Abstract
Hydrogen peroxide (H2O2) is a broad-range chemical catalyst that is receiving rapidly increasing attention recently due to its role as a signaling molecule in various plant physiological and biochemical processes. A study was carried out to investigate the effects of H2O2 on the plant physiology, root growth, mineral nutrient accumulation, root anatomy, and nematode control of Ficus deltoidea, a slow growing shade tolerant and nematode susceptible medicinal plant. H2O2 at 0 (control), 15, 30, 60, and 90 mM was injected into the root zone of plants weekly. The results showed that the treatment of H2O2 enhanced the accumulation of pigments, photosynthetic characteristics, and quantum yield (Fv/Fm) of F. deltoidea. H2O2 at a 90 mM treatment significantly increased seedling height, leaf number, syconium number, biomass yield, relative water content, leaf dry matter, leaf moisture, and live line fuel moisture of the plant by 1.35-, 3.02-, 3.60-, 5.13-, 1.21-, 1.12-, 1.79- and 1.06-fold, respectively, over the control plant. In addition, root growth, which includes root crown diameter, root length, root volume, root tips, number of roots and root biomass, also exhibited the highest values with an application of 90 mM of H2O2. Heavy metals arsenic (As+) and antimony (Sb+) content in the leaves decreased by 4.08-and 1.63-fold, respectively, in the 60 mM H2O2 treated plant when compared to the control plant. In addition, 90 mM H2O2 was the best treatment for magnesium (Mg2+), calcium (Ca2+), and sodium (Na+) mineral accumulation in the syconium of F. deltoidea. Treatments with 60 mM H2O2 increased magnesium (Mg2+), calcium (Ca2+), and potassium (K+) content in leaves by 14%, 19%, and 15%, respectively, over the control plant. In the study of controlling root-knot nematode, both control and 15 mM treatments produced many root galls, whereas, 60 mM H2O2 treatment produced fewer tiny root galls and 90 mM of H2O2 showed no root gall formation. H2O2 treatments reduced root gall size, root/shoot ratio, and increased the shoot biomass of plants. The treated root developed an epidermal suberin, root periderm, resin duct, cortex, druses, and a well-developed vascular system compared to the control plants. Furthermore, no nematodes were observed in the roots of treated plants with 30–90 mM H2O2. The study concluded that injections of 60–90 mM H2O2 to the root zone weekly improved plant physiology, increased mineral accumulation, root growth and development, reduced root gall formation, improved root cellular structure, and controlled root-knot nematode of F. deltoidea plants.
1. Introduction
Nowadays, people tend to use herbal products rather than synthetic remedies due to their safety when consumed by humans. One herb that has gained recent attention is Ficus deltoidea (Moraceae). F. deltoidea, commonly known as the mistletoe fig, is a species of shrub or small tree native to Southeast Asia and is widely distributed throughout Malaysia, the Moluccas, Java, Kalimantan, Sumatra, Sulawesi, and Thailand [1]. It has been reported that the whole plant part of F. deltoidea has been traditionally used as a remedy for healing, and is said to hold palliative and preventative medicine properties [2]. Besides, it was also reported that F. deltoidea has been used for regulating blood pressure [3], increasing and recovering sexual desire, for womb contraction after delivery [4], reducing cholesterol levels, reducing blood sugar levels [5], in the treatment of migraines, as an aid in toxin removal, to delay menopause, treat nausea, joint pains, piles pain, and to improve blood circulation [4]. To date, F. deltoidea can be found in the form of leaf and bark extracts, coffee drinks, herbal drinks, massage oil, and capsules, which are widely available in the market. According to Adam et al. [6], the root, bark, leaves, and syconium (fruit) of F. deltoidea have medicinal properties.
Ficus deltoidea is a slow-growing plant with growth of 0.3 to 3.0 m in typical commercial use, displaying a low photosynthetic rate during vegetative and reproductive growth [7]. In addition, the growth and development of the plant can also be affected by nematode infestation. Thus, hydrogen peroxide (H2O2), a growth promoting chemical, was used in this study to increase the photosynthetic and other physiological activities of F. deltoidea to promote both plant and root growth and development. H2O2 can be used as a signal molecule involved in adaptive signaling, triggering tolerance contrary to various environmental stresses [8]. According to Kolla [9], H2O2 is the most steady reactive oxygen species (ROS) since the production of H2O2 occurs through various paths in plant cells (NADPH oxidase, lipid peroxidation, and photosynthetic electron transport chain) and can diffuse rapidly across the cell membrane [10]. Recent research suggests exogenous H2O2 has a stimulatory effect on plant physiology, fruit growth, and fruit ripening of the Syzygium samarangense [11]. During photosynthesis and photorespiration, H2O2 is produced naturally in plant cells and plays a role as a growth regulator and terminator in various types of plants. H2O2 plays a dual role in plants at one to five mmol/g fresh weight (low and normal concentrations) and acts as a messenger molecule in adaptive signaling and triggering tolerance against stresses, and at above seven mmol/g fresh weight, orchestrates cell death [12]. Nurnaeimah et al. [13] stated that exogenous H2O2, as a foliar spray, improves plant growth and development and stimulates the bioactive compounds of F. deltoidea seedlings. It has also been reported that 60 mM H2O2 as a foliar spray reduced net photosynthetic rate, stomatal conductance, seedling growth, phenols, and flavonoid content of mas cotek plants [13]. H2O2 treatment as a spray, on the young fruits of Kyoho berries, promotes ripening by regulating the expression levels of several genes and photosynthetic pathways [14]. It has been reported that exogenous H2O2 increased the formation and growth of adventitious roots of seedling explants in mungbean [15]. The use of H2O2 for the environmental control of pathogens has also received much attention.
Root-knot nematode is one of the most harmful groups of plant-parasitic nematodes that infect almost all major crops [16]. Root-knot nematodes (RKNs, Meloidogyne spp.) attack a wide range of crop species, and about 5% of the world crop production is destroyed by Meloidogyne species yearly [17,18,19]. The root-knot nematode can retard plant growth and can expose the plant to other root diseases and eventually kill herb plants like F. deltoidea [20]. An increased intensity of root-knot nematode infection leads to a significant reduction of plant growth and biomass [21]. Gustin et al. [22] reported that H2O2 produced by some bacteria could kill nematodes due to its toxicity. Studies by Gustin et al. [22] and Jansen et al. [23] demonstrated that H2O2 has the potential to become an agent for the environmental control of pathogens. F. deltoidea plants are susceptible to root-knot nematode infection. In Beach Ridges Interspersed with Swales (BRIS) soil, nematode infestation affects the plant growth; thus, the plants will wilt and then die. Little is known about how H2O2 alleviates the root-knot nematode attack on herbs like F. deltoidea.
Currently, no research has been reported about the regulatory effect of exogenous H2O2 treatment in the rhizopheric soil on the photosynthesis, plant growth, root development, mineral accumulation, root anatomical structure, and root-knot nematode control of F. deltoidea. Two main objectives of this study were to determine the effects of H2O2 on the plant physiology, root architecture, and mineral absorption as well as to promote root growth, cell proliferation, and nematode control in nematode infested problematic soil. In this project, first, we investigated the regulatory role of H2O2 on plant growth, photosynthesis capacity, mineral absorption and accumulation as well as biomass yield of F. deltoidea. Second, we examined the effects of H2O2 on root growth, root-knot development, root cellular structure and control of root-knot nematode under nematode infested soil of the F. deltoidea. The study proposes that injection of H2O2 in the rhizopheric soil can improve the photosynthesis, biomass and mineral accumulation, leaf hydrological properties, architecture and cellular structure of roots, reduce root gall formation, and induce resistance against root-knot nematode of F. deltoidea. The novel findings from this study will be beneficial in improving the photosynthetic capacity, plant growth and development processes, and controlling root-knot nematode of the F. deltoidea, thus making it a more commercially viable plant.
2. Materials and Methods
2.1. Experimental Site, Plant Materials and Treatment Setting
The experiments were conducted at a research plot at a farm at the Universiti Sultan Zainal Abidin (UniSZA), Besut Campus, Besut, Terengganu, Malaysia, between the months of February 2015 and December 2016. The mother plant of the Ficus deltoidea var. deltoidea was collected from Sungai Nibong, Batu Pahat, Johor. All cuttings were propagated from the collected mother plant with the accession number of FD301. Thirty five uniform F. deltoidea plants were used in the experiments. A completely randomized design (CRD), with seven replications, was used for the treatment application. All experiments consisted of five treatments including the control (0, 15, 30, 60, and 90 mM H2O2) in seven replicates with a single seedling taken as an experimental unit. Stem cuttings of F. deltoidea were transplanted into polybags containing growing media cocopeat, paddy husk, and perlite at the ratio 4:2:1, and each polybag was filled with 4.5 kg growing media. Two weeks before treatment application, 15 g of nutrients (N:P:K, ratio at 5:5:5) was applied per seedling. Plants were watered daily 15 min in the morning around 9 am through the sprinkled method. This experiment was conducted under a sunlight proof shade house: temperature 21–37 °C, maximum PAR 500–1000 µEm−2 s−1, and relative humidity 50–90%. In the treatment procedure, 10 mL H2O2 was injected into three different points of the growing media of the seedlings root zone where intense biological and chemical activity is influenced by compounds exuded by the root. H2O2 was applied immediately after irrigation weekly from the vegetative to the reproductive stage of the plants during the experimental period. A needle injection syringe was used to inject the H2O2 solution into the rhizopheric growing media of the seedlings. H2O2 was injected weekly into the rhizopheric growing media a total of 15 times before being harvested four months after transplanting. The H2O2 treatment selection followed that described in a previous study conducted by Ozaki et al. [23]. However, in their study, they applied (0–50 mM) H2O2 to melon seedlings as a soil treatment at the rate 300 mL per day for three weeks. Treated plants were harvested four months after transplanting for the plant biomass, root growth, and other yield data analysis.
2.2. Measurement of Growth, Photosynthesis, Chlorophyll Content, Chlorophyll Fluorescence and Photosynthetic Yield
Growth parameters including seedling height, leaf number, and syconium number were measured manually for each of the four weeks of treatment application. Photosynthetic characteristics for both the control and treated plants were measured by using a portable CI-340 handled photosynthesis system (Bioscience, Camas, WA, USA) and the measurements were carried out according to the method described by Khandaker et al. [11]. The leaf chlorophyll content of the F. deltoidea was determined using a chlorophyll content meter (CCM). A PEA (plant efficiency analyzer) (Hansatech Instruments Ltd., King’s Lynn, UK) was used to measure chlorophyll fluorescence and the photosynthetic yield (Fv/Fm) of the treated and untreated plants.
2.3. Determination of Chlorophyll A, B and Carotenoid Content
For the determination of pigment content, 0.25 g leaf samples were homogenized in 10 mL of 80% acetone in a mortar and pestle. The homogenate leaf sample was centrifuged at 2500 rpm for ten minutes and filtered with Whatman No. 1 filter paper. The absorbance of the extracted filtrate was measured at 480 nm, 645 nm, and 663 nm. At 480 nm, the wavelength of 80% acetone was used as the blank to zero the spectrophotometer. The level of chlorophyll a, chlorophyll b, and carotenoid content of the leaves were calculated using the following formulas [24]:
Chlorophyll a = 12.7 × Absorbance at 663 nm − 2.69 × Absorbance at 645 (mM concentration)
Chlorophyll b = 22.9 × Absorbance at 645 nm − 4.68 × Absorbance at 663 (mM concentration)
Carotenoid = (Absorbance at 480 + (0.114 × Absorbance at 663) − (0.638 × Absorbance at 645)) ÷ 112.5 (mM concentration)
2.4. Measurements of Leaf Water Content, Leaf Dry Matter, Leaf Moisture, Live Line Fuel Moisture and Biomass Content
Three leaf physiological traits were assessed: relative water content of leaf (RWC), leaf dry matter content (LDMC), and leaf moisture (LM). The method used followed Saura-Mas and Lloret [25]. In addition, live fine fuel moisture (LFFM) content was also measured from shoots <6 mm in diameter of the experimental plants. Leaf and shoot samples from the untreated and treated plants were collected around 12.00 pm to 2.00 pm on a sunny day during the experimental period. Relative water content (RWC) of the leaf was determined according to the method described by Munné-Bosch and Peñuelas [26]. Leaves were brought to the lab directly after collection from the experimental field. Sampled leaves were stored in ice box conditions. To saturate the leaves with water, a plastic jar filled with water was kept in the icebox (for each treatment level a previously weighed plastic jar filled with water was used) and was stored for six to nine hours. The leaf fresh weight was recorded after saturation with water. Then, the plastic jars were closed and were kept in ice-box conditions to avoid the loss of water. Meanwhile, the leaves were weighed outside the jar to obtain their saturated weight. Finally, all of the sample leaves were oven dried for 48 h at 70 °C and weighed with an electronic balance. LDMC and LM content of the sample leaves were determined following the same procedure as RWC.
LFFM was determined following the procedures outline by Viegas et al. [27]; shoots (<6 mm diameter) from five different plant treatments were collected and closed in sealed plastic bags and stored in refrigerated conditions. After weighing the sample, shoots were oven-dried for 48 h at 70 °C and weighed again (fresh weight and dry weight, with a precision of 0.01 g). The formulas for measuring LRWC (leaf relative water content), LDMC (Leaf dry matter content), LM (leaf moisture) and LLFM (live line fuel moisture) are presented below:
The RWC (%) was calculated as W = 100 × [(Mf − Md)/(Mt − Md)], where, Mf = fresh mass, Mt = turgid mass after rehydrating of the leaves and Md = dry mass of oven dried leaves. The RWC of a leaf takes into account the leaf turgid mass, and it is the proportion of the leaf water content related to the maximum water content, that can potentially be achieved by the leaf.
LDMC = Md/Mt (mg g−1). LDMC is the proportion of the leaf dry matter content without water, related to the mass of the leaf with the maximum water content.
Leaf moisture (L) (%) and live fine fuel moisture (F) (%) of leaves and shoots, respectively, were calculated as L or F = 100 × [(Mf − Md)/Md]. The shoot and root biomass were measured using an electronic balance (Model: Mettle PJ3000, Tokyo, Japan) at the end of the experiment.
2.5. Assessment of Root Architecture and Profiles
After harvesting the experimental plants, root samples were cleaned and washed manually with distilled water to remove the soil particles. The root growth pattern of control and treated Ficus deltoidea plants was determined by examining the branching pattern or architecture as in the method described by Yen [28]. A WinRHIZO (Version 2008a, Reagent Instruments Inc., QC, Canada) system was used, connected to an Epson XL 10,000 professional scanner, which was equipped with an additional light unit (TPU). A 400 (dpi) resolution was used for measuring root morphology. The root architecture and profile analyses were performed immediately after images were acquired and saved in TIFF format so that the images could later be accessed from an Excel spreadsheet with integrated XL-Rhizo system. The total root length (cm), root volume (cm3), mean root diameter (mm), and root length per diameter class (cm) were determined.
