, N.; Onodera, H.; Kashiwagi, T.; Ujiie, K.; Shimizu, B.; Onishi, A.; et al. Loss of function of the IAA-glucose hydrolase gene TGW6 enhances rice grain weight and increases yield. Nat. Genet. 2013, 45, 707–711. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Y.; Fang, Y.; Zeng, L.; Xu, J.; Yu, H.; Shi, Z.; Pan, J.; Zhang, D.; Kang, S.; et al. A Rare Allele of GS2 Enhances Grain Size and Grain Yield in Rice. Mol. Plant 2015, 8, 1455–1465. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Wu, K.; Yuan, Q.; Liu, X.; Liu, Z.; Lin, X.; Zeng, R.; Zhu, H.; Dong, G.; Qian, Q.; et al. Control of grain size, shape and quality by OsSPL16 in rice. Nat. Genet. 2012, 44, 950–954. [Google Scholar] [CrossRef]
- Liu, Q.; Han, R.; Wu, K.; Zhang, J.; Ye, Y.; Wang, S.; Chen, J.; Pan, Y.; Li, Q.; Xu, X.; et al. G-protein βγ subunits determine grain size through interaction with MADS-domain transcription factors in rice. Nat. Commun. 2018, 9, 852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.S.; Li, Q.F.; Zhang, C.Q.; Zhang, C.; Yang, Q.Q.; Pan, L.X.; Ren, X.Y.; Lu, J.; Gu, M.H.; Liu, Q.Q. GS9 acts as a transcriptional activator to regulate rice grain shape and appearance quality. Nat. Commun. 2018, 9, 1240. [Google Scholar] [CrossRef] [PubMed]
- Si, L.; Chen, J.; Huang, X.; Gong, H.; Luo, J.; Hou, Q.; Zhou, T.; Lu, T.; Zhu, J.; Shangguan, Y.; et al. OsSPL13 controls grain size in cultivated rice. Nat. Genet. 2016, 48, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Liu, X.; Wang, M.; Meyer, R.S.; Luo, X.; Ndjiondjop, M.N.; Tan, L.; Zhang, J.; Wu, J.; Cai, H.; et al. A single-nucleotide polymorphism causes smaller grain size and loss of seed shattering during African rice domestication. Nat. Plants 2017, 3, 17064. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Hou, Q.; Si, L.; Huang, X.; Luo, J.; Lu, D.; Zhu, J.; Shangguan, Y.; Miao, J.; Xie, Y.; et al. The PLATZ Transcription Factor GL6 Affects Grain Length and Number in Rice. Plant Physiol. 2019, 180, 2077–2090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Xiong, H.; Zhu, X.; Zhang, H.; Li, H.; Miao, J.; Wang, W.; Tang, Z.; Zhang, Z.; Yao, G.; et al. OsLG3 contributing to rice grain length and yield was mined by Ho-LAMap. BMC Biol. 2017, 15, 28. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Fan, C.; Xing, Y.; Jiang, Y.; Luo, L.; Sun, L.; Shao, D.; Xu, C.; Li, X.; Xiao, J.; et al. Natural variation in GS5 plays an important role in regulating grain size and yield in rice. Nat. Genet. 2011, 43, 1266–1269. [Google Scholar] [CrossRef]
