Comparison of Productivity and Physiological Traits of Faba Bean (Vicia faba L.) Varieties under Conditions of Boreal Climatic Zone
Abstract
1. Introduction
2. Materials and Methods
2.1. Site and Soil Description
2.2. Experimental Details and Agronomic Management
2.3. Plant Sampling, Measurements, and Calculations
2.3.1. Physiological Parameters
2.3.2. Gas Exchanges Parameters
2.4. Incidence of Chocolate Spot (Botrytis fabae Sard.)
2.5. Seed Yield (SY) and Seed Quality Analyses
2.6. Statistical Analysis
2.7. Meteorological Conditions
3. Results
3.1. Variation of Physiological Parameters of Faba Bean Varieties
3.2. Variation of LAI and PAR Capture Ratio and Penetration Ratio of Faba Bean Varieties
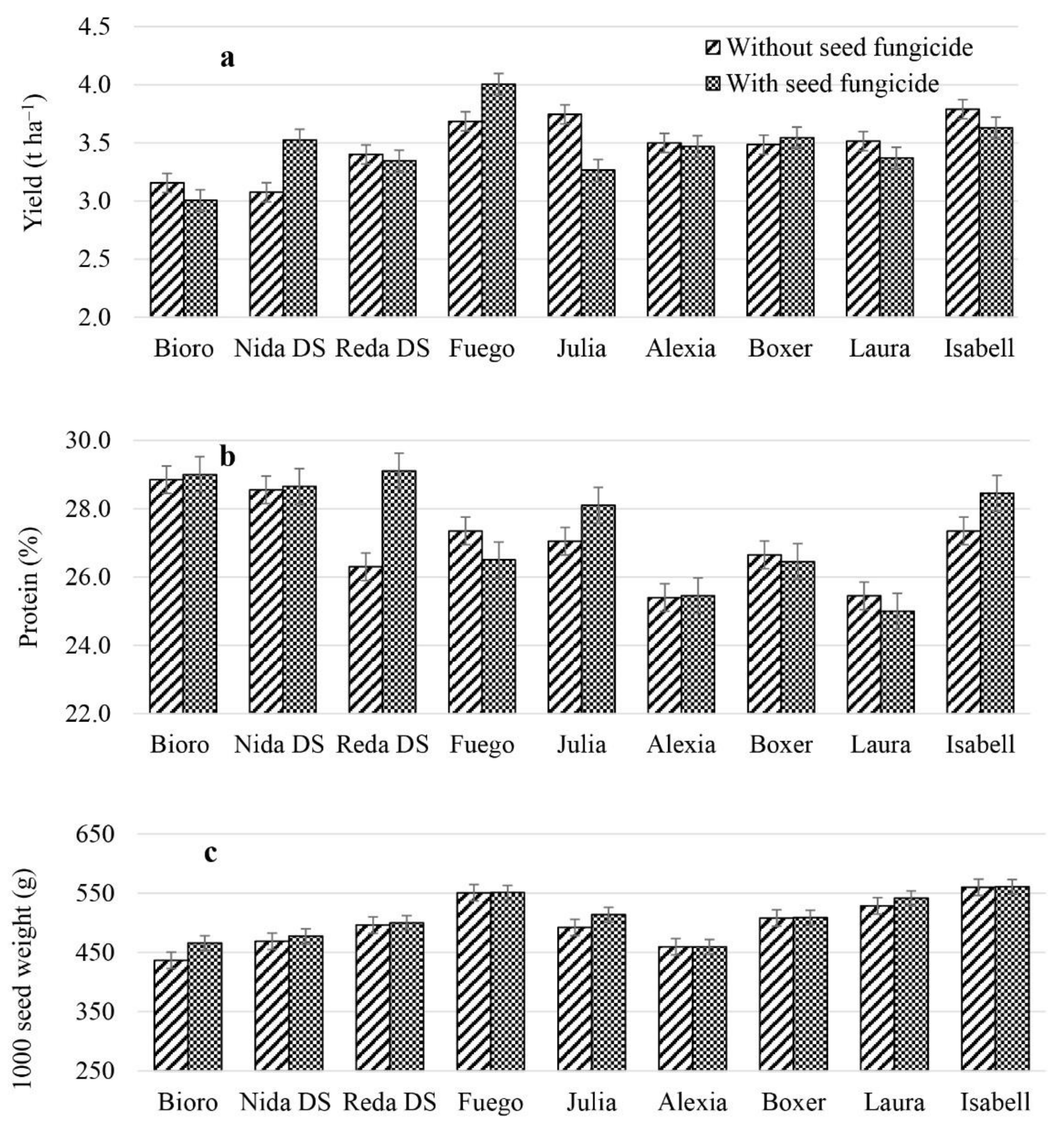
3.3. Variation of Seed Yield and Quality Parameters of Faba Bean Varieties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crepon, K.; Marget, P.; Peyronnet, C.; Carroue, B.; Arese, P.; Duc, G. Nutritional value of faba bean (Vicia faba L.) seeds for feed and food. Field Crop. Res. 2010, 115, 329–339. [Google Scholar] [CrossRef]
- Lizarazo, C.I.; Lampi, A.M.; Liu, J.; Sontag-Strohm, T.; Piironen, V.; Stoddard, F.L. Nutritive quality and protein production from grain legumes in a boreal climate. J. Sci. Food Agric. 2015, 95, 2053–2064. [Google Scholar] [CrossRef]
- Lepse, L.; Dane, S.; Zeipina, S.; Domínguez-Perles, R.; Rosa, E.A.S. Evaluation of vegetable-faba bean (Vicia faba L.) intercropping under Latvian agro-ecological conditions. J. Sci. Food Agric. 2017, 97, 4334–4342. [Google Scholar] [CrossRef]
- O’Sullivan, D.M.; Angra, D. Advances in faba bean genetics and genomics. Front. Genet. 2016, 7, 150. [Google Scholar] [CrossRef] [PubMed]
- De Cillis, F.; Leoni, B.; Massaro, M.; Renna, M.; Santamaria, P. Yield and quality of faba bean (Vicia faba L. var. major) genotypes as a vegetable for fresh consumption: A comparison between Italian landraces and commercial varieties. Agriculture 2019, 9, 253. [Google Scholar] [CrossRef]
- Peltonen-Sainio, P.; Jauhiainen, L. Unexploited potential to diversify monotonous crop sequencing at high latitudes. Agric. Syst. 2019, 174, 73–82. [Google Scholar] [CrossRef]