2.6. Nutrient Analysis
The mineral accumulation in leaves and syconium analysis was carried out at the Malaysian Nuclear Agency (ANM), Bangi, Selangor. In this study, seven mineral nutrients were analyzed including, arsenic (As+), antimony (Sb+), calcium (Ca2+), iron (Fe2+), magnesium (Mg+), potassium (K+), and sodium (Na+). The method used to analyze the mineral uptake and accumulation was neutron activation analysis (NAA), following the method of Nashriyah et al. [29].
2.7. H2O2 on Root Anatomy, Root-Knot Formation and Controlling Root-Knot Nematode of F. deltoidea
Another study was conducted to investigate the regulatory role of H2O2 on the root gall development, plant biomass production, cellular structure of roots, and control of root-knot nematode under a root-knot nematode (Meloidogyne incognita) containing growing medium.
2.7.1. Nematode Inoculums
Root-knot nematode inoculum was obtained from one and a half year old F. deltoidea plants grown in BRIS soil and the identified species was M. incognita. This study adopted the preparation step for nematode extraction, as suggested by Tahery et al. [30]. Root-knot nematode (M. incognita) eggs for inoculums were extracted from infected F. deltoidea roots by agitating in 0.05% NaOCl for 2 to 3 min in a 1 L Schott bottle. The eggs were collected and rinsed with tap water on nested No. 200 mesh (75 µm) and No. 500 mesh (25 µm) sieves. The roots and soil particles were held in the No. 200 sieve while the egg masses were collected in the No. 500 sieve. Eggs were put into a big beaker containing distilled water for the next procedure. Hatching of nematode eggs was performed following the procedure proposed by Atamian et al. [31]. A clean metal basket with a few layers of Kimwipe paper was fitted on a glass Petri dish with a 1-cm distance between the bottom of the basket and the dish. Then, the extracted nematode eggs were poured onto the paper in the wire basket. Enough liquid was added so that the bottom of the wire basket touched the water surface, but was not immersed in the water. The wire basket top was covered with a plastic lid. The water level was checked daily to prevent the eggs from drying.
2.7.2. Soil Sterilizing and Treatment Settings
Soil consisting of 95% sand (BRIS soil) was prepared and bulked on a plastic bed. For sterilizing, the soil was kept moist and covered with plastic. BASAMID granules were mixed thoroughly at recommended rates (150 to 220 g/m3) and the soil was blended to obtain uniformity. The soil was then sealed with plastic sheets and aerated for five to seven days after treatment. After aeration, the soil was left for two weeks to release BASAMID gases from the soil before planting. This experiment adopted Tahery et al.’s [30] method for soil sterilization.
After one week of seedling establishment, water that contained J2 stage from the Petri dishes was collected in the beaker and the air was supplied using a small aquarium pump. Then, the collected J2 stage was counted and 50 mL of suspension containing 3000 J2 stage per polybag was transferred and inoculated into the sterilized polybag growing media. After nematode inoculation, H2O2 treatments at the rate of 0, 15, 30, 60, and 90 mM were applied weekly. A 5 mL of H2O2 solution was applied per polybag at three different points, done through an injection method, to the root zone BRIS soil of F. deltoidea seedlings. A CRD with thirty uniform F. deltoidea seedlings was used in the experiment. The study consisted of five treatments, the same as the experimental one including the control (0 mM H2O2), in six replicates with a single seedling taken as an experimental unit. After three months, all the seedlings were harvested and root and root biomass were measured. The number of root galls and size of root galls were also recorded. Infected (root gall) and non-infected middle parts of lateral roots were collected and prepared for scanning by electron microscopy analysis.
2.8. Electron Microscopy Scanning of Infected and Non-Infected Roots
Root segments containing gall nematodes (0–60 mM H2O2) and middle segments without root gall (90 mM H2O2) of secondary roots were cultured and placed in vials containing a 0.1 M sodium cacodylate buffer with 2.5% glutaraldehyde, at pH 7, for 3 h at 30 °C. After that, samples were rinsed in a ring three times for a 15 min duration each using a 0.1 M sodium cacodylate buffer maintaining pH 7.2. After the post chemical fixation, the infected and non-infected parts of the root were dehydrated in a graded series of ethanol, from 35% to 100%. Dehydrated samples were put into a critical point dryer system to dry the samples by replacing ethanol with the liquid carbon dioxide under pressure. All the root segments were mounted on stubs and coated with 15–30 nm of gold-palladium in a K550 sputter coater. The coated samples were viewed with an analytical scanning electron microscope (model JEOL JSM-6360 LA) following the standard procedures written by Rohini et al. [32] at the Institute of Oceanography (INOS), Universiti Malaysia Terengganu, Terengganu. In this study, the cellular damage due to the presence of nematodes and root structural improvement by exogenous H2O2 were observed for the different treatments under potted conditions.
2.9. Statistical Analysis
A CRD with seven replications was used for the treatments in all experiments. The root anatomical and nematode control experiment was a CRD with six replicates. The data were analyzed using SPSS-17 statistical software. A one way repeated ANOVA was used to evaluate significant differences within the parameters studied in all the experiments. Tukey’s (HSD) test was used to compare between the mean and rank them at p = 0.05.
3. Results
3.1. Vegetative and Reproductive Growth
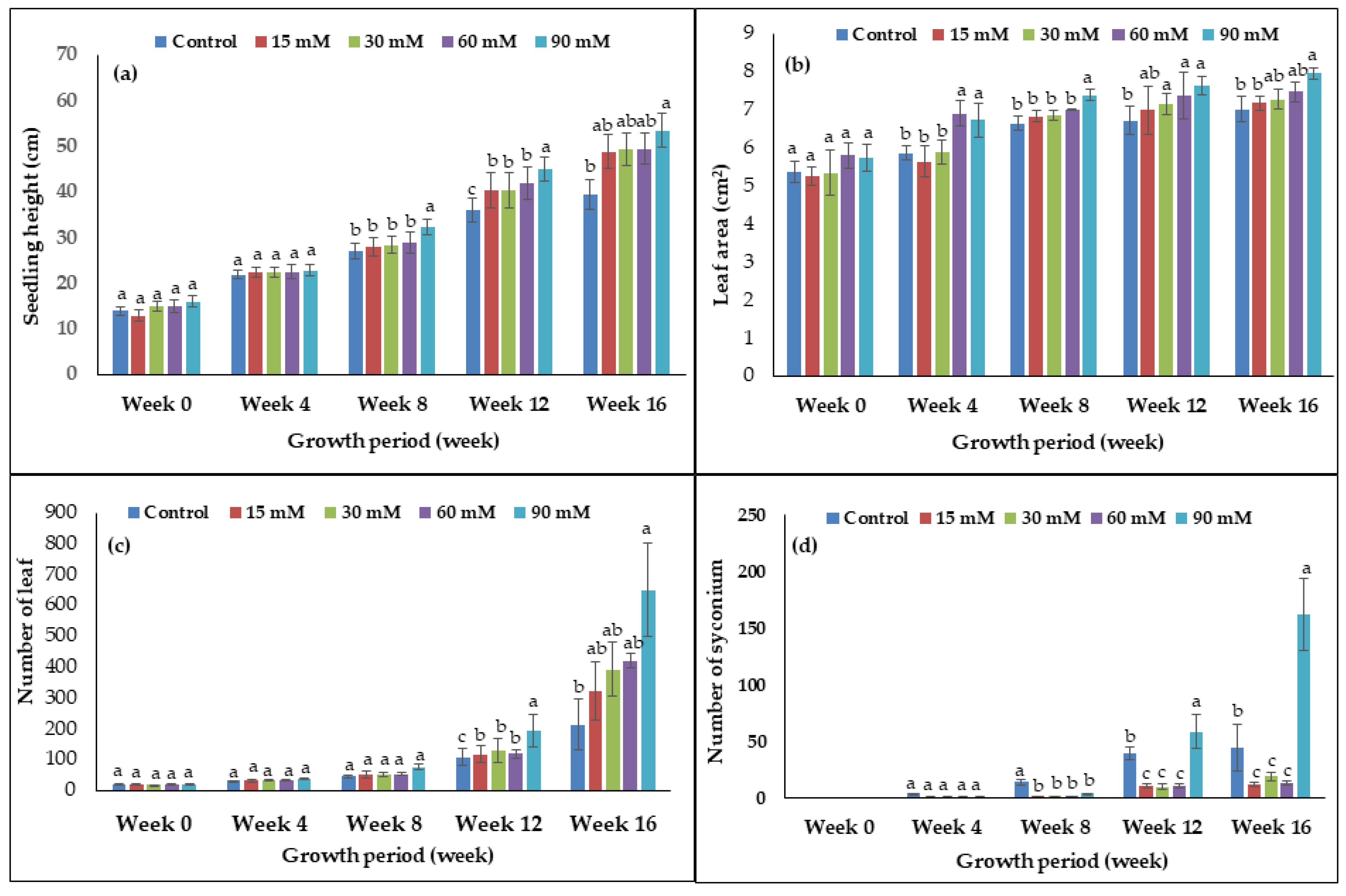
Several vegetative and reproductive parameters were recorded for evaluating the effects of hydrogen peroxide (H2O2) on the plant growth and development of F. deltoidea. Figure 1 represents the effects of H2O2 on seedling height, leaf area of mature leaves, leaf number, and number of syconium at the 16th week of observation using different concentrations of H2O2 as treatments. As seen in the bar graph (Figure 1), there was no significant difference in the seedling height, leaf number, and syconium number between treated and untreated plants from week 0 until week 4. However, starting from week 8 onward, there was a significant effect seen in the plant growth parameters. The results showed that 90 mM H2O2 was the best concentration for increasing the seedling height of the F. deltoidea followed by treatments of 60, 30, and 15 mM of H2O2 from week 8 until week 16. At week 16, the 90 mM treated plant had increased 26% more in seedling height compared to the untreated control seedling and it was statistically significant (Figure 1a). From week 12 until week 16, 30–90 mM treated plants displayed a significant increase in leaf area and number of leaves. The treatment with 90 mM H2O2 showed the highest leaf area of almost 8 cm2 as the average for week 16, which was statistically greater than the control (Figure 1b and Figure 2A). As seen in the bar graph (Figure 1c), there was no difference in the leaf number during week 0 until week 8. However, starting from week 12 onward, there was an increase in the leaf number of all treated plants. Treatment with 90 mM H2O2 produced the highest number of leaves at week 16 with almost 650 leaves per plant, which was three times greater than the control plant (Figure 1c and Figure 2B) and the difference was statistically significant. There was no difference in the syconium number during week 0 until week 4 (Figure 1d). For the number of syconium at week 16, treatment with 90 mM H2O2 yielded 260% more syconium when compared to the control plant, which was statistically significant (Figure 1d).
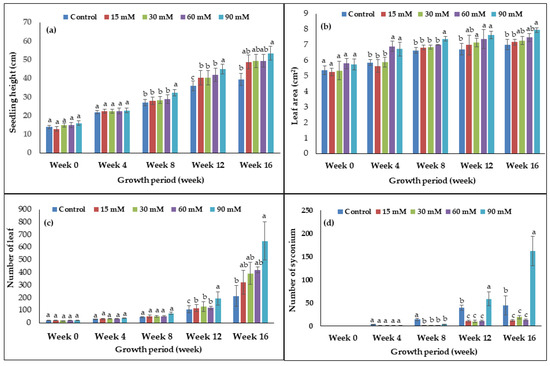
Figure 1.
The effects of H2O2 on (a) seedling height, (b) leaf area of mature leaves, (c) number of leaf, and (d) number of syconium of F. deltoidea plants. Bars represent means (n = 7) ± standard error (S.E.). a, b, and c indicate significant differences within each growth period (p < 0.05, Tukey’s Honestly Significant Difference (HSD)-test)).
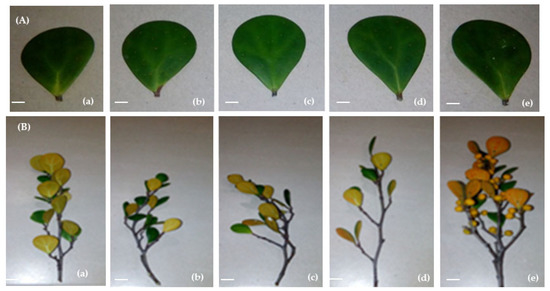
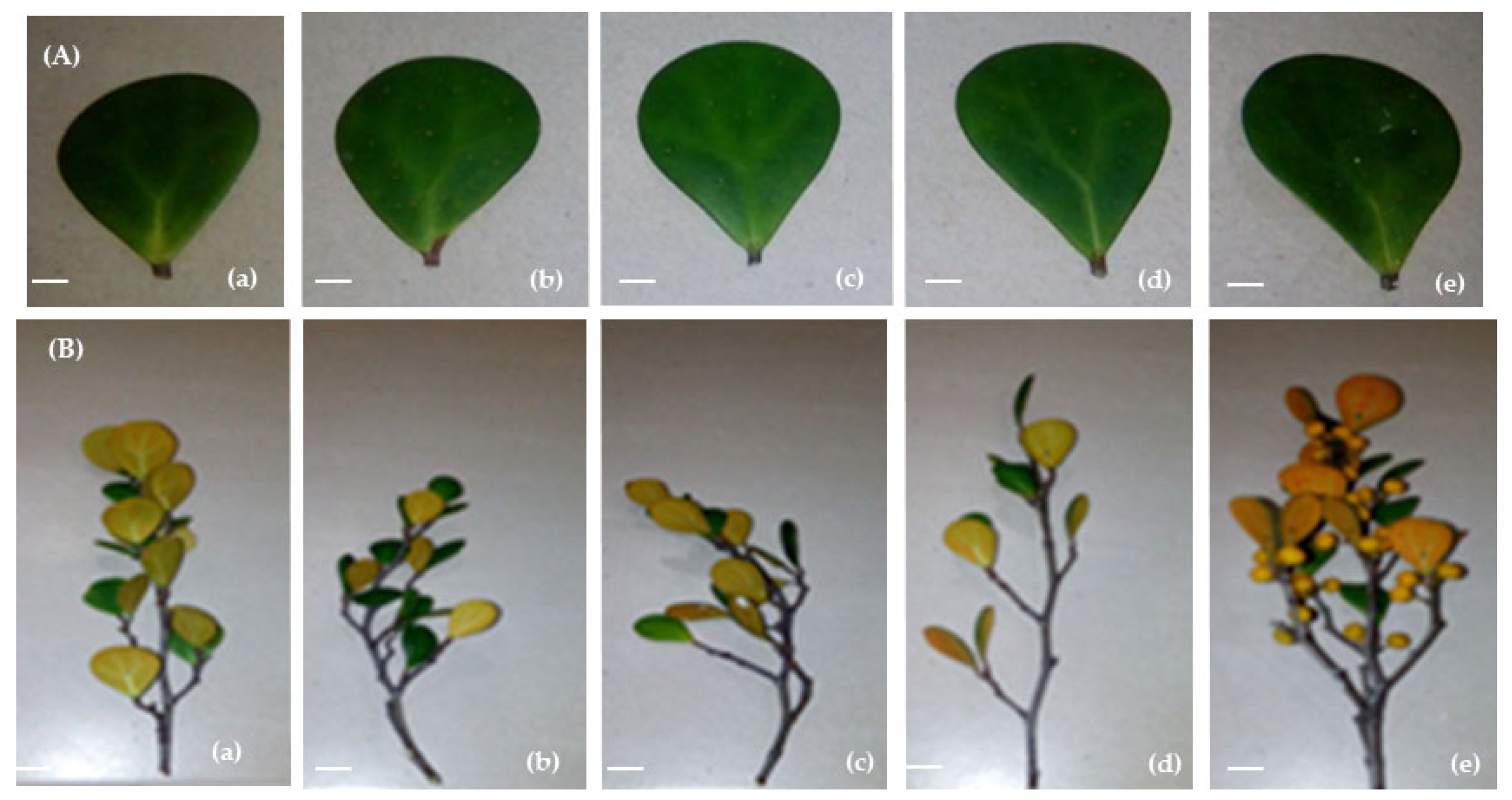
Figure 2.