- Shi, C.L.; Dong, N.Q.; Guo, T.; Ye, W.W.; Shan, J.X.; Lin, H.X. A quantitative trait locus GW6 controls rice grain size and yield through the gibberellin pathway. Plant J. 2020, 103, 1174–1188. [Google Scholar] [CrossRef]
- Wang, Y.; Xiong, G.; Hu, J.; Jiang, L.; Yu, H.; Xu, J.; Fang, Y.; Zeng, L.; Xu, E.; Xu, J.; et al. Copy number variation at the GL7 locus contributes to grain size diversity in rice. Nat. Genet. 2015, 47, 944–948. [Google Scholar] [CrossRef]
- Ying, J.Z.; Ma, M.; Bai, C.; Huang, X.H.; Liu, J.L.; Fan, Y.Y.; Song, X.J. TGW3, a Major QTL that Negatively Modulates Grain Length and Weight in Rice. Mol. Plant 2018, 11, 750–753. [Google Scholar] [CrossRef] [Green Version]
- Song, X.J.; Kuroha, T.; Ayano, M.; Furuta, T.; Nagai, K.; Komeda, N.; Segami, S.; Miura, K.; Ogawa, D.; Kamura, T.; et al. Rare allele of a previously unidentified histone H4 acetyltransferase enhances grain weight, yield, and plant biomass in rice. Proc. Natl. Acad. Sci. USA 2015, 112, 76–81. [Google Scholar] [CrossRef] [Green Version]
- Dong, N.Q.; Sun, Y.; Guo, T.; Shi, C.L.; Zhang, Y.M.; Kan, Y.; Xiang, Y.H.; Zhang, H.; Yang, Y.B.; Li, Y.C.; et al. UDP-glucosyltransferase regulates grain size and abiotic stress tolerance associated with metabolic flux redirection in rice. Nat. Commun. 2020, 11, 2629. [Google Scholar] [CrossRef]
- Li, N.; Xu, R.; Duan, P.; Li, Y. Control of grain size in rice. Plant Reprod. 2018, 31, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Peng, R.; Peng, J. Overview of Rice Quantitative Traits (QTL) Mapping Main Construction Population and Statistical Methods. Hunan Agric. Sci. 2016, 7, 120–123, (In Chinese with English Abstract). [Google Scholar]
- Hongge, L.I.; Zhaoe, P.; Shoupu, H.E.; Yinhua, J.; Xiaoli, G.; Baojun, C.; Liru, W.; Baoyin, P.; Xiongming, D.U. QTL mapping of agronomic and economic traits for four F2 populations of upland cotton. J. Cotton Res. 2021, 4, 3. [Google Scholar]
- Kabange, N.R.; Park, S.; Shin, D.; Lee, S.; Jo, S.; Kwon, Y.; Cha, J.; Song, Y.; Ko, J.; Lee, J. Identification of a Novel QTL for Chlorate Resistance in Rice (Oryza sativa L.). Agriculture 2020, 10, 360. [Google Scholar] [CrossRef]
- Yan, X.; Zheng, H.; Zhang, P.; Weldu, G.T.; Li, Z.; Liu, D. QTL mapping of adult plant resistance to stripe rust in the Fundulea 900× Thatcher RIL population. Czech J. Genet. Plant 2021, 57, 1–8. [Google Scholar] [CrossRef]