Photographs showing the effect of H2O2 on the (A) leaf area of mature leaves and (B) number of mature leaves of F. deltoidea (a) 0 mM H2O2 (control), (b) 15 mM H2O2, (c) 30 mM H2O2, (d) 60 mM H2O2, and (e) 90 mM H2O2. Larger leaf area and the highest number of leaves were visible in the treated plants compared to the control. Scale bars = 1.2 cm (A) and 3.0 cm (B).
3.2. Leaf Chlorophyll Content, Chlorophyll Fluorescence and Carotenoid Content
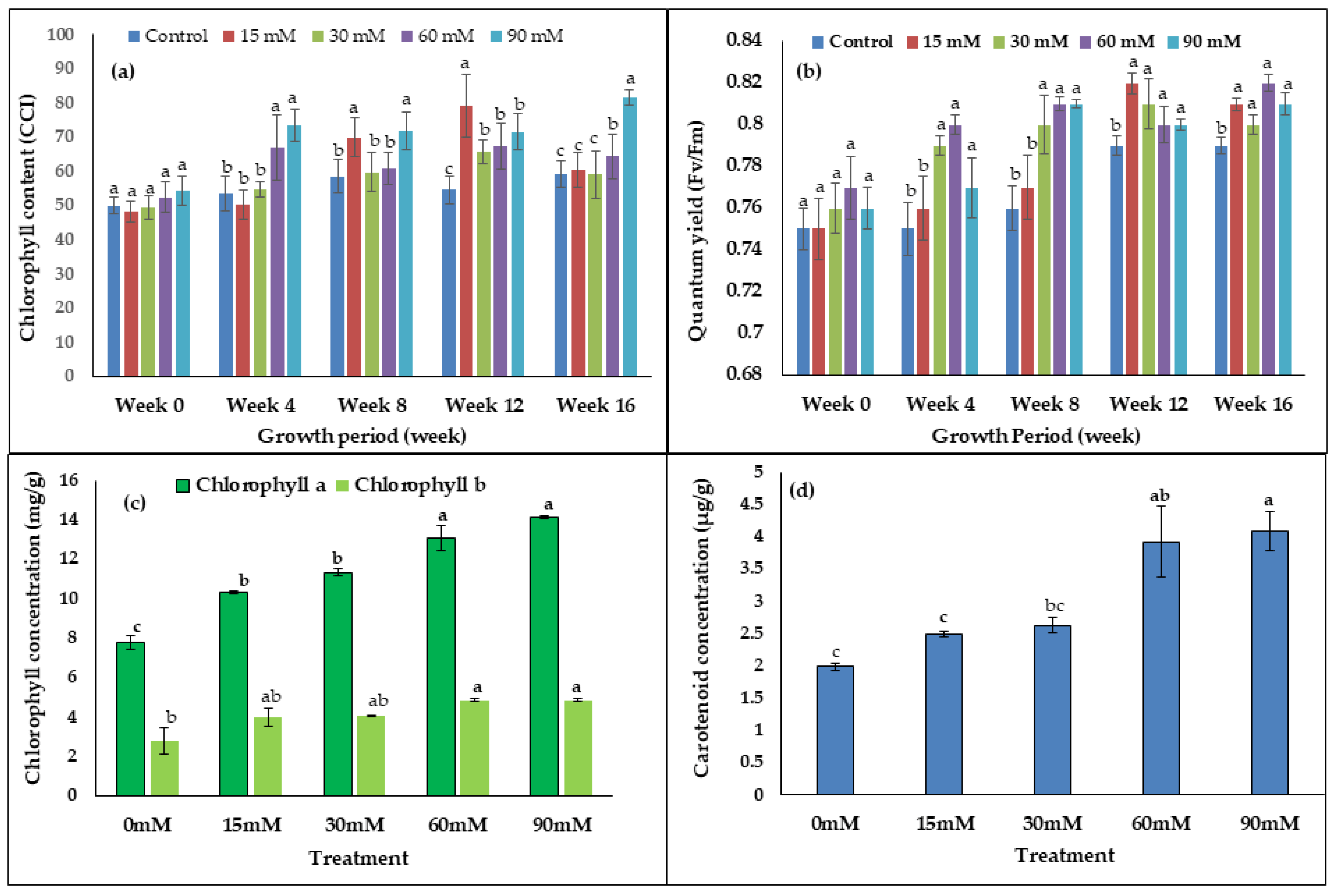
As seen in the bar chart (Figure 3a,b), there were no differences in the chlorophyll content (CCI) and quantum yield during week 0. However, the chlorophyll content (CCI) of the 60–90 mM treated leaves was significantly higher than the control from week 12 until week 16 (Figure 3a). At week eight, chlorophyll content was 29% and 33% higher in the 15 mM and 90 mM treated plants, respectively, compared to the control. The quantum yield (Fv/Fm) of 30–90 mM treated plants was significantly higher than the control from week 4 until week 16. In Figure 3b, it was apparent that treatment with 60 mM H2O2 showed the highest value of quantum yield (Fv/Fm) compared to the control plant with the following percentage change increases; week 4 (7%), week 8 (7%), week 12 (3%), and week 16 (4%). The leaf chlorophyll (a, b) and carotenoid contents were significantly increased by the H2O2 treatments used in this study (Figure 3c,d). Chlorophyll a showed the highest value compared to chlorophyll b in all treatments. The highest amount of chlorophyll a (14.12 mg/g) was recorded in the 90 mM treated plants, while the lowest amount (7.9 mg/g) was recorded in the control plants (Figure 3c). Furthermore, chlorophyll b content was 75% higher in the 60 mM treated plants compared to the control. In addition, total carotenoid content in the 90 mM H2O2 treated plants was 2.05-fold higher compared to the control plants (Figure 3d).
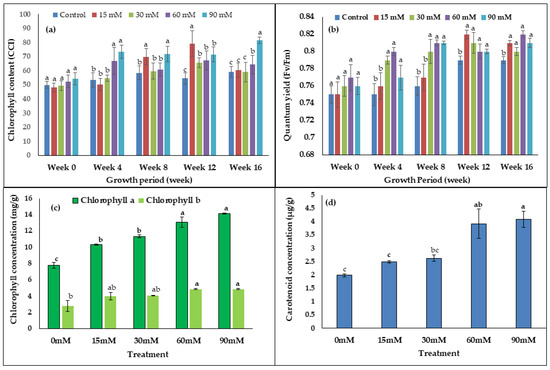
Figure 3.
The effects of H2O2 on (a) chlorophyll content, (b) chlorophyll florescence, (c) concentration of chlorophyll a and chlorophyll (b) and (d) total carotenoid content. Bars represent means (n = 7) ± standard error (S.E.). a, b, and c indicate significant differences within each growth period (p < 0.05, Tukey’s HSD-test).
3.3. Photosynthetic Characteristics
H2O2 treatments increased the leaf net photosynthetic activity considerably of the plants, which was statistically significant. Leaf net photosynthetic activity was the highest with the 90 mM H2O2 treatments, followed by 60 mM, 15 mM, and 30 mM H2O2. The lowest activity was for no H2O2 application. The net photosynthetic rate was 3.12-fold higher in the 90 mM H2O2 treated plants over the untreated plants and this was statistically significant (Table 1). In addition, the transpiration rates and the leaf stomatal conductance of the 60 and 90 mM H2O2 treated plants were significantly higher than the control plants (Table 1). Leaf stomatal conductance was four times higher in the 90 mM H2O2 treated plants when compared with the control plants (Table 1). The results for internal CO2 con centration were insignificant between the treated and untreated plants (Table 1).

Table 1.
Effects of different treatments of H2O2on net photosynthetic rate, transpiration rate, leaf stomatal conductance, and internal CO2 of F. deltoidea plants.
3.4. Leaf Hydrological Properties and Dry Matter Content
The leaf’s relative water and dry matter content was affected significantly by the exogenous H2O2 application at the rhizopheric growing media. Leaf relative water content in treatments of 90 mM H2O2 was 22% higher when compared to the control plants (Table 2). The lowest value for relative water content (%) was recorded in the control plants. The treatments with 90 mM H2O2 yielded the highest leaf dry matter content, 1.12-fold higher over the control plants (Table 2). Similarly, soil injection of H2O2 in the root zone produced a significant effect on leaf moisture and live line fuel moisture content of F. deltoidea (Table 2). The highest leaf moisture content was determined in 90 mM H2O2 treated plants followed by treatments of 60 mM, 30 mM, and 15 mM H2O2 with values of 1.79-, 1.12-, 1.11- and 1.11-fold, respectively, compared to the control plants. In addition, live line fuel moisture content was recorded as highest in the 90 mM H2O2 treated plants, which was 1.07-times higher over the control plants (Table 2).

Table 2.
Effects of different treatments of H2O2 on relative water content, leaf dry matter content, leaf moisture, live line fuel moisture of F. deltoidea.
3.5. Root Growth and Development
Observations and measurements are shown for the whole adventitious root system; root biomass for both fresh and dry weight, root crown diameter, and root traits, which include root length, root volume, and number of root tips. Results showed that H2O2 application played a significant role on root growth and development of the F. deltoidea. In Table 3, the data shows the significant effects of different concentrations of H2O2 on root biomass and root crown diameter of the F. deltoidea plants. Treatments with 90 mM of H2O2 showed the highest value of root biomass with 128.5 g for fresh and 59.5 g for dry. The root biomass in 90 mM treated plants was 3.55- and 2.28 fold higher for fresh and dry biomass, respectively, when compared to the control plants. For the root crown diameter, the biggest root crown was recorded in the 90 mM H2O2 treated plants, which was 88% larger than the control plants (Table 3).

Table 3.
Effects of different concentrations of H2O2 on root biomass, root crown diameter, and root traits of F. deltoidea plants.
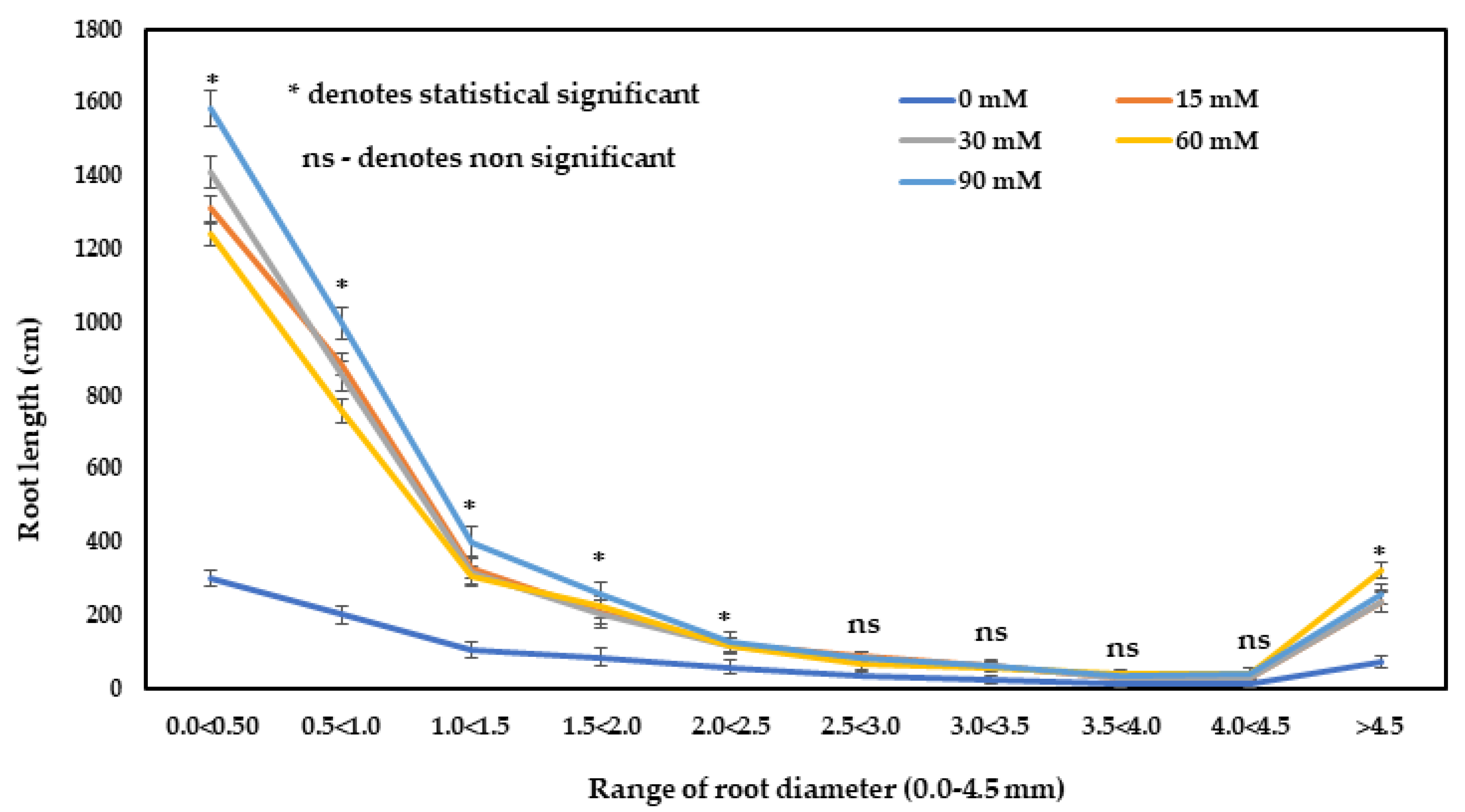
The values recorded for the root length and root volume were significantly higher in all treated plants compared to the untreated control plants (Table 3). Meanwhile, the highest root length and root volume were observed with the application of 90 mM H2O2. The root length was 4.21- and root volume was 2.12-fold higher in the plants with 90 mM H2O2 treatment over the control plants. H2O2 treatment produced a significant effect on the number of root tips of F. deltoidea plants. The highest root tip was recorded in the 30 mM H2O2 treatment, which was 3.82-fold higher over the control (Table 3). It can be seen from Figure 4 that as the range of the diameter of the root increased, the root lengths decreased. This means that at a larger size root diameter, the root length is shorter. The root length of 0.0–2.5 mm diameter roots was significantly higher in all treated plants compared to the control plants (Figure 4). The control plants had the lowest root lengths compared to the treated group, whereas the highest root lengths of all categories were recorded on the plants treated with 90 mM of H2O2, followed by other H2O2 treatments (Figure 4).
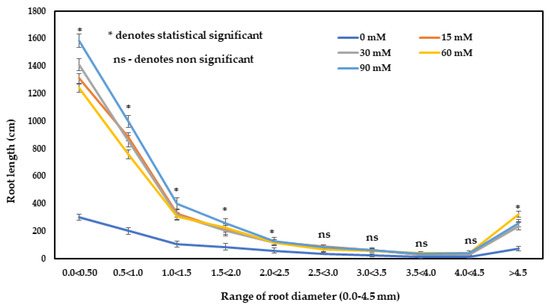
Figure 4.
The influence of H2O2 on the fine root length (total) of F. deltoidea plants. Fine root length was classified according to various diameter classes; fine root (>0.0–2.0 mm) and thin roots (>2.0 mm). Fine root length was higher in all treated plants compared to the control group. Error bars indicate ± standard error (S.E.).
Figure 5 displays images of the root growth of the F. deltoidea affected by different treatments of H2O2. There were lower numbers of roots on the control plants compared to the other treated plants. Plants treated with 15, 30, 60, and 90 mM H2O2 showed higher numbers of roots and higher fine root biomass. From the photographs, it can be seen that plants treated with 30 and 90 mM showed the highest number of roots and fine root biomass (Figure 5).

Figure 5.
Effect of H2O2 on root growth of F. deltoidea under polybagged condition after plant harvesting and processing. (a) 0 mM H2O2 (control), (b) 15 mM H2O2, (c) 30 mM H2O2, (d) 60 mM H2O2, and (e) 90 mM H2O2. Photographs show the higher number of root and fine root biomass in all treated plants compared to the untreated control plants.
3.6. Plant Biomass, Heavy Metals and Mineral Nutrients Accumulation
Table 4 shows the significant effect of H2O2 on the plant biomass content of the F. deltoidea. It clearly shows that plants treated with 90 mM H2O2 had the highest values for both fresh and dry weights, which was 5.13-fold more for fresh weight and 4.67-fold more for dry weight when compared to the control plants, followed by plants treated with 60 mM, 30 mM, 15 mM, and 0 mM of H2O2. Meanwhile, the control plants produced the lowest amounts of fresh and dry biomass. The applications of H2O2 into the rhizopheric soil significantly reduced the accumulation of heavy metals in the plant parts (Table 4). The highest content of As+ in leaf and syconium (4.08- and 6.84 fold higher) were recorded in the control plants compared to the 60 mM treated plants (Table 4). The plants treated with 60 mM H2O2 accumulated the lowest amount of As+ in leaf and syconium. In the case of Sb+, the highest concentrations were recorded in the control plants, which were 9-fold higher than the 30 mM treated plants. Additionally, in the syconium, Sb+ accumulations were affected significantly by the H2O2 treatments. These results showed that all the treated plants had significantly lower concentrations of As+ and Sb+ compared to the control plants.

Table 4.
Plant biomass, arsenic (As+) and antimony (Sb+) content in leaf and syconium of F. deltoidea plants affected by exogenous H2O2.
Table 5 shows the nutrient content for Fe2+, Mg2+, Ca2+, Na+ and K+ in both thee leaves and syconium of the F. deltoidea for the control group and treated group. The results showed that Fe2+ accumulation was significantly higher in the 15 mM H2O2 treatment for leaves and syconium, which was 1.21- and 1.39-fold higher when compared to the control plants. For Mg2+ content in leaves, treatment of 60 mM H2O2 accumulated the highest amounts, which were a significant 1.14-fold higher than the control group. The leaves’ Mg2+ content accumulation decreased in the 90 mM H2O2, treated plants while in syconium, a 48% higher Mg2+ content was recorded for the 90 mM H2O2 treatment when compared to the control group, which was statistically significant (Table 5).

Table 5.
Effects of H2O2 on the nutrient content (Fe2+, Mg2+, Ca2+, Na+, and K+) accumulation in the leaf and syconium of F. deltoidea plants.
The Ca2+ concentration in leaves had the same significant results with the highest amounts recorded for the 60 mM H2O2 treatments, which had an increase of 1.19-fold higher than the control. Meanwhile in syconium, the highest amount was recorded in treatments of 90 mM of H2O2 with a significant increase of 1.49-fold compared to the control (Table 5). The results also showed that Na+ concentration was significant with the highest in the leaves and syconium of the 90 mM H2O2 treated plants compared to other treatments and the control with values of 750 µg/g and 760 µg/g dry mass, respectively. A significant increase in K+ content was observed in the 60 mM treated plants compared to the control plants (Table 5). For K+ accumulations in the leaves and syconium, plants treated with 60 mM H2O2 showed the highest amounts of 1.45-fold higher for leaves and 1.15-fold higher for syconium compared to the control plants (Table 5).
3.7. Plant Biomass Production and Root Gall Development
As presented in Table 6, H2O2 significantly increased the weight of the roots and shoots of F. deltoidea seedlings. For both weights of roots and shoots, seedlings treated with 90 mM H2O2 displayed the highest value rather than those treated with 60 mM H2O2, 30 mM H2O2, 15 mM H2O2, and the control. The root and shoot weight ratio was the highest in untreated plants that showed an increase in root, but decrease in shoot weight of the plants. The lowest root and shoot ratio with the highest amount of shoot biomass was recorded in the 60 and 90 mM treated plants (Table 6). In this study, nematode infestation significantly reduced the plant growth, specifically, the plant height, leaf area, number of leaves, number of syconium, number of branches, and root lengths of the control (without H2O2) F. deltoidea plants (data not shown). Table 6 also presents the effects of H2O2 on the total number of root galls found in the root of F. deltoidea.

Table 6.
Effects of different concentrations of H2O2 on the weights of roots and shoots, number of root galls, and root gall size of F. deltoidea plants.
The presence of root galls in the roots indicates that the plants were infested with the root-knot nematode (M. incognita). Table 6 shows that the highest total number of root galls recorded was in the untreated plants. With the application of an increase in H2O2 concentration, the total number of root galls decreased drastically in the treated plants. In contrast, no root galls were found in plants treated with 90 mM of H2O2. Furthermore, the plants were healthy and no root-knot nematode infestation was found in the roots of the plants. The result showed that H2O2 significantly affected the total number of root galls in the plants’ roots. As shown in Table 6, the size of root galls was larger in the control plants at 0.6 cm in width and 7.1 cm in length. The smallest root gall was found in the 60 mM treated plants with only a 0.1 cm width and 1.3 cm length. These findings suggest that the number of root-knot nematodes present in the untreated plant was higher than in the others. This result is also a good indication that H2O2 may significantly control the root gall size in plants.
Figure 6 shows the formation of root galls caused by the root-knot nematode. In Figure 6, many galls formed in the untreated plants. The abundance of root galls was higher in the middle parts of the lateral roots of harvested seedlings. The colors of the galls in the photos are yellow and white. The numbers of galls seemed to be reduced with the increase in H2O2 concentrations injected into the roots. For plants treated with 15 mM of H2O2, numerous root-knot nematode infestations were still present. In contrast, for plants treated with 30 and 60 mM H2O2, the galls could be hardly seen, while there were no galls in the plants treated with 90 mM H2O2 (Figure 6).

Figure 6.
Photographs of the root system of treated and untreated F. deltoidea plants. (a) 0 mM H2O2 (control), (b) 15 mM H2O2, (c) 30 mM H2O2, (d) 60 mM H2O2, and (e) 90 mM H2O2. Many root galls (abnormal growth in the root) caused by root-knot nematode are visible in the control and 15 mM H2O2 treated plants, whereas, a lower number and tiny root galls in 60 mM treatment and no root gall in the 90 mM H2O2 treated plants were seen. A well-developed root system with many lateral roots were visible in both the 60 and 90 mM H2O2 treatments. Abbreviations: RG—Root gall, TRG—Tiny root gall, and NRG—No root gall.
3.8. Root Anatomical Structure in Nematode-Infested Soil
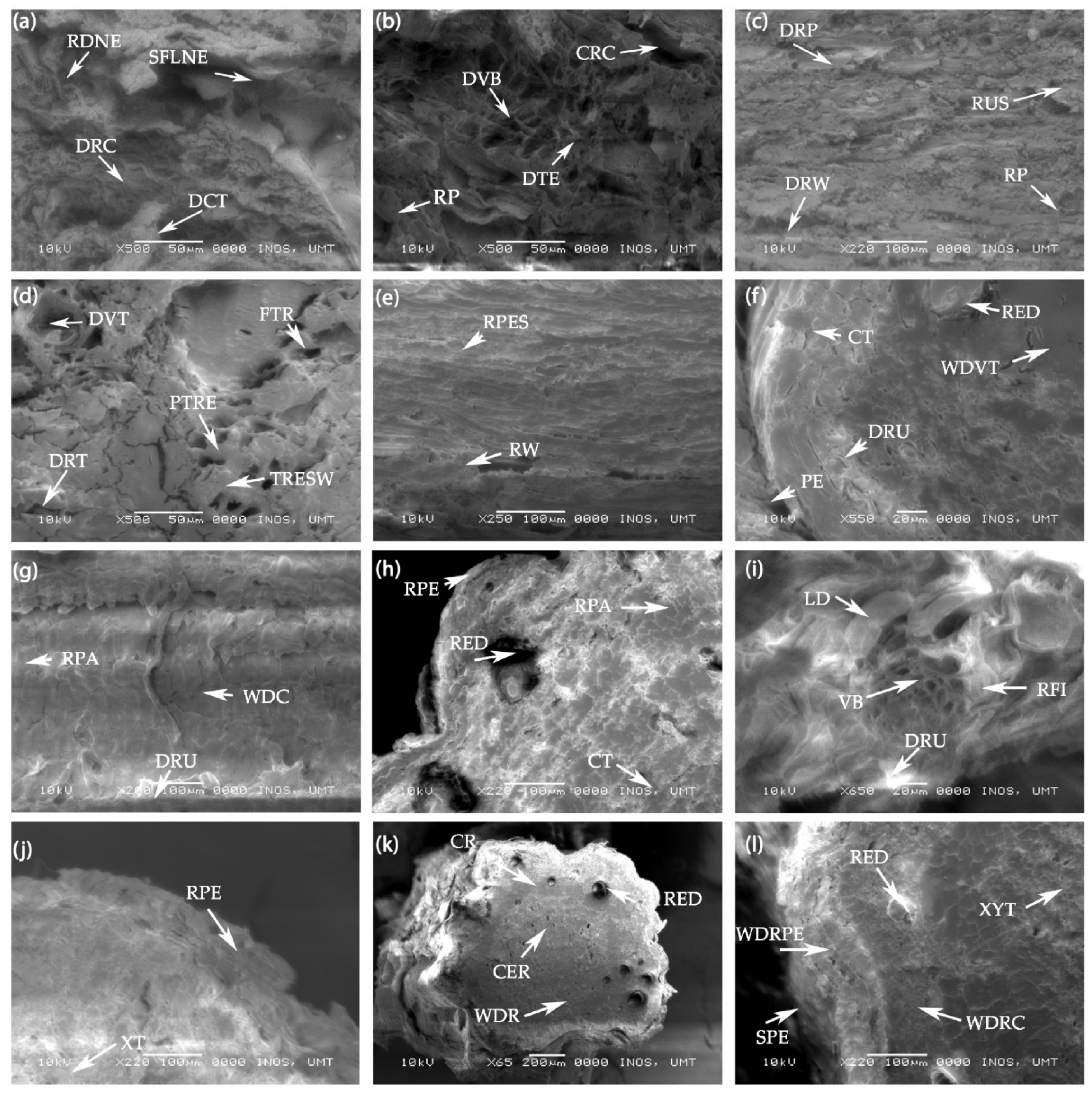
In the scanning electron microscopy (SEM) analysis, ruptured periderm and damaged root cells as well as damaged cortical and vascular tissue are shown in the root cross sections of the control treatment. Close magnification of the root’s cross section showed that roots were completely damaged including the peridermal wax/suberin with signs of the forward locomotion of the nematode visible in the root infection area of the untreated plants (Figure 7a,b). There were slightly damaged tracheary elements with thickened secondary wall, root periderm, and ruptured suberin found in the 15 mM H2O2 treated plants. Fractured tracheids, disorganized cortex, and slightly damaged vascular bundles were also visible in the roots treated with the lowest concentrations of H2O2. However, the anatomical structure of the infected roots was comparatively better than the control treatment (Figure 7c,d). At the root cross section of the 30 mM H2O2 treated plants, there were indications of root peridermal suberin, cortical cells, root parenchyma, and druses (Ca oxalate deposition) in the cortical region of the roots. Well-developed root periderm, cortex, resin ducts, and vascular tissue (xylem and phloem) were visible in the roots of 30 mM treated plants (Figure 7e–g). Figure 7h,j shows a well-developed root periderm, vascular system, root fiber, druses, and resin duct in the peripheral region of the roots. A well-established cortex, root parenchyma, and lignified vascular tissue were also seen in the treated roots. Furthermore, when looking at the SEM cross section of the roots, there was not much difference between the roots of plants treated with the 30 and 60 mM H2O2 treatments. However, at 30 and 60 mM of H2O2, the root anatomical structure appeared much better than the root anatomy of the control plants. As seen in Figure 7k,l, a developed root system with cortex and stele, and no adverse effects appeared with higher concentrations of H2O2 on the anatomical root structure of F. deltoidea plants. Distinct regions of suberized root periderm, embedded resin ducts, developed cortex, phloem, xylem, and the central region were also visible in the anatomy of the 90 mM treated roots.
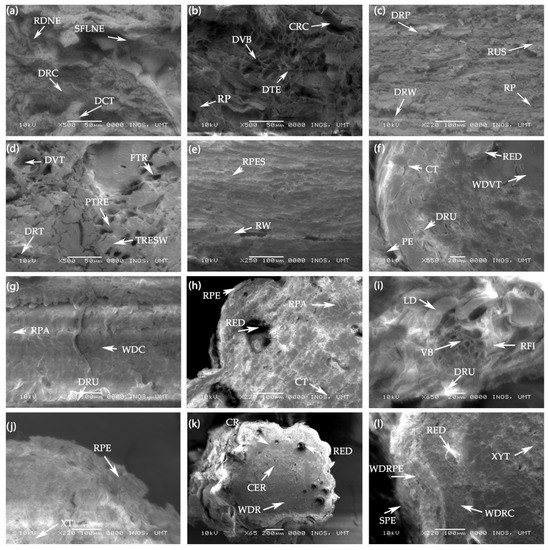
Figure 7.