- Tang, G.P.; Cheng, X.R.; Zhu, C.L.; Peng, X.S.; He, X.P.; Fu, J.R.; Bian, J.M.; Hu, L.F.; Ouyang, L.J.; He, H.H. Identification Low Nitrogen Tolerance of XieqingzaoB//XieqingzaoB/Dongxiang wild Rice BC1F13 Populations and Some Restorers of Hybricl Rice. Acta Agric. Univ. Jiangxiensis 2017, 39, 214–222, (In Chinese with English Abstract). [Google Scholar]
- Zhang, H.; Zhu, Y.J.; Zhu, A.D.; Fan, Y.Y.; Huang, T.X.; Zhang, J.F.; Xie, H.A.; Zhuang, J.Y. Fine-mapping of qTGW2, a quantitative trait locus for grain weight in rice (Oryza sativa L.). PeerJ 2020, 8, e8679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Yuan, Z.; Wang, Y.; Sun, W.; Tang, X.; Sun, Y.; Yu, S. Genetic Dissection of Seed Dormancy in Rice (Oryza sativa L.) by Using Two Mapping Populations Derived from Common Parents. Rice 2020, 13, 52. [Google Scholar] [CrossRef]
- Zhang, B.; Shang, L.; Ruan, B.; Zhang, A.; Yang, S.; Jiang, H.; Liu, C.; Hong, K.; Lin, H.; Gao, Z.; et al. Development of Three Sets of High-Throughput Genotyped Rice Chromosome Segment Substitution Lines and QTL Mapping for Eleven Traits. Rice 2019, 12, 33. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, D.; Liu, W.; Li, D.; Liao, Y.; Li, J.; Fu, C.; Fu, F.; Huang, H.; Zeng, X.; et al. QTL mapping using an ultra-high-density SNP map reveals a major locus for grain yield in an elite rice restorer R998. Sci. Rep. 2017, 7, 10914. [Google Scholar] [CrossRef]
- Huang, X.; Feng, Q.; Qian, Q.; Zhao, Q.; Wang, L.; Wang, A.; Guan, J.; Fan, D.; Weng, Q.; Huang, T.; et al. High-throughput genotyping by whole-genome resequencing. Genome Res. 2009, 19, 1068–1076. [Google Scholar] [CrossRef] [Green Version]
- van Os, H.; Andrzejewski, S.; Bakker, E.; Barrena, I.; Bryan, G.J.; Caromel, B.; Ghareeb, B.; Isidore, E.; de Jong, W.; van Koert, P.; et al. Construction of a 10,000-marker ultradense genetic recombination map of potato: Providing a framework for accelerated gene isolation and a genomewide physical map. Genetics 2006, 173, 1075–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Wu, L.; Wang, J.; Sun, J.; Xia, X.; Geng, X.; Wang, X.; Xu, Z.; Xu, Q. Genome sequencing of rice subspecies and genetic analysis of recombinant lines reveals regional yield-and quality-associated loci. BMC Biol. 2018, 16, 102. [Google Scholar] [CrossRef] [Green Version]