(a,b) Scanning electron microscopy (SEM) photographs of the untreated (control) root cross sections of the F. deltoidea plant. The photographs show damaged root cells, dented cortical tissue, ruptured periderm, and damaged vascular bundles. The sign of forward locomotion of the nematode and cortical lesion are visible in the roots of the control plants. Abbreviations: RDNE—Root damage by nematode, SFLNE—Sign of forward locomotion of the nematode, DRC—Damaged root cells, DCT—Damaged cortical tissue, CRC—Cavity in root cortex, DVB—Damaged vascular bundle, DTE—Damaged tracheary elements, and RP—Ruptured periderm. (c,d) SEM photographs showing the ruptured suberin, outside of root periderm, and fractured tracheids in roots. Damaged root periderm and vascular bundle, disorganized root cortex, and tracheary elements with thickened secondary wall are also visible in the root cross section of the 15 mM H2O2 treated plants. Abbreviations: RP—Root periderm, RUS—Ruptured suberin, DRP—Damaged root periderm, DRW—Damaged root wax, DVT—Damaged vascular tissue, FTR—Fractured tracheid, PTRE—Pitted tracheary elements, TRESW—Tracheary elements with secondary wall thickening, and DRT—Damaged root tissue. (e–g) SEM photographs showing the peridermal suberin, cortical tissue, vascular tissues and druses (Ca oxalate deposition) in the root. A well-developed root periderm, cortex, root parenchyma in cortex, and resin duct (oil gland) are visible in the 30 mM H2O2 treated root. Abbreviations: RPES—Root peridermal suberin, RW—Root wax, PE—periderm, CT—cortical tissue, DRU—Druses (Ca oxalate deposition), RED—Resin duct, WDVT—Well developed vascular tissue, RPA—Root parenchyma, and WDC—Well developed cortex. (h–j) SEM photographs of root periderm, a well-developed vascular system, root fiber, and druses in the fiber cells of 60 mM treated root. Cortical tissue, root parenchyma, resin duct, and suberin deposition in the root vascular cells are visible at the cross section of the 60 mM H2O2 treated root. Abbreviations: RPE—Root periderm, RED—Resin duct, RPA—Root parenchyma, CT—Cortical tissue, VB—Vascular bundle, RFI—Root fiber, LD—Lignin deposition, DRU—Druses, RPE—Root periderm, and XT—Xylem tissue. (k,l) SEM photographs of a well-developed root system (cortex and stele) including suberized root periderm. Embedded resin ducts, developed cortex, phloem, xylem tissues and root central region are visible in 90 mM H2O2 treated root of F. deltoidea plants. Abbreviations: CR—Cortical region, RED—Resin duct, CER—Central region, WDR—Well developed root, SPE—Suberized periderm, WDRPE—Well developed root periderm, WDRC—Well-developed root cortex, and XYT—Xylem tissue.
From the above results (Figure 7e–l), the plants treated with 30–90 mM H2O2 improved the root periderm, cellular structure in cortex, druses, resin ducts, and vascular tissue in the presence of nematodes. Our study confirmed that exogenous H2O2 at the root zone protects plant roots from root-knot nematode infestation and improves the root structure of plants grown in problematic soils. It also appears that higher concentrations of H2O2 do not produce any adverse effects on the roots’ anatomical structure or vascular system of F. deltoidea plants.
3.9. Presence of Nematode in Treated and Untreated Plants
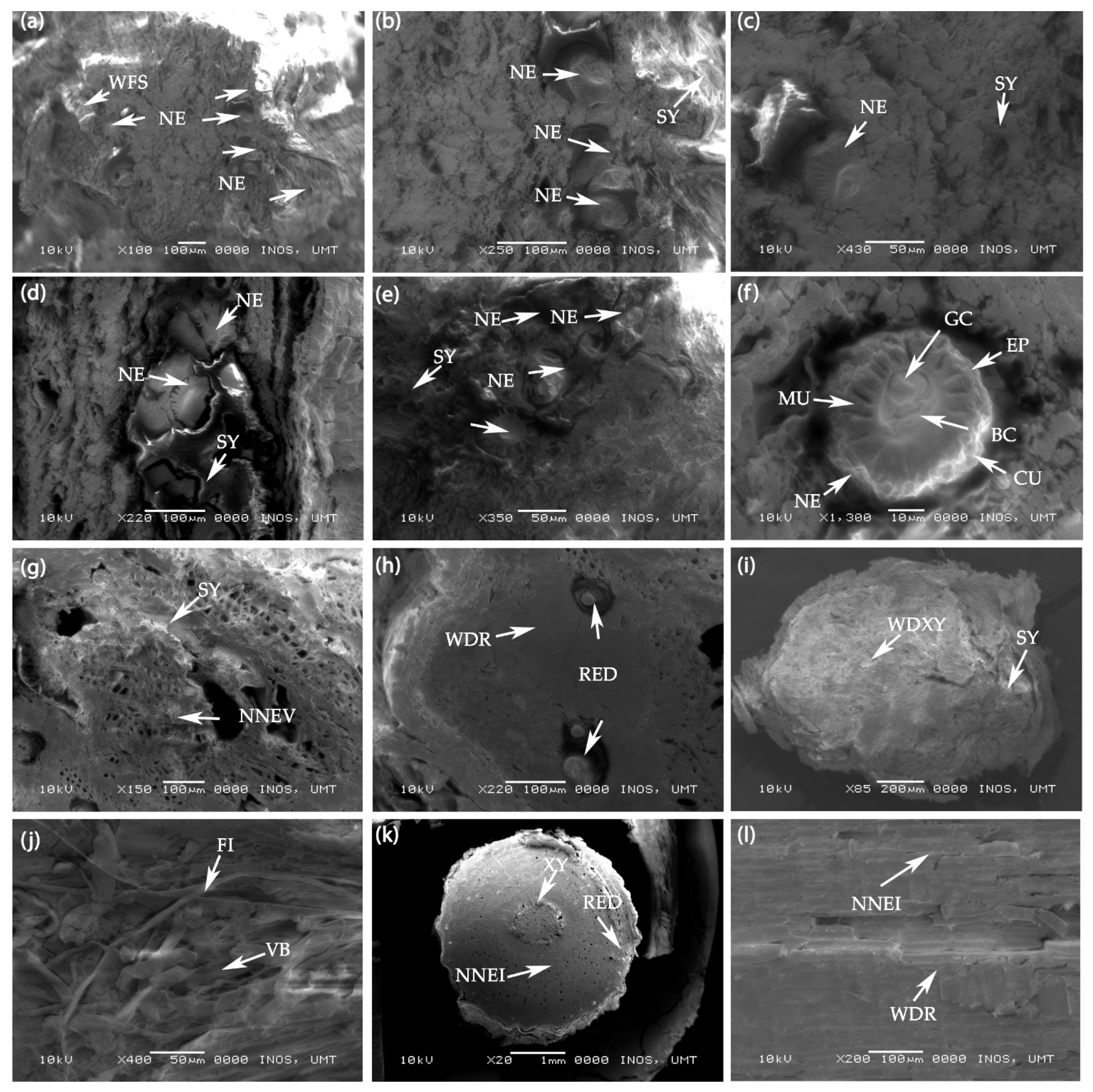
Figure 8a–c shows the cross section of swollen galls of untreated or control plants without H2O2 injection. Figure 8a–c illustrates a well-formed syncytium consisting of several cells in the vascular cylinders of the infected roots. These cells are known as syncytia cells (Figure 8a–c), characterized by large-sized cells with dense contents, and they can easily be distinguished from the smaller and less dense cells in normal vascular tissue. Figure 8a–c also shows the cross section of the root-knot nematode in the syncytia cells. With the larger size of swollen galls, many root-knot nematodes (Meloidogyne spp.) inside it trigger the more extensive formation of the syncytium.
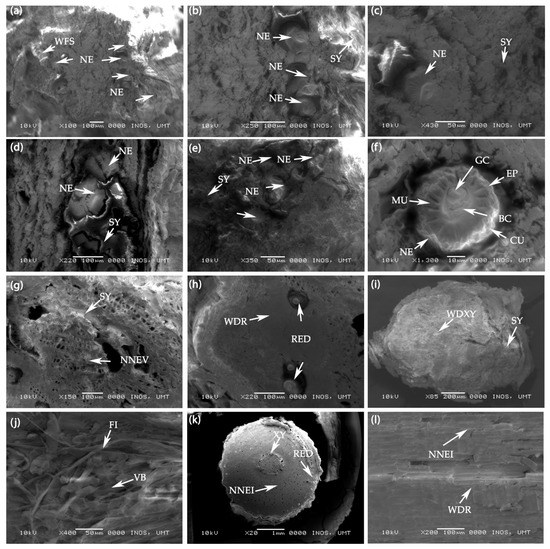
Figure 8.
(a–c) Scanning electron microscopy (SEM) photographs of the swollen galls (cross section) in the root of untreated F. deltoidea plant. Syncytia cells and many nematodes are visible in the control plants. Abbreviations: WFS—Well-formed syncytium, NE—Nematode, and SY—Syncytium (d–f) SEM photographs of swollen galls (cross section) of the F. deltoidea root treated with 15 mM H2O2. Syncytia cells and a smaller number of nematodes are visible in the root galls. Abbreviations: NE—Nematode, SY—Syncytium, MU—Muscle, GC—Gut cavity, EP—Epidermis, BC—Body cavity, and CU—Cuticle, (g,h) SEM photographs of swollen galls (cross section) of the F. deltoidea plant root treated with 30 mM H2O2. Syncytia and resin duct are visible in the galls of the treated plant. No nematode is present in the gall cross section. Abbreviations: NNEV—No nematode visible, WDR—Well developed root, and RED—Resin duct. (i,j) SEM photographs of swollen galls (cross section) of the root of F. deltoidea plant treated with 60 mM H2O2. Syncytia and wood developed xylem cells are evident in the root galls. No nematode is visible in the gall cross section. Abbreviations: WDX—Well developed xylem, FI—Fiber, and VB—Vascular bundle. (k,l) SEM Photographs of the root cross section of F. deltoidea plant treated with 90 mM H2O2. Resin duct and well-developed cells are visible. No root gall and nematode are visible in cross section of root. Abbreviations: XY—Xylem, NNEI—No nematode infection, and WDR—Well developed root.
Figure 8d–f shows the images of syncytium in plants treated with 15 mM H2O2 compared with the control plants, where this syncytium size was smaller, and was comprised of a few syncytia cells. These cells contain numerous small spherical bodies resembling vacuoles. Figure 8d–f displays the cross section of the root-knot nematode found in this syncytium. Figure 8g–h illustrates the cross section of the swollen galls of the plants treated with 30 mM H2O2. The syncytia cells, being larger in size, resulted from its proliferation and from being accompanied by numerous small vacuoles that characterized the syncytium. This image also shows the explicit presence of the resin duct, but no presence of the nematode. In contrast, the galls in the untreated plants had more syncytia cells, and the resin ducts were invisible. In Figure 8i,j, the roots treated with 60 mM H2O2 treatment induced a few galls with small sizes, and only a few syncytia cells were present. The cells did not proliferate extensively, resulting in the small syncytium formation. Figure 8i,j also shows the well-developed xylem cells in the treated root of F. deltoidea, and there was no root-knot nematode in the galls. These observations suggest that as the result of H2O2 application, the nematode perhaps died or moved away from the root gall.
Figure 8k,l illuminates the plant root injected with 90 mM H2O2 and shows that the injection of 90 mM H2O2 at the root zone of F. deltoidea induced a good cellular structure and root shape, indicating no presence of root-knot nematode infestation. The xylem and phloem were also in good positions, suggesting that these plants were healthy and may grow well. Figure 8i,k shows that the resin duct was more apparent than those in the control and 15 mM H2O2, where most of their galls were full of syncytium, showing a noticeable presence of root-knot nematode.
3.10. Relationship among Studied Parameters
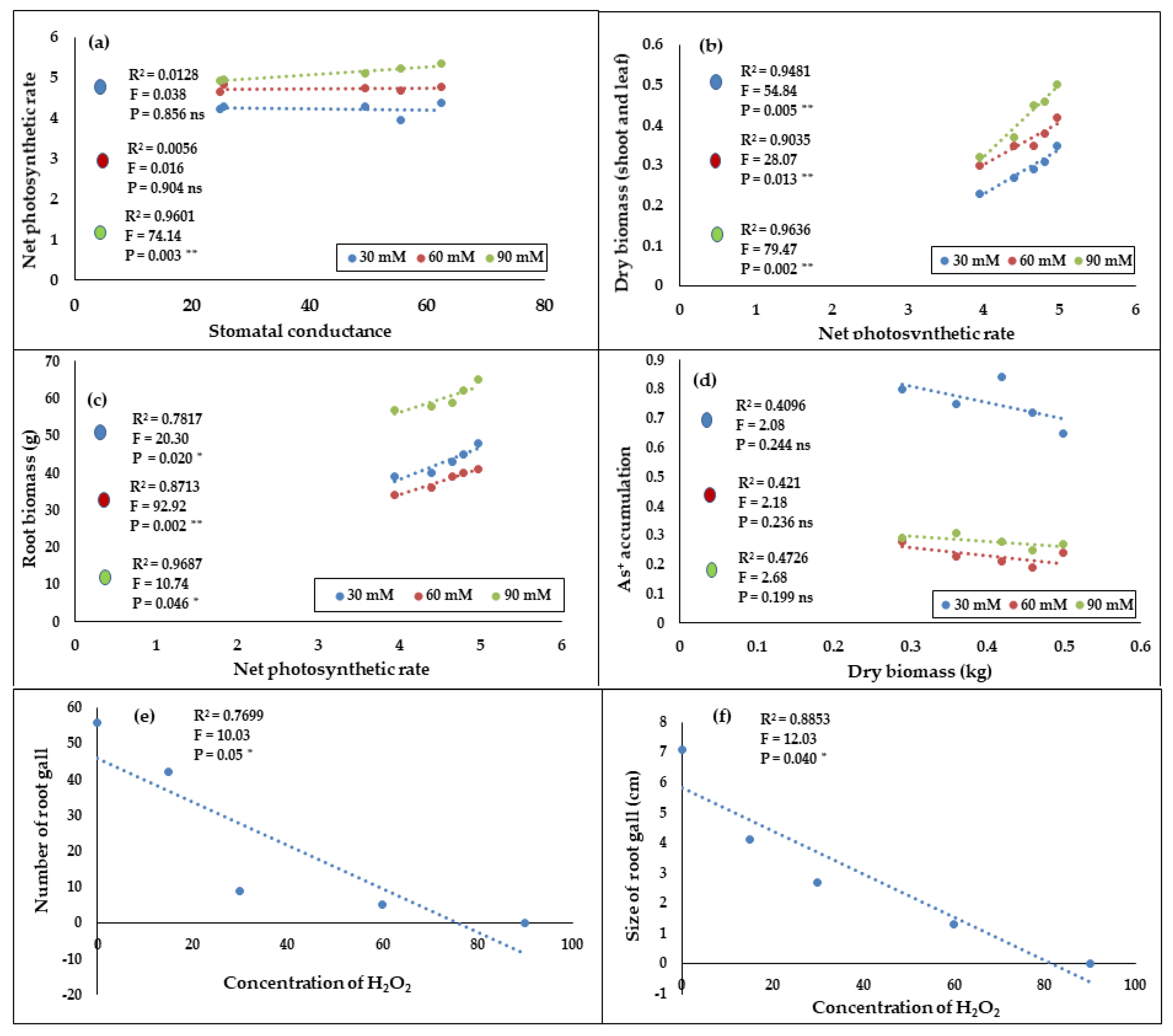
The results showed that net photosynthetic rate and stomatal conductance were significantly correlated in the 90 mM H2O2 treated plants (R2 = 0.96, p = 0.003). However, no correlations were found in the 30 and 60 mM treated plants (Figure 9a). A significant high correlation was observed between the dry biomass content and net photosynthetic rate in the 30 to 90 mM treated plants, with the highest in the 90 mM treated plants with a R2 value of 0.96 (p = 0.002). The regression slopes indicate that dry biomass increased with net photosynthetic rates (Figure 9b). Similarly, a significant relationship was observed between root biomass content and net photosynthetic rate in the 30, 60, and 90 mM treated F. deltoidea plants with R2 values of 0.96, 0.87, and 0.78 (p = 0.02, p = 0.002, and p = 0.046, Figure 9c), respectively. The regression equation shows that root biomass increased with the net photosynthetic rate of the treated plants (Figure 9c). Apart from that, there was no correlation between As+ accumulation and dry biomass content in the plants treated with 30–90 mM H2O2 (Figure 9d). Plant biomass decreased with the increase in As+ accumulation, and the accumulation of As+ was the highest in the control treatment, followed by the 30 mM treated plants. Figure 9e,f shows a significantly negative correlation between H2O2 concentrations with the number of root gall and gall size (p = 0.05 and p = 0.04, respectively). It was observed that the number of root gall and gall size decreased with the H2O2 concentrations.
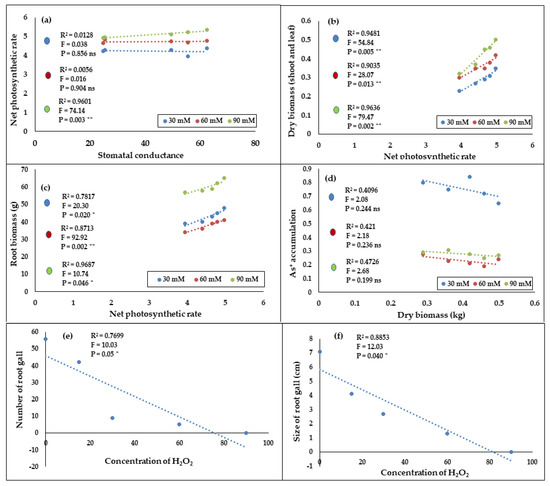
Figure 9.
Relationship between different physiological parameters of H2O2 treated plants. (a) Stomatal conductance (mmol/m2/s) with net photosynthetic rates (µmol/m2/s); (b) Net photosynthetic rate (µmol/m2/s) with dry biomass content; (c) Net photosynthetic rate (µmol/m2/s) with root biomass (g); (d) Dry biomass (kg) with As+ accumulation (μg/g) in H2O2 treated plants; (e) Number of root gall with H2O2 concentration (mM), and (f) Root gall size with H2O2 concentration (mM). **, and * indicate significance at p = 0.020 < 0.01, and p = 0.020 < 0.05, respectively. The value of linear regression slope was obtained according to the homogeneity test of regression coefficient.
4. Discussion
In this study, different concentrations of H2O2 were injected into the growing media of rhizophere to investigate the effects of exogenous H2O2 on the photosynthetic characteristics, root growth and architecture, mineral accumulation, and root anatomy of F. deltoidea plants. H2O2 was injected into the root zone growing media instead of other aerials parts of the plants because exogenous H2O2 may improve root structure, and the developed root can regulate the plant physiological and biochemical processes. This study confirmed that exogenous rhizopheric H2O2 improved the photosynthetic characteristics, enhanced plant growth and biomass production, maintained better leaf hydrological properties, developed the root system, increased mineral accumulation, and improved the root cellular structure of F. deltoidea plants. Our results show that inoculant treatment with H2O2 can induce plant vegetative and reproductive growth of F. deltoidea. We found that exogenous H2O2 increased the seedling height, leaf area, number of leaves, and syconium numbers. The positive results may be due to the role of H2O2 as a signaling molecule and plant growth stimulant. Guzel and Terzi [33] also reported that growth, water percentage, mineral absorption, total sugar, proline content, dry matter, and soluble protein content increased in the leaves of H2O2 treated maize seedlings. H2O2 may also regulate basic developmental processes and defenses, and can increase stress tolerance in plants [4]. It may be that the exogenous H2O2 in the root zone increased the availability of oxygen to the plant roots, which played a significant role in the plant growth and development processes. The applications of H2O2 may have also elevated the metabolic activity in favor of plant growth and development [34]. Currently, it has been reported that H2O2 acts as a signaling molecule and regulates the expression of various genes in cells [35].
Bojovic and Stojanovic [36] stated that in most plants, chlorophyll a and b ratios are at a 3:1 ratio, which is due to chlorophyll’s function in plant growth and development. This study showed that applications of H2O2 elevated the level of chlorophyll content of the F. deltoidea plants. It was also reported in the study of Khandaker et al. [11] that H2O2 treatment positively affected the pigment content in the leaves of the wax apple plant. H2O2 may protect chloroplast ultrastructure and increase chlorophyll content in the mesophyll tissue and stimulate photosynthesis. In this study, it was found that treated plants had the highest values for total carotenoid content. The increase in total carotenoid content may be due to the role of H2O2 as a signaling molecule during the biosynthesis of carotene. This supports the supposition that with the increase in carotenoid content, the photosynthetic activity also increases in F. deltoidea plants, thus increasing the biomass of the plants. Carotenoids play a significant role in the photosynthesis process of plants [36]. Exogenous H2O2 produced a significant positive effect on chlorophyll fluorescence (Fv/Fm) of the F. deltoidea plants, which means that soil applications of H2O2 enhanced the growth of the plants and improved their stress tolerance ability. H2O2 may regulate chlorophyll fluorescence by affecting electron acceptors in the photosynthetic pathway, downstream of PSII, notably plastoquinone, and in particular, QA. Soil applications of H2O2 into the rhizophere did not produce any adverse effects on the roots or on plant health. Recently, it was reported that H2O2 treatments increased the drought tolerance of soybean plants by maintaining the water content of its leaves [37]. H2O2 acts as a key regulator in a broad range of physiological and biochemical processes such as growth and development, photorespiration and photosynthesis, stomata movement, cell cycle, stress tolerance, and senescence [38].
In this study, all of the H2O2 treated plants showed a higher net photosynthetic rate and stomatal conductance compared to the control plants. This may be due to the H2O2 treatment, which increases the stomatal aperture with the availability of CO2. H2O2 is an essential signal molecule that mediates stomatal aperture by induced abscisic acid (ABA) via the activation Ca-permeable channels in the plasma membrane [39]. Ozaki et al. [23] also stated that foliar application of H2O2 to melon seedlings increased the net photosynthetic rate and increased sugar accumulation in the fruit. H2O2 induces the activity of sucrose phosphate synthase (SPS), an enzyme that regulates the formation of sucrose from triose phosphates during and after photosynthesis in plants at the transcriptional level [40]. The elevated levels of H2O2 may also regulate the metabolic and antioxidant enzyme activity, which enhances the physiological processes of the plants. In this study, the highest transpiration rates were recorded in plants treated with 60–90 mM of H2O2. The increase in the concentration of H2O2 also increases the rate of transpiration. However, Pilar et al. [41] did not notice any potential effect of H2O2 on the assimilation of CO2, stomatal aperture, transpiration, or xylem water potential. They also reported that xylem vessel diameter and xylem/phloem ratio tended to be greater for trees with soil injections of H2O2 rather than for the control plants. Our study indicated that H2O2 treated plants absorbed and transported more water and nutrients when compared to the control group. A plant with a higher transpiration rate indicates a greater stomatal conductance. As stomatal conductance changes, rates of transpiration and photosynthesis will fluctuate because the stoma (pore) size will provide a consistent resistance to the diffusion of CO2 into, and H2O out of, the leaf. The results of this study report that soil injections of H2O2 significantly increased the stomatal aperture of the F. deltoidea leaves. Furthermore, there was no recognizable significant effect of H2O2 on the internal carbon dioxide of F. deltoidea.
A leaf’s relative water content is positively correlated to stomatal conductance and the photosynthetic rates of plants [42]. In this study, leaf water content of F. deltoidea was significantly higher in all treated plants. This may be due to the positive effects of exogenous H2O2 on the levels of osmatic solutes in the leaves and stomatal aperture [33]. Dry matter content in the leaf was also used as an indicator of plant resource use. This trait is related to a leaf’s lifespan and it involves the important transaction between the speedy production of biomass and an effective maintenance of nutrient elements [43]. For leaf dry matter content, H2O2 treated plants showed higher leaf dry matter content when compared to the control. Perhaps the improved photosynthetic rates in treated plants favor the greater accumulation of leaf dry matter. H2O2 treatments increased the leaf moisture content significantly in F. deltoidea. In addition, our results showed that exogenous applications of H2O2 significantly affected the live line fuel moisture of the leaves of F. deltoidea. It was recorded in this study that the fresh biomass and dry matter content of F. deltoidea plants increased with the dose of H2O2 injected into the root zone. This may be due to improvements in the availability of soil oxygen, thus increasing root aeration and absorption as well as the biomass of the aerial portions of the plants [41]. Treated plants accumulated more dry matter due to greater photosynthetic rates, faster seedling growth, and a well-developed root system in the presence of H2O2. The application of H2O2 increased the dry matter content of maize plants under copper stress conditions [33]. Different growing media was used to grow the F. deltoidea seedlings in two different experiments of the study. The root-knot nematode cannot survive in a mixture of cocopeat, paddy husk, and perlite, which is why BRIS soil was used in the second experiment. The plant growth and biomass accumulation were very high in the first experiment compared to the second one due to the higher amount of H2O2 and suitable growing media. The dry matter accumulation in plants grown in cocopeat, paddy husk, and perlite was also greater compared to plants grown in BRIS soil.
Our study reports that root biomass of the F. deltoidea increased significantly with H2O2 treatments. Larger root crown diameters were also recorded in all H2O2 treated plants and crown diameters increased with the concentration applied. This is due to the role of H2O2 as a signal molecule, which may mediate signals, on root cells and plays a key role in coordinating root cellular functions. Exogenous treatment of H2O2 promotes the growth of lateral roots, root hairs, and the primary root [44]. For root traits, the results showed that root length, root volume, and root tip numbers were significantly affected by exogenous root zone H2O2, which correlated with the higher root biomass production. H2O2 injection into the soil alleviated root asphyxia of avocado trees in slow draining clay soils, or a flood prone area [41]. When injected into the soil, H2O2 typically breaks down into water and oxygen (one half mole O2 per mole of H2O2). The natural decomposition of H2O2 may supply additional oxygen needed for the growth, development, and aerobic metabolism of roots. This natural decomposition of H2O2, catalyzed by iron (Fe2+) or by the enzyme catalase, is universal in aerobic organisms [45]. The application of H2O2 with irrigation water in a heavy clay soil increased the biomass content and yield of cotton, soybean, and zucchini [46]. This study confirmed that the injection of H2O2 to the root elongation zone plays a significant role in root length, crown diameter, and root tip number of the F. deltoidea plants. This concludes that exogenous applications of H2O2 increased the number of roots and fine root biomass, which promoted plant growth and development by absorption of water as well as higher nutrient element absorption from the soil.
Our results showed that H2O2 applications through growing media injections reduced the accumulation of arsenic and antimony in both leaves and syconium of F. deltoidea plants compared to the control. This is because the use of H2O2 pre-oxidizes As3+ to As5+, which may assist conventional arsenic removal processes using iron co-precipitation. This has shown a beneficial effect of H2O2 in reducing the presence of As+ and Sb+, which are considered toxic to plants. Improved root cellular structure such as well-developed periderm and suberin deposition as well as thickened vascular tissues were found in the treated plants. Our findings related to a well-developed root system are also an indication of lower absorption and accumulation of heavy metals. It can be reported from the study that exogenous H2O2 can protect the plant from cytotoxicity and protect humans from heavy metal toxicity. In this study, we recorded 55 to 120 µg/g Fe2+ content in the leaf tissue of the F. deltoidea with the Fe2+ content in the syconium a little bit higher than the leaf. This level of Fe2+ was the same as in the edible tissue of maize, which was 10 to 160 mg/kg; in rice, it was 60 to 122 mg/kg; and 15 to 360 mg/kg in wheat [47]. The higher level of Fe2+ in the plant tissues is an indication of improved physiological functions and elevated levels of the key cellular processes in the treated plants.
In our study, all of the treated plants accumulated a higher amount of Mg2+ than the control. The net photosynthetic rate was positively affected by the treatment of H2O2 in this study, which may be related to the elevated level of Mg2+ content in the leaves as Mg2+ is the main component of the chlorophyll molecule, which is involved in the CO2 assimilation reactions and regulates RuBP carboxylase activity. A suitable proportion of Mg2+ in the cell also regulates cellular pH and the cation–anion balance. This elevated level of Mg2+ may improve photosynthesis and assimilate supply, which enhances plant growth, development, and biomass accumulation of F. deltoidea. The Ca2+ concentration in leaves and syconium of F. deltoidea was greater in all treated plants compared to the control group. A suitable concentration triggers Ca2+ accumulation and Ca2+ is also recognized as a signal molecule that induces plant growth and development. The better root systems and higher plant growth are likely linked with Ca2+ accumulation in the presence of H2O2. The elevated level of Ca2+ within the plant may improve the cellular structure of roots, which induces tolerance from the infection of root-knot nematode.
Hydrogen peroxide at 60 mM treatment decreased the accumulation of Na+ in the plant leaves, which was 390 μg/g. The Na+ concentration should be less than 1.0 mg/kg in plant tissue because if higher than that, it can disrupt plant growth and development [48]. In addition, K+ content in the leaves improved in all treatments. This may be due to the H2O2 treatment increasing the number of root hairs, thus more minerals were absorbed by the expanded surface area of absorption. H2O2 accumulates in a discrete area of the distal part of roots that plays a significant role in active K+ uptake and translocation through the plant [49]. It was also reported that H2O2 might play a role in the cellular signaling of K+ deprivation. Exogenous H2O2 may alleviate the levels of endogenous enzymatic antioxidants, which increase the K+ and Na+ fluxes in plants [50]. This increased level of K+ and Na+ may increase the turgor in guard cells and thereby drive stomatal opening. These results prove why the net photosynthetic rates and transpiration rates were higher in all treated plants since a higher K+ and Na+ content in leaves increases stomatal aperture and improves net photosynthetic rate and biomass accumulation.