- Ashikari, M.; Sakakibara, H.; Lin, S.; Yamamoto, T.; Takashi, T.; Nishimura, A.; Angeles, E.R.; Qian, Q.; Kitano, H.; Matsuoka, M. Cytokinin oxidase regulates rice grain production. Science 2005, 309, 741–745. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, Z.; Chen, Y.; Cao, Y.; Deng, C.; Xue, P.; Zhan, X.; Cheng, S.; Cao, L.; Zhang, Y. Finding new addictive QTL for yield traits based on a high-density genetic map in hybrid rice. Plant Growth Regul. 2021, 93, 105–115. [Google Scholar] [CrossRef]
- Salunkhe, A.S.; Poornima, R.; Prince, K.S.; Kanagaraj, P.; Sheeba, J.A.; Amudha, K.; Suji, K.K.; Senthil, A.; Babu, R.C. Fine mapping QTL for drought resistance traits in rice (Oryza sativa L.) using bulk segregant analysis. Mol. Biotechnol. 2011, 49, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Abe, A.; Yoshida, K.; Kosugi, S.; Natsume, S.; Mitsuoka, C.; Uemura, A.; Utsushi, H.; Tamiru, M.; Takuno, S.; et al. QTL-seq: Rapid mapping of quantitative trait loci in rice by whole genome resequencing of DNA from two bulked populations. Plant J. 2013, 74, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Abe, A.; Takagi, H.; Fujibe, T.; Aya, K.; Kojima, M.; Sakakibara, H.; Uemura, A.; Matsuoka, M.; Terauchi, R. OsGA20ox1, a candidate gene for a major QTL controlling seedling vigor in rice. Theor. Appl. Genet. 2012, 125, 647–657. [Google Scholar] [CrossRef]
- Cheng, S.H.; Cao, L.Y.; Zhuang, J.Y.; Chen, S.G.; Zhan, X.D.; Fan, Y.Y.; Zhu, D.F.; Min, S.K. Super Hybrid Rice Breeding in China: Achievements and Prospects. J. Integr. Plant Biol. 2007, 49, 805–810. [Google Scholar] [CrossRef]
- Zhang, Q. Rice planting and pest control techniques. Mod. Agric. Res. 2020, 8, 139–140, (In Chinese with English Abstract). [Google Scholar]
- McCouch, S.R. Gene Nomenclature System for Rice. Rice 2008, 1, 72–84. [Google Scholar] [CrossRef] [Green Version]
- Rogers, S.O.; Bendich, A.J. Extraction of DNA from milligram amounts of fresh, herbarium and mummified plant tissues. Plant Mol. Biol. 1985, 5, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [Green Version]
- Cingolani, P.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, N.; Xu, R.; Huang, L.; Zhang, B.; Duan, P.; Li, N.; Luo, Y.; Li, Y. SMALL GRAIN 11 Controls Grain Size, Grain Number and Grain Yield in Rice. Rice 2016, 9, 64. [Google Scholar] [CrossRef] [Green Version]
- Wan, X.Y.; Wan, J.M.; Weng, J.F.; Jiang, L.; Bi, J.C.; Wang, C.M.; Zhai, H.Q. Stability of QTLs for rice grain dimension and endosperm chalkiness characteristics across eight environments. Theor. Appl. Genet. 2005, 110, 1334–1346. [Google Scholar] [CrossRef]
- Li, Z.F.; Wan, J.M.; Xia, J.F.; Zhai, H.Q. Mapping quantitative trait loci underlying appearance quality of rice grains (Oryza sativa L.). J. Genet. Genom. 2003, 30, 251–259. [Google Scholar]
- Xue, P.; Zhang, Y.; Lou, X.; Zhu, A.; Chen, Y.; Sun, B.; Yu, P.; Cheng, S.; Cao, L.; Zhan, X. Mapping and genetic validation of a grain size QTL qGS7.1 in rice (Oryza sativa L.). J. Integr. Agr. 2019, 18, 1838–1850. [Google Scholar] [CrossRef]
- Li, J.; Xiao, J.; Grandillo, S.; Jiang, L.; Wan, Y.; Deng, Q.; Yuan, L.; McCouch, S.R. QTL detection for rice grain quality traits using an interspecific backcross population derived from cultivated Asian (O. sativa L.) and African (O. glaberrima S.) rice. Genome 2004, 47, 697–704. [Google Scholar] [CrossRef]
- Li, X.; Wei, Y.; Li, J.; Yang, F.; Chen, Y.; Chen, Y.; Guo, S.; Sha, A. Identification of QTL TGW12 responsible for grain weight in rice based on recombinant inbred line population crossed by wild rice (Oryza minuta) introgression line K1561 and indica rice G1025. BMC Genet. 2020, 21, 10. [Google Scholar] [CrossRef] [Green Version]
- Qi, L.; Ding, Y.; Zheng, X.; Xu, R.; Zhang, L.; Wang, Y.; Wang, X.; Zhang, L.; Cheng, Y.; Qiao, W.; et al. Fine mapping and identification of a novel locus qGL12.2 control grain length in wild rice (Oryza rufipogon Griff.). Theor. Appl. Genet. 2018, 131, 1497–1508. [Google Scholar] [CrossRef]
- Ni, X.; Xia, Q.; Zhang, H.; Cheng, S.; Li, H.; Fan, G.; Guo, T.; Huang, P.; Xiang, H.; Chen, Q.; et al. Updated foxtail millet genome assembly and gene mapping of nine key agronomic traits by resequencing a RIL population. Gigascience 2017, 6, 1–8. [Google Scholar] [CrossRef]
- Sun, B.; Zhan, X.; Lin, Z.; Gao, Z.; Yu, P.; Liu, Q.; Shen, X.; Zhang, Y.; Chen, D.; Cheng, S.; et al. Correlation Analysis and QTL Mapping of Grain Shape and Grain Weight in Rice. Mol. Plant Breed. 2015, 13, 2663–2672. [Google Scholar]
- Quist, T.M.; Sokolchik, I.; Shi, H.; Joly, R.J.; Bressan, R.A.; Maggio, A.; Narsimhan, M.; Li, X. HOS3, an ELO-like gene, inhibits effects of ABA and implicates a S-1-P/ceramide control system for abiotic stress responses in Arabidopsis thaliana. Mol. Plant 2009, 2, 138–151. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Zhang, Y.; Ding, P.; Johnson, K.; Li, X.; Zhang, Y. The ankyrin-repeat transmembrane protein BDA1 functions downstream of the receptor-like protein SNC2 to regulate plant immunity. Plant Physiol. 2012, 159, 1857–1865. [Google Scholar] [CrossRef] [Green Version]