The results of the scanning electron microscopy of root cross sections showed that exogenous treatment of H2O2 improved the cellular structure in the root periderm, cortex, and vascular system of the plant root. The accumulation of Ca2+ in treated plants increased significantly, thus the elevated level of Ca2+ may improve the cellular structure of the roots. Calcium plays an important role in the structure and function of cells and in maintaining the integrity of cell walls and membrane structures [51,52]. Scanning electron microscope image analysis indicated a well-developed root cuticular wax, suberin, druses, and glands (resin duct) in the cellular structure of the roots of plants treated with H2O2. H2O2 improved root cellular structure, which may be due to its role in preventing oxidative damage to the cell by biological agents, lignification, and the cross linking of cell wall structural proteins. The results paralleled a study conducted by Jamaludin et al. [53], who reported that H2O2 treatment as a foliar spray improved vascular structure and promoted cell proliferation as well as thickened minor veins in cell proliferation. Applications of H2O2 increased the number and size of tracheary elements in the vascular systems [54] and cell wall thickening of the inter-fascicular fiber [55] as well as solidified the cell wall of root fibers in tomato plants [56]. The improved vascular system transports water and minerals efficiently and creates an impact on plant physiological and developmental processes. Nasir et al. [40] stated that foliar application of 30 mM H2O2 produced a better cell structure and histology of F. deltoidea leaves. However, a treatment dose of 100 mM H2O2 or higher than that directly causes severe cytotoxicity within as little as 15 min of exposure [57]. This study showed that H2O2 treatment increased the deposition of suberin and lignin in the periderm and vascular tissue of the root. The endogenous H2O2 enhanced the suberin formation in the plant parts during suberization [58]. H2O2 restrained within the walls of xylem vessels, fibers, and other surrounding cells caused secondary thickening [59]. Presumably, the deposition of suberin in the root periderm and vascular tissue restrict nematode entry in the plant roots.
Our experimental results affirmed the devastating effect of root-knot nematode infection in untreated plants. In this study, nematode infection reduced the root and shoot biomass, and increased the number and size of root gall in the control plants. The lower concentrations of 15–30 mM H2O2 for treated plants demonstrated a much lower effect of root-knot nematode infection, but higher growth than the control plants. The effect of root-knot nematode was very low in 60 mM treated plants. No negative effect of nematode related to growth and development was found in 90 mM treated plants. This result may be caused by the exogenous application of H2O2, which induced a direct deleterious effect on nematode reproduction and pathogenicity. H2O2 also acts as an antiseptic due to its cytotoxic effects on many bacterial strains and microorganisms [60,61]. Karajeh [62] stated that H2O2 treatment of the growing media (0–1000 mM) produced negative effects on nematode reproduction and protected tomato roots from nematode infection. Exogenous H2O2 may affect plant parasitic nematodes directly by its oxidizing capacity or indirectly as an elicitor, triggering defense of the host plant.
Many large-sized galls were formed in the root of the untreated plants. The number of root galls in the treated F. deltoidea plants decreased with the increase in H2O2 injected into the soil near the root zone. This study recorded that plants treated with 15 mM and 30 mM H2O2 concentrations still had galls attached to their roots, whereas in the 60 mM H2O2 treated plants, the galls were tiny. In contrast, there were no root galls in the plants treated with 90 mM H2O2. Gustin et al. [22] claimed that H2O2 vapor could kill the eggs of Caenohabditis elegans in vitro, a free-living transparent nematode in soil. Streptococci can produce sufficient amounts of H2O2 to kill Caenohabditis elegans, killing kinetics similar to those of equimolar concentrations of pure H2O2 [63,64]. Terzi et al. [65] stated that exogenous H2O2 increased the endogenous H2O2 content in the treated plants. It may be that the elevated level of endogenous H2O2 reduced the nematode infection and protected the plant from root gall formation. Our study suggests that root-knot nematode cannot survive at a higher concentration of H2O2 (60 to 90 mM) and it also indicated that plants can combat root-knot nematode infestation with the presence of exogenous H2O2.
Most galls of the control plants showed the presence of root-knot nematode. There was a well-formed syncytium, comprising several cells in the vascular cylinders of the roots. Syncytia, a specialized feeding structure, form by the plant parasitic nematode in the host roots, which serve as nutrient source for the reproduction and development of nematode [66]. The results of scanning electron microscopy showed that many nematodes were found in the root gall syncytia of the control and 15 mM treated plants. However, nematode was not visible in root cross sections of the 30–90 mM treated plants. This result may be due to the cytotoxic effects of exogenous and endogenous H2O2. H2O2 regulates the hypersensitive response in plant cells and cell death as a defense response against pathogen attack [67]. There were no galls in the roots of plants with the 90 mM H2O2 treatment, suggesting that the plants were healthy and survived the nematode infestation. Perhaps the higher concentrations of H2O2 strengthen the root, which then combats the nematode, and makes the roots resistant through enhanced deposition of suberin and improved cellular structure.
Our results report that the net photosynthetic rate positively correlated with leaf stomatal conductance of the H2O2 treated plants. The stomatal conductance may control the influx of CO2 in the mesophyll cells, a critical component of photosynthesis, and allow for the excess oxygen to exit, thus elevating the levels of the net photosynthetic rate of the plants. Stomatal limitations such as photoinhibition, photorespiration, and reduced rubisco activation under high temperature can cause a reduction in photosynthesis [68]. The findings of this study suggest that exogenous rhizopheric H2O2 maintained the stomatal conductance, and that the net photosynthetic capacities seem to be positively regulated by stomatal conductance. Dry matter content in above ground plant parts and roots increased with the net photosynthetic rates and this was positively correlated in this study. Exogenous H2O2 enhanced the level of rubisco gene expression [35] and the high expression of this gene may enable the enhancing of photosynthesis capacity and dry matter accumulation in the aerial parts and roots. The findings of this study show that H2O2 treatment enhanced the level of photosynthesis and increased the carbon sink such as root crown diameter, root length, root volume, and number of root tips of the treated plants. The enhancement of root systems under H2O2 treatments suggests improved root metabolic activities. Thus, the below ground carbon sink may receive a greater amount of photo-assimilates and increase root biomass. This higher level of biomass in the root system may significantly contribute to maximizing the plant growth and development. In this study, arsenic (As+) accumulation was negatively correlated with plant dry matter production and the accumulation of As+ decreased with increasing H2O2 concentrations. Previously, it was reported that As+ can reduce root extension, damage leaf chlorophyll, and cause necrosis in aerial plant parts [69]. As+ may severely impede plant growth by slowing mineral translocation and arresting biomass accumulation [70]. Our results show that the number of root galls and gall size in treated plants correlated negatively with concentration of H2O2. Karajeh [62] stated that nematode reproduction eggs/g fresh root in tomato plants decreased with H2O2 concentrations. Based on this discussion, it can be summarized that an injection of H2O2 near the root zone of F. deltoidea plants enhanced the growth, biomass accumulation, and created a nematicidal effect on the root-knot nematode M. incognita in the BRIS soil.
5. Conclusions
The main objective of this study was to find the best way of enhancing the process of growth and development, and combat root-knot nematode within the F. deltoidea plants by using rhizopheric H2O2. The application of exogenous H2O2, in particular treatments with 60 and 90 mM of H2O2, increased the seedling height, leaf area, number of leaves, syconium number, photosynthetic pigment content, photosynthetic characteristics, and quantum yield of F. deltoidea plants. At the same time, root growth, which includes root crown diameter, root length, root volume, root tips, number of roots, and root biomass (fresh and dry), also increased with applications of H2O2. In addition, exogenous rhizopheric H2O2 decreased heavy metals (As+ and Sb+) and increased mineral (Fe2+, Na+, Mg+, Ca+, and K+) accumulation, which showed a significant increase in the plant biomass. Furthermore, H2O2 treatment, particularly 60 and 90 mM as a soil injection near the root zone, reduced the number of root galls and gall size, improved plant biomass, root periderm, root cellular structure, and vascular system, thus preventing root damage caused by root-knot nematode in problematic BRIS soil. H2O2 treatments (60–90 mM) reduced nematode reproduction, produced cytotoxic effects, and combated the root-knot nematode of F deltoidea. Root gall formation and size of gall was negatively correlated with the concentration of H2O2. As a result, it can be concluded that 60–90 mM H2O2 treatments into the rhizopheric soil could enhance plant growth, mineral accumulation, root growth and development, reduce root gall formation, and combat root-knot nematode infestation by improving root structure, function, and creating nematicidal effects.
Author Contributions
Data curation, N.H.A.A.R. and M.M.K.; Funding, M.M.K.; Methodology, M.M.K; Supervision, M.M.K.; Validation, K.S.M., A.M.F., and A.M.; Writing original draft, N.H.A.A.R. and M.M.K.; Root Analysis, M.S. and N.O.; Writing—review & editing, N.A.B., K.S.M., K.M., N.Y., Z.M.N. and M.M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported from the Fundamental Research Grant Scheme, FRGS Grant No. FRGS/2/2014/SG03/UNISZA/02/01, Ministry of Higher Education Malaysia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.
Acknowledgments
The authors would like to thank Universiti Sultan Zainal Abidin (UniSZA), Terengganu, Malaysia for supporting the FRGS project (FRGS/2/2014/SG03/UNISZA/02/01). We also acknowledge the editing and partial publication support of Taif University researchers in supporting project numbers TURSP-2020/110 & TURSP-2020/46 at Taif University, Taif, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- USDA-ARS National Genetic Resources Program, Germplasm Resources Information Network (GRIN) Database; National Germplasm Resources Laboratory: Beltsville, MD, USA, 2007.
- Abdulla, M.A.; Ahmed, K.A.; Abu-Luhoom, F.M.; Muhanid, M. Role of Ficus deltoidea Extract in the Enhancement of Wound Healing in Experimental Rats. Biomed. Res. 2010, 21, 241–245. [Google Scholar]
- Abolmaesoomi, M.; Aziz, A.A.; Junit, S.M.; Ali, J.M. Ficus deltoidea: Effects of Solvent Polarity on Antioxidant and Anti-Proliferative Activities in Breast and Colon Cancer Cells. Eur. J. Integr. Med. 2019, 28, 57–67. [Google Scholar] [CrossRef]
- Nazarni Che Isa, M.; Ajit, A.; Naila, A.; Sulaiman, A.Z. Effect of Microwave Assisted Hydro-Distillation Extraction on Extracts of Ficus Deltoidea. Mater. Today Proc. 2018, 5, 21772–21779. [Google Scholar] [CrossRef]
- Misbah, H.; Abdul Aziz, A.; Aminudin, N. Antidiabetic and Antioxidant Properties of F. deltoidea Fruit Extracts and Fractions. BMC Complement. Altern. Med. 2013, 13, 118. [Google Scholar]
- Adam, Z.; Hamid, M.; Ismail, A.; Khamis, S. Effect of Ficus deltoidea Aqueous Extract on Blood Glucose Level in Normal and Mild Diabetic rats. Malays. J. Health Sci. 2007, 5, 9–16. [Google Scholar]
- Awang, N.A.; Hasan, S.M.Z.; Shafie, M.S.B. Morphological Study of Ficus deltoidea Jack in Malays. J. Agric. Sci. Technol. B 2013, 3, 144–150. [Google Scholar]
- Slesak, I.; Libik, M.; Karpinska, B.; Karpinski, S.; Miszalski, Z. The Role of Hydrogen Peroxide in Regulation of Plant Metabolism and Cellular Signalling in Response to Environmental Stresses. Acta Biochim. Pol. 2007, 54, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Kolla, V.A.; Vavasseur, A.; Raghavendra, A.S. Hydrogen Peroxide Production is an Early Event During Bicarbonate Induced Stomatal Closure in Abaxial Epidermis of Arabidopsis. Planta 2006, 225, 1421–1429. [Google Scholar] [CrossRef]
- Rojkind, M.; Domínguez-Rosales, J.-A.; Nieto, N.; Greenwel, P. Role of Hydrogen Peroxide and Oxidative Stress in Healing Responses. Cell. Mol. Life Sci. 2002, 59, 1872–1891. [Google Scholar] [CrossRef]