- Nardini, M.; Dijkstra, B.W. Alpha/beta hydrolase fold enzymes: The family keeps growing. Curr. Opin. Struct. Biol. 1999, 9, 732–737. [Google Scholar] [CrossRef]
- Dlakić, M. Functionally unrelated signalling proteins contain a fold similar to Mg2+-dependent endonucleases. Trends Biochem. Sci. 2000, 25, 272–273. [Google Scholar] [CrossRef]
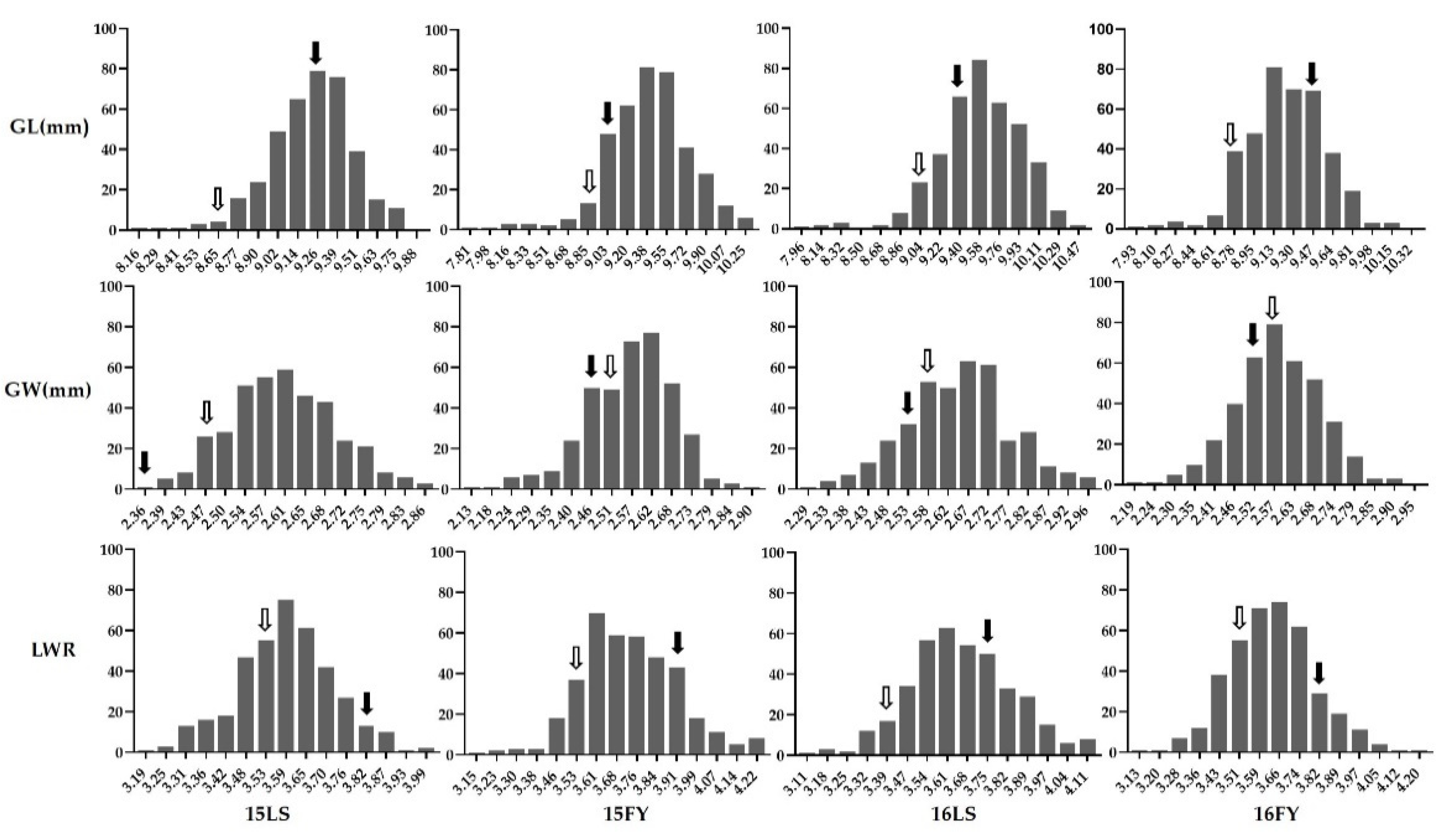
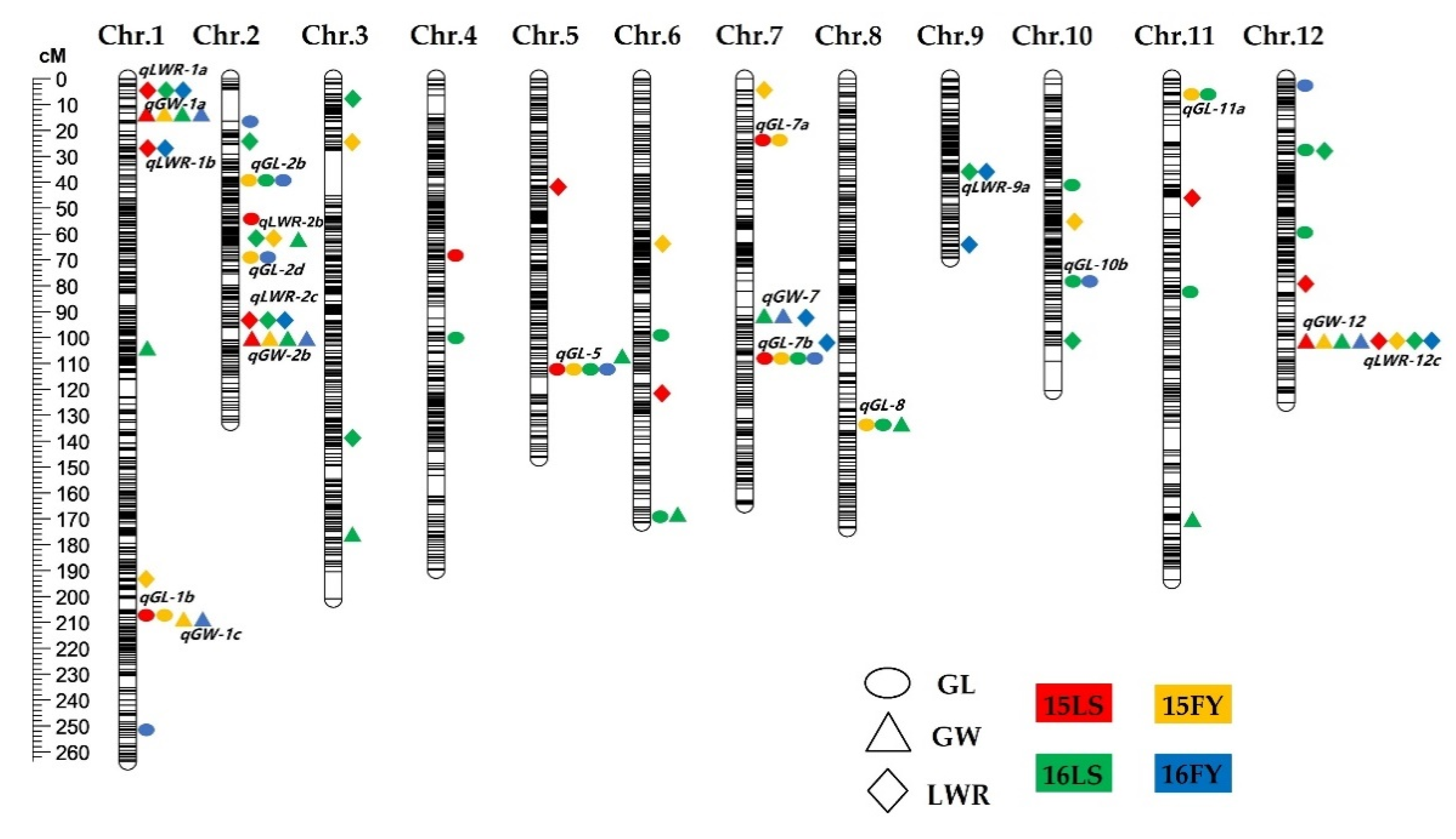
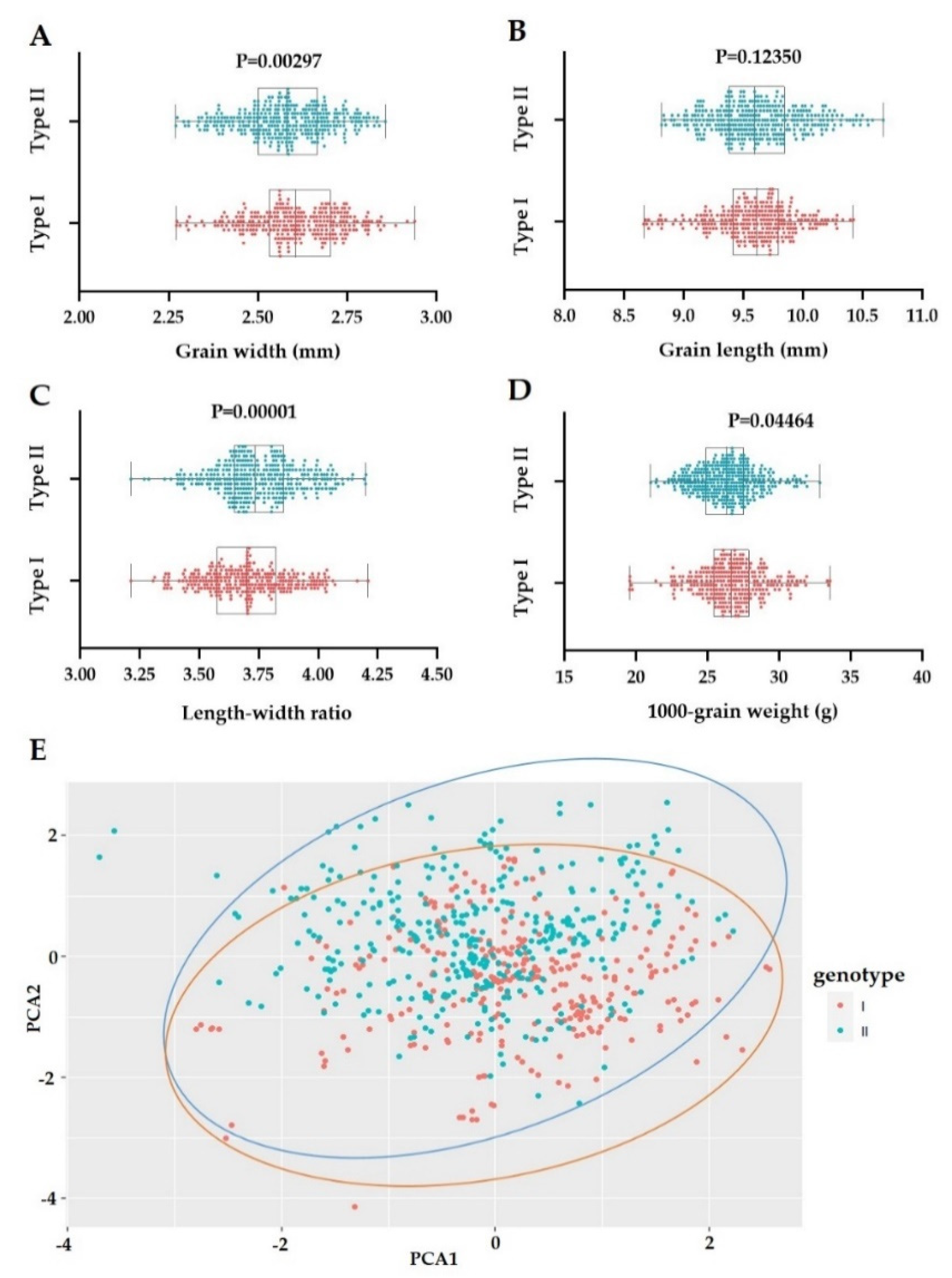
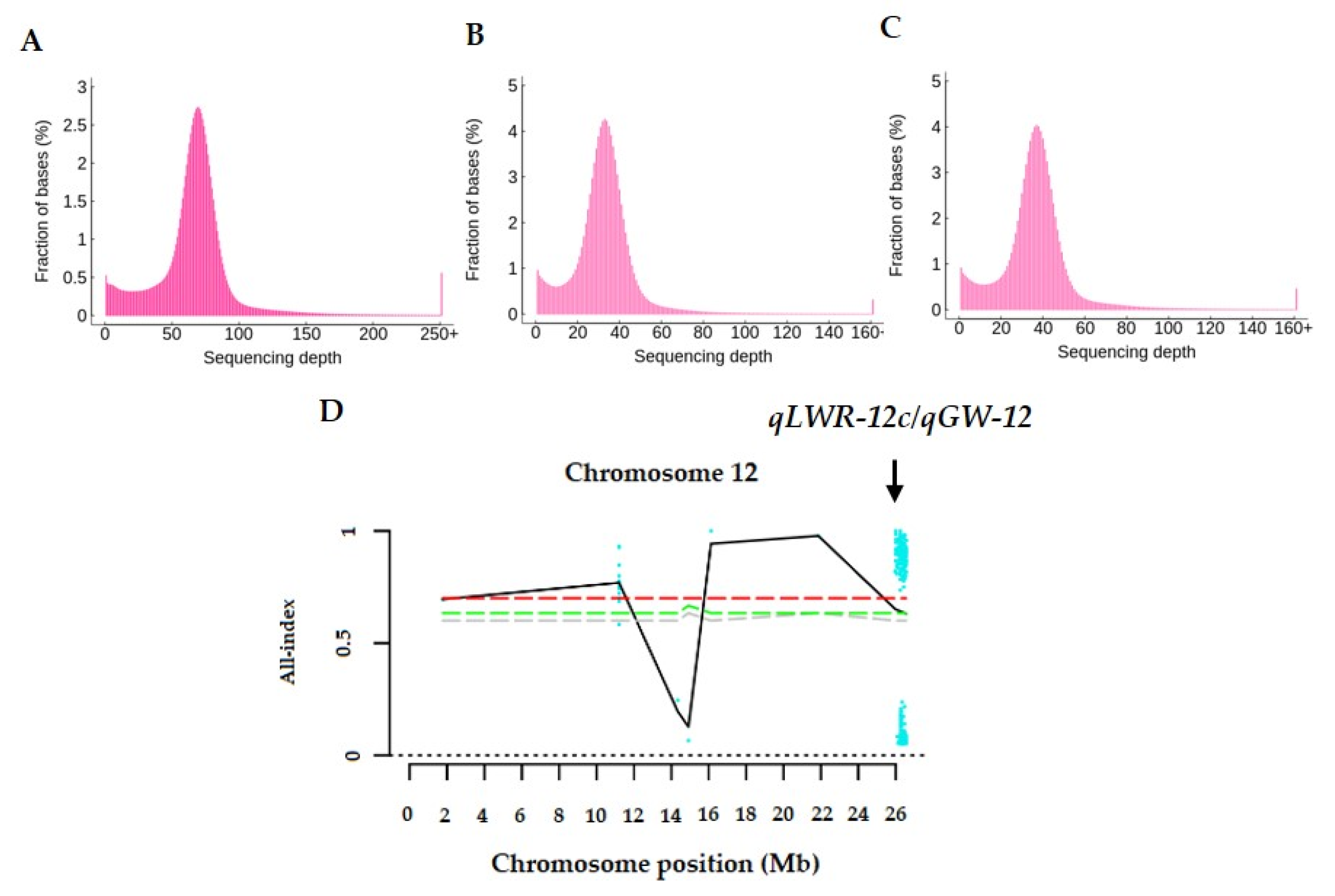
| QTLs | Chr. | Physical (Mb) a | Env. b | Peak Bin | LOD c | PVE(%) d | Add e |
|---|---|---|---|---|---|---|---|
| qGL-1b | 1 | 34.45–37.25 | 15LS | Bin336 | 2.66 | 2.5 | −0.04 |
| 16FY | Bin337 | 5.93 | 5 | −0.08 | |||
| qGL-2b | 2 | 4.95–6.55 | 15FY | Bin474 | 2.62 | 2.76 | −0.07 |
| 16LS | Bin472 | 4.44 | 4.13 | −0.84 | |||
| 16FY | Bin471 | 2.98 | 3.51 | −0.07 | |||
| qGL-2d | 2 | 26.05–28.85 | 16FY | Bin605 | 5.21 | 4.59 | 0.08 |
| 15FY | Bin606 | 2.73 | 2.56 | 0.06 | |||
| qGL-5 | 5 | 26.25–28.95 | 16FY | Bin1547 | 10.67 | 9.89 | −0.11 |
| 15LS | Bin1548 | 5.66 | 5.57 | −0.06 | |||
| 15FY | Bin1553 | 6.4 | 6.32 | −0.09 | |||
| 16LS | B1553 | 10.48 | 10.04 | −1.23 | |||
| qGL-7a | 7 | 1.55–2.05 | 15FY | Bin1904 | 2.64 | 2.55 | 0.06 |
| 15LS | Bin1904 | 3.22 | 3.21 | 0.05 | |||
| qGL-7b | 7 | 19.95–23.65 | 15LS | Bin2054 | 3.3 | 3.32 | 0.05 |
| 15FY | Bin2055 | 3.52 | 3.43 | 0.07 | |||
| 16LS | Bin2055 | 4.82 | 4.28 | 0.07 | |||
| 16FY | Bin2059 | 4.69 | 4.33 | 0.8 | |||
| qGL-8 | 8 | 22.70–22.05 | 15FY | Bin2318 | 4.09 | 3.98 | 0.08 |
| 16LS | Bin2318 | 3.36 | 3.01 | 0.68 | |||
| qGL-10b | 10 | 20.55–23.35 | 16FY | Bin2756 | 5.26 | 4.66 | 0.08 |
| 16LS | Bin2756 | 2.53 | 2.55 | 0.6 | |||
| qGL-11a | 11 | 0.15–1.85 | 15FY | Bin2791 | 2.7 | 2.54 | 0.06 |
| 16FY | Bin2806 | 3.07 | 2.6 | 0.06 | |||
| qGW-1a | 1 | 0.05–4.25 | 15FY | Bin7 | 4.67 | 4.2 | 0.03 |
| 16FY | Bin12 | 4.91 | 4.74 | 0.03 | |||
| 15LS | Bin20 | 2.99 | 2.98 | 0.02 | |||
| 16LS | Bin26 | 5.54 | 4.71 | 0.29 | |||
| qGW-1c | 1 | 34.25–40.65 | 15FY | Bin337 | 3.3 | 2.83 | −0.02 |
| 16FY | Bin337 | 2.74 | 2.53 | −0.02 | |||
| qGW-2b | 2 | 32.25–36.25 | 16FY | Bin663 | 4.3 | 4.15 | −0.02 |
| 15FY | Bin664 | 4.74 | 4.26 | −0.03 | |||
| 15LS | Bin666 | 4.58 | 4.6 | −0.02 | |||
| 16LS | Bin666 | 7.59 | 6.58 | −0.33 | |||
| qGW-7 | 7 | 18.85–20.25 | 16LS | Bin2030 | 3.97 | 3.26 | 0.24 |
| 16FY | Bin2033 | 3.81 | 3.65 | 0.02 | |||
| qGW-12 | 12 | 23.55–25.85 | 15FY | Bin3254 | 4.57 | 4.08 | 0.003 |
| 16LS | Bin3254 | 13.52 | 11.93 | 0.045 | |||
| 16FY | Bin3256 | 3.82 | 3.68 | 0.002 | |||
| 15LS | Bin3270 | 3.7 | 3.69 | 0.002 | |||
| qLWR-1a | 1 | 0.05–1.05 | 16LS | Bin3 | 5.11 | 4.61 | −0.043 |
| 16FY | Bin4 | 2.65 | 2.38 | −0.029 | |||
| 15FY | Bin8 | 2.96 | 2.95 | −0.034 | |||
| qLWR-1b | 1 | 1.45–4.25 | 15LS | Bin28 | 5.51 | 5.45 | −0.033 |
| 16FY | Bin31 | 6.28 | 6.46 | −0.033 | |||
| qLWR-2b | 2 | 1.35–3.15 | 16LS | Bin443 | 5.67 | 5.14 | −0.044 |
| 16FY | Bin441 | 2.65 | 2.38 | −0.026 | |||
| qLWR-2c | 2 | 31.05–34.75 | 15FY | Bin652 | 3.25 | 3.06 | 0.033 |
| 16FY | Bin52 | 5.49 | 5.19 | 0.038 | |||
| 16LS | Bin665 | 6.13 | 5.56 | 0.044 | |||
| qLWR-9a | 9 | 7.75–18.35 | 16FY | Bin2521 | 3.8 | 3.54 | −0.033 |
| 16LS | Bin2520 | 3.45 | 3.07 | 0.034 | |||
| qLWR-12c | 12 | 23.15–26.55 | 16LS | Bin3250 | 8.88 | 8.21 | −0.054 |
| 15LS | Bin3251 | 5.4 | 5.37 | −0.033 | |||
| 15FY | Bin3252 | 4.34 | 4.13 | −0.038 | |||
| 16FY | Bin3253 | 5.33 | 5.04 | −0.038 |
| Sample | Pool-High | Q42 | Q43 |
|---|---|---|---|
| Clean reads | 234,038,246 | 107,415,386 | 122,679,378 |
| Clean bases (bp) | 35,105,736,900 | 16,112,307,900 | 18,401,906,700 |
| Mapped reads | 232,122,373 | 106,376,621 | 121,604,676 |
| Mapped bases (bp) | 34,818,355,950 | 15,956,493,150 | 18,240,701,400 |
| Mapping rate | 99.18% | 99.03% | 99.12% |
| Duplicate reads | 44,313,210 | 19,409,663 | 23,628,250 |
| Duplicate rate | 19.09% | 18.25% | 19.43% |
| Mismatch bases (bp) | 242,427,045 | 128,415,115 | 132,032,810 |
| Mismatch rate | 0.70% | 0.80% | 0.72% |
| Average sequencing depth | 70.2 | 32.76 | 36.95 |
| Coverage | 97.24% | 96.07% | 95.91% |
| Coverage at least 4X | 95.89% | 93.51% | 93.46% |
| Coverage at least 10X | 93.57% | 89.62% | 89.75% |
| Coverage at least 20X | 90.31% | 82.45% | 83.98% |
| Coverage at least 50X | 78.44% | 6.35% | 10.69% |
| R498 ID | Genebank | Type | Ref. | Q42 | Q43 | Function |
|---|---|---|---|---|---|---|
| OsR498G1221877800 | LOC4352512 | upstream | G | G | A | RNA pseudouridine synthase 1-like |
| OsR498G1221877900 | ABA98931 | upstream | - | - | - | expressed protein |