- Khandaker, M.M.; Boyce, A.N.; Osman, N. The Influence of Hydrogen Peroxide on the Growth, Development and Quality of Wax Apple (Syzygium Samarangense, [Blume] Merrill & L.M. Perry var. jambu madu) Fruits. Plant Physiol. Biochem. 2012, 53, 101–110. [Google Scholar] [CrossRef]
- Cheeseman, J.M. Hydrogen Peroxide Concentrations in Leaves Under Natural Conditions. J. Exp. Bot. 2006, 57, 2435–2444. [Google Scholar] [CrossRef] [PubMed]
- Nurnaeimah, N.; Mat, N.; Mohd, K.S.; Badaluddin, N.A.; Yusoff, N.; Sajili, M.H.; Mahmud, K.; Adnan, A.F.M.; Khandaker, M.M. The Effects of Hydrogen Peroxide on Plant Growth, Mineral Accumulation, as Well as Biological and Chemical Properties of Ficus deltoidea. Agronomy 2020, 10, 599. [Google Scholar] [CrossRef]
- Guo, D.-L.; Wang, Z.-G.; Pei, M.-S.; Guo, L.-L.; Yu, Y.-H. Transcriptome Analysis Reveals Mechanism of Early Ripening in Kyoho Grape with Hydrogen Peroxide Treatment. BMC Genom. 2020, 21, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xue, L.; Xu, S.; Feng, H.; An, L. Hydrogen Peroxide Acts as a Signal Molecule in the Adventitious Root Formation of Mung Bean Seedlings. Environ. Exp. Bot. 2009, 65, 63–71. [Google Scholar] [CrossRef]
- Gill, H.K.; Mcsorley, R. Cover Crops for Managing Root-Knot Nematodes; University of Florida: Gainesville, FL, USA, 2011; pp. 1–6. [Google Scholar]
- Sasser, J.N.; Eisenback, J.D.; Carter, C.C.; Triantaphyllou, A.C. The International Meloidogyne Project-Its Goals and Accomplishments. Ann. Rev. Phytopathol. 1983, 21, 271–288. [Google Scholar] [CrossRef]
- Barker, K.R.; Sasser, J.N.; Carter, C.C. An Advanced Treatise on Meloidogyne; North Carolina State University Graphics: Raleigh, NC, USA, 1985; Volume 2. [Google Scholar]
- Ralmi, N.H.A.A.; Khandaker, M.M.; Mat, N. Occurrence and control of root knot nematode in crops: A review. Aus. J. Crop. Sci. 2016, 10, 1649–1654. [Google Scholar] [CrossRef]
- Musa, Y. Evaluation of Growth Performance and Yield Potential of Selected Mas Cotek (Ficus deltoidea) Accessions on Bris Soils. J. Trop Agril. Food Sci. 2006, 34, 229–235. [Google Scholar]
- Ishwar, P.S.; Sharma, A.K. Effects of Initial Inoculums Levels of Meloidogyne Incognita J2 on Development and Growth of Tomato cv. PT-3 Under Control Conditions. Afr. J. Microbiol. Res. 2015, 9, 1376–1380. [Google Scholar] [CrossRef][Green Version]
- Gustin, E.J.; McDonnell, G.E.; Mullen, G.; Gordon, B.E. The Efficacy of Vapour Phase Hydrogen Peroxide against Nematode Infestation of the Caenohabditis Elegans Model. In Proceedings of the 53rd AALAS National Meeting, San Antonio, TX, USA, 27–31 October 2002. [Google Scholar]
- Ozaki, K.; Uchida, A.; Takabe, T.; Shinagawa, F.; Tanaka, Y.; Takabe, T.; Hayashi, T.; Hattori, T.; Rai, A.K.; Takabe, T. Enrichment of Sugar Content in Melon Fruits by Hydrogen Peroxide Treatment. J. Plant Physiol. 2009, 166, 569–578. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper Enzymes in Isolated Chloroplasts Polyphenol Oxidase in Beta Vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Saura-Mas, S.; Lloret, F. Leaf and Shoot Water Content and Leaf Dry Matter Content of Mediterranean Woody Species with Different Post-Fire Regenerative Strategies. Ann. Bot. 2007, 99, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Munné-Bosch, S.; Peñuelas, J. Drought-Induced Oxidative Stress in Strawberry Tree (Arbutus unedo L.) Growing in Mediterranean Field Conditions. Plant Sci. 2004, 166, 1105–1110. [Google Scholar] [CrossRef]
- Viegas, D.X.; Piñolb, J.; Viegas, M.T.; OgayaD, R. Estimating Live Fine Fuels Moisture Content Using Meteorologically-Based Indices. Int. J. Wildland Fire 2001, 10, 223. [Google Scholar] [CrossRef]
- Yen, C.P. Tree Root Patterns and Erosion Control. In Proceedings of the International Workshop on Soil Erosion and its Coun-termeasures, Soil and Water Conservation Society of Thailand, Bangkok, Thailand, 19 November 1987; pp. 92–111. [Google Scholar]
- Nashriyah, M.; Zaini, H.; Mazleha, M.; Abdul, K.W. Mineral Uptake by Taro (Colocasia Esculenta) in Swamp Agroecosystem Following Gramoxone® (Paraquat) Herbicide Spraying. J. Nuclear Related Technol. 2006, 3, 59–68. [Google Scholar]
- Tahery, Y.N.; Shukor, A.A.; Abdul-Hamid, H.; Abdullah, M.P.; Norlia, B. Status of Root Knot Nematode Disease on Kenaf Cultivated on Bris Soil in Kuala Terengganu, Malaysia. World Appl. Sci. J. 2011, 15, 1287–1295. [Google Scholar]
- Atamian, H.S.; Roberts, P.A.; Kaloshian, I. High and Low Throughput Screens with Root-Knot Nematodes Meloidogyne spp. J. Visualised Exp. 2012, 61, 3629. [Google Scholar] [CrossRef] [PubMed]
- Gowtham, H.G.; Hariprasad, P.; Singh, S.B.; Niranjana, S.R. Biological Control of Phomopsis Leaf Blight of Brinjal (Solanum melongena L.) with Combining Phylloplane and Rhizosphere Colonizing Beneficial Bacteria. Biol. Control. 2016, 101, 123–129. [Google Scholar] [CrossRef]
- Guzel, S.; Terzi, R. Exogenous Hydrogen Peroxide Increases Dry Matter Production, Mineral Content and Level of Osmotic Solutes in Young Maize Leaves and Alleviates Deleterious Effects of Copper Stress. Bot. Stud. 2013, 54, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Eniu, L.; Eliao, W. Hydrogen Peroxide Signaling in Plant Development and Abiotic Responses: Crosstalk with Nitric Oxide and Calcium. Front. Plant Sci. 2016, 7, 230. [Google Scholar] [CrossRef]
- Quan, L.-J.; Zhang, B.; Shi, W.-W.; Li, H.-Y. Hydrogen Peroxide in Plants: A Versatile Molecule of the Reactive Oxygen Species Network. J. Integr. Plant Biol. 2008, 50, 2–18. [Google Scholar] [CrossRef]
- Bojovic, B.; Stojanovic, J. Chlorophyll and Carotenoid Content in Wheat Cultivars as a Function of Mineral Nutrition. Arch. Biol. Sci. 2005, 57, 283–290. [Google Scholar] [CrossRef]
- Anjum, N.A.; Sharma, P.; Gill, S.S.; Hasanuzzaman, M.; Khan, E.A.; Kachhap, K.; Mohamed, A.A.; Thangavel, P.; Devi, G.D.; Vasudhevan, P.; et al. Catalase and Ascorbate Peroxidase—Representative H2O2-Detoxifying Heme Enzymes in Plants. Environ. Sci. Pollut. Res. 2016, 23, 19002–19029. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.Y.; Li, Z.H.; Xiang, H.; Huang, J.H.; Jia, S.H.; Miao, X.X.; Huang, Y.P. Preliminary Studies on Differential Defense Responses Induced During Plant Communication. Cell Res. 2005, 15, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Neill, S.J.; Desikan, R.; Clarke, A.; Hancock, J.T. Nitric Oxide Is a Novel Component of Abscisic Acid Signaling in Stomatal Guard Cells. Plant Physiol. 2002, 128, 13–16. [Google Scholar] [CrossRef]
- Nasir, N.N.N.M.; Khandaker, M.M.; Mohd, K.S.; Badaluddin, N.A.; Osman, N.; Mat, N. Effect of Hydrogen Peroxide on Plant Growth, Photosynthesis, Leaf Histology and Rubisco Gene Expression of the Ficus deltoidea Jack Var. Deltoidea Jack. J. Plant Growth Regul. 2020, 1–22. [Google Scholar] [CrossRef]
- Pilar, M.G.M.; Raul, F.E.; Cristian, B.M.; Carlos, Z.E.; Luis, G.R. Effect of Injecting Hydrogen Peroxide into Heavy Clay Loam Soil on Plant Water Status, NET CO2 Assimilation, Biomass, and Vascular Anatomy of Avocado Trees. Chil. J. Agric. Res. 2009, 69, 97–106. [Google Scholar] [CrossRef]
- Peñuelas, J.; Munné-Bosch, S.; Llusià, J.; Filella, I. Leaf Reflectance and Photo- and Antioxidant Protection in Field-Grown Summer-Stressed Phillyrea Angustifolia. Optical Signals of Oxidative Stress? New Phytol. 2004, 162, 115–124. [Google Scholar] [CrossRef]
- Poorter, H.; Garnier, E. Ecological Significance of Inherent Variation in Relative Growth Rate and its Components. In Pugnaire FI, Handbook of Functional Plant Ecology; Valladares, F., Ed.; Marcel Dekker: New York, NY, USA, 1999; pp. 81–120. [Google Scholar]
- Zolla, G.; Heimer, Y.M.; Barak, S. Mild Salinity Stimulates a Stress-Induced Morphogenic Response in Arabidopsis Thaliana Roots. J. Exp. Bot. 2009, 61, 211–224. [Google Scholar] [CrossRef]
- Petigara, B.R.; Blough, N.V.; Mignerey, A.C. Mechanisms of Hydrogen Peroxide Decomposition in Soils. Environ. Sci. Technol. 2002, 36, 639–645. [Google Scholar] [CrossRef]
- Bhattarai, S.P.; Huber, S.; Midmore, D.J. Aerated Subsurface Irrigation Water Gives Growth and Yield Benefits to Zucchini, Vegetable Soybean and Cotton in Heavy Clay Soils. Ann. Appl. Biol. 2004, 144, 285–298. [Google Scholar] [CrossRef]
- Briat, J.-F.; Dubos, C.; Gaymard, F. Iron Nutrition, Biomass Production, and Plant Product Quality. Trends Plant Sci. 2015, 20, 33–40. [Google Scholar] [CrossRef]
- Jia, Y.; Kong, X.; Weiser, M.D.; Lv, Y.; Akbar, S.; Jia, X.; Tian, K.; He, Z.; Lin, H.; Bei, Z.; et al. Sodium Limits Litter Decomposition Rates in a Subtropical Forest: Additional Tests of the Sodium Ecosystem Respiration Hypothesis. Appl. Soil Ecol. 2015, 93, 98–104. [Google Scholar] [CrossRef]
- Shin, R.; Schachtman, D.P. Hydrogen Peroxide Mediates Plant Root Cell Response to Nutrient Deprivation. Proc. Natl. Acad. Sci. USA 2004, 101, 8827–8832. [Google Scholar] [CrossRef]
- Maksimović, J.D.; Mojović, M.; Maksimović, V.; Römheld, V.; Nikolic, M. Silicon Ameliorates Manganese Toxicity in Cucumber by Decreasing Hydroxyl Radical Accumulation in the Leaf Apoplast. J. Exp. Bot. 2012, 63, 2411–2420. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Calcium in Plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Sanders, D.; Pelloux, J.; Brownlee, C.; Harper, J.F. Calcium at the Crossroads of Signaling. Plant Cell 2002, 14, S401–S417. [Google Scholar] [CrossRef] [PubMed]
- Jamaludin, R.; Mat, N.; Mohd, K.S.; Badaluddin, N.A.; Mahmud, K.; Sajili, M.H.; Khandaker, M.M. Influence of Exogenous Hydrogen Peroxide on Plant Physiology, Leaf Anatomy and Rubisco Gene Expression of the Ficus Deltoidea Jack var. Deltoidea. Agronomy 2020, 10, 497. [Google Scholar] [CrossRef]
- İşeri, Ö.D.; Körpe, D.A.; Sahin, F.I.; Haberal, M. Hydrogen Peroxide Pretreatment of Roots Enhanced Oxidative Stress Response of Tomato Under Cold Stress. Acta Physiol. Plant. 2013, 35, 1905–1913. [Google Scholar] [CrossRef]
- López-Delgado, H.; Zavaleta-Mancera, H.A.; Mora-Herrera, M.E.; Vázquez-Rivera, M.; Flores-Gutiérrez, F.X.; Scott, I.M. Hydrogen Peroxide Increases Potato Tuber and Stem Starch Content, Stem Diameter, and Stem Lignin Content. Am. J. Potato Res. 2005, 82, 279–285. [Google Scholar] [CrossRef]
- Thordal-Christensen, H.; Zhang, Z.; Wei, Y.; Collinge, D.B. Subcellular Localization of H2O2 in Plants. H2O2 Accumulation in Papillae and Hypersensitive Response During the Barley-Powdery Mildew Interaction. Plant J. 1997, 11, 1187–1194. [Google Scholar] [CrossRef]
- Lin, K.-Y.; Chung, C.-H.; Ciou, J.-S.; Su, P.-F.; Wang, P.-W.; Shieh, D.-B.; Wang, T.-C. Molecular Damage and Responses of Oral Keratinocyte to Hydrogen Peroxide. BMC Oral Health 2019, 19, 1–10. [Google Scholar] [CrossRef]
- Bernards, M.A.; Razem, F.A. The Poly(Phenolic) Domain of Potato Suberin: A Non-Lignin Cell Wall Bio-Polymer. Phytochemistry 2001, 57, 1115–1122. [Google Scholar] [CrossRef]
- Barceló, A.R. Xylem Parenchyma Cells Deliver the H2O2 Necessary for Lignification in Differentiating Xylem Vessels. Planta 2005, 220, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Linley, E.; Denyer, S.P.; McDonnell, G.; Simons, C.; Maillard, J.-Y. Use of Hydrogen Peroxide as a Biocide: New Consideration of its Mechanisms of Biocidal Action. J. Antimicrob. Chemother. 2012, 67, 1589–1596. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. The Chemistry of Free Radicals and Related Reactive Species. In Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 1999; pp. 6–104. [Google Scholar]
- Karajeh, M. Interaction of Root-Knot Nematode (Meloidogyne Javanica) and Tomato As Affected By Hydrogen Peroxide. J. Plant Prot. Res. 2008, 48, 181–187. [Google Scholar] [CrossRef]
- Jansen, W.T.M.; Bolm, M.; Balling, R.; Chhatwal, G.S.; Schnabel, R. Hydrogen Peroxide-Mediated Killing of Caenorhabditis Elegans by Streptococcus Pyogenes. Infect. Immun. 2002, 70, 4757–4761. [Google Scholar] [CrossRef]
- Bolm, M.; Jansen, W.T.M.; Schnabel, R.; Chhatwal, G.S. Hydrogen Peroxide-Mediated Killing of Caenorhabditis Elegans: A Common Feature of Different Streptococcal Species. Infect. Immun. 2004, 72, 1192–1194. [Google Scholar] [CrossRef] [PubMed]
- Terzi, R.; Kadioglu, A.; Kalaycioglu, E.; Saglam, A. Hydrogen Peroxide Pretreatment Induces Osmotic Stress Tolerance by Influencing Osmolyte and Abscisic Acid Levels in Maize Leaves. J. Plant Interact. 2014, 9, 559–565. [Google Scholar] [CrossRef]
- Zhang, L.; Lilley, C.J.; Imren, M.; Knox, J.P.; Urwin, P.E. The Complex Cell Wall Composition of Syncytia Induced by Plant Parasitic Cyst Nematodes Reflects Both Function and Host Plant. Front. Plant Sci. 2017, 8, 1087. [Google Scholar] [CrossRef]
- Klessig, D.F.; Durner, J.; Noad, R.; Navarre, D.A.; Wendehenne, D.; Kumar, D.; Zhou, J.M.; Shah, J.; Zhang, S.; Kachroo, P.; et al. Nitric Oxide and Salicylic Acid Signaling in Plant Defense. Proc. Natl. Acad. Sci. USA 2000, 97, 8849–8855. [Google Scholar] [CrossRef] [PubMed]
- Koyama, K.; Takemoto, S. Morning Reduction of Photosynthetic Capacity before Midday Depression. Sci. Rep. 2014, 4, 4389. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, A.A.; Aarabi, M.; Delaune, R.; Gambrell, R.; Patrick, W. Bioavailability and Uptake of Arsenic by Wetland Vegetation: Effects on Plant Growth and Nutrition. J. Environ. Sci. Health Part A 1998, 33, 45–66. [Google Scholar] [CrossRef]
- Garg, N.; Singla, P. Arsenic Toxicity in Crop Plants: Physiological Effects and Tolerance Mechanisms. Environ. Chem. Lett. 2011, 9, 303–321. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).