| OsR498G1222155700 | LOC4352862 | 3′UTR | T | T | C | ras-related protein RABF2a |
| OsR498G1222156200 | ABA99931 | upstream | C/T | C/T | A/C | zinc finger family protein, putative, expressed |
| 5′UTR | A | A | G | |||
| OsR498G1222164100 | ABA99947 | 5′UTR | A | A | G | expressed protein |
| OsR498G1222165500 | ABA99953 | upstream | T/TAA | T/TAA | C/T | F-box domain containing protein |
| OsR498G1222170400 | LOC4352887 | upstream | A | T | A/ | elongation of fatty acids protein 3-like |
| NM | A | G | A | |||
| OsR498G1222170500 | ABA99966 | upstream | T/T/C/T/T/A/A/T/T | C/C/T/C/C/G/G/C/C | T/T/C/T/T/A/A/T/T | retrotransposon protein, putative, LINE subclass |
| NM | C/T | C/C | T/T | |||
| FS | A | ATCGTTTCT | A | |||
| OsR498G1222171700 | LOC4352888 | upstream | C/C | C/C | T/T | E3 ubiquitin-protein ligase RNF5 |
| 5′UTR | C/C | C/C | A/G | |||
| OsR498G1222171900 | LOC4352889 | NM | T | T | C | ankyrin-2 |
| OsR498G1222173600 | LOC9267278 | downstream | - | - | - | expressed protein |
| OsR498G1222173400 | LOC4352891 | upstream | G | G | A | epoxide hydrolase A |
| NM | C | C | T | |||
| OsR498G1222177200 | LOC4352903 | upstream | C | T | C | expressed protein |
| OsR498G1222178200 | LOC4352905 | 5′UTR | T | C | T | protein NRT1/ PTR FAMILY 2.11 |
| OsR498G1222185100 | - | NM | T | T | C | |
| OsR498G1222185500 | LOC4352920 | upstream | G | G | A | vesicle-associated membrane protein 721 |
| OsR498G1222185300 | LOC4352919 | downstream | - | - | - | beta-glucuronosyltransferase GlcAT14A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, Y.; Zhang, M.; Zhang, Y.; Wu, W.; Xue, P.; Zhan, X.; Cao, L.; Cheng, S.; Zhang, Y. Genetic Mapping of Grain Shape Associated QTL Utilizing Recombinant Inbred Sister Lines in High Yielding Rice (Oryza sativa L.). Agronomy 2021, 11, 705. https://doi.org/10.3390/agronomy11040705
Kang Y, Zhang M, Zhang Y, Wu W, Xue P, Zhan X, Cao L, Cheng S, Zhang Y. Genetic Mapping of Grain Shape Associated QTL Utilizing Recombinant Inbred Sister Lines in High Yielding Rice (Oryza sativa L.). Agronomy. 2021; 11(4):705. https://doi.org/10.3390/agronomy11040705
Chicago/Turabian StyleKang, Yiwei, Miao Zhang, Yue Zhang, Weixun Wu, Pao Xue, Xiaodeng Zhan, Liyong Cao, Shihua Cheng, and Yingxin Zhang. 2021. "Genetic Mapping of Grain Shape Associated QTL Utilizing Recombinant Inbred Sister Lines in High Yielding Rice (Oryza sativa L.)" Agronomy 11, no. 4: 705. https://doi.org/10.3390/agronomy11040705