- Knudsen, M.T.; Hermansen, J.E.; Olesen, J.E.; Topp, C.F.E.; Schelde, K.; Angelopoulos, N.; Reckling, M. Climate impact of producing more grain legumes in Europe. In Proceedings of the 9th International Conference on Life Cycle Assessment in the Agri-Food Sector, San Francisco, CA, USA, 8–10 October 2014; Schenck, R., Huizenga, D., Eds.; American Center for Life Cycle Assessment: Vashon, WA, USA, 2014; pp. 641–646. Available online: http://lcacenter.org/lcafood2014/proceedings/LCA_Food_2014_Proceedings.pdf (accessed on 17 December 2020).
- Karkanis, A.; Ntatsi, G.; Lepse, L.; Fernández, J.A.; Vågen, I.M.; Rewald, B.; Alsina, I.; Kronberga, A.; Balliu, A.; Olle, M.; et al. Faba bean cultivation—Revealing novel managing practices for more sustainable and competitive European cropping systems. Front. Plant Sci. 2018, 9, 1115. [Google Scholar] [CrossRef]
- Sanchez-Navarro, V.; Zarnoza, R.; Faz, A.; Fernandez, J.A. Comparison of soil organic carbon pools, microbial activity and crop yield and quality in two vegetable multiple cropping systems under mediterranean conditions. Sci. Hortic. 2020, 261, 109025. [Google Scholar] [CrossRef]
- Ruisi, P.; Amato, G.; Badagliacca, G.; Frenda, A.S.; Giambalvo, D.; Miceli, G. Agro-ecological benefits of faba bean for rainfed Mediterranean cropping systems. Ital. J. Agron. 2017, 12, 233–245. [Google Scholar] [CrossRef]
- Etemadi, F.; Hashemi, M.; Zandvakili, O.; Dolatabadian, A.; Sadeghpour, A. Nitrogen contribution from winter-killed faba bean cover crop to spring-sown sweet corn in conventional and no-till systems. Agron. J. 2018, 110, 455–462. [Google Scholar] [CrossRef]
- Cernay, C.; Ber-Ari, T.; Pelzer, E.; Meynard, J.M.; Makowski, D. Estimating variability in grain legume yields across Europe and the Americas. Sci. Rep. 2015, 5, 11171. [Google Scholar] [CrossRef]
- Maalouf, F.; Hu, J.; O’Sullivan, D.; Zong, X.; Hamwieh, A.; Kumar, S.; Baum, M. Breeding and genomics status in faba bean (Vicia faba). Plant Breed. 2019, 138, 465–473. [Google Scholar] [CrossRef]
- Lake, L.; Godoy-Kutchartt, D.E.; Calderini, D.F.; Verrell, A.; Sadras, V.O. Yield determination and the critical period of faba bean (Vicia faba L.). Field Crop. Res. 2019, 241, 107575. [Google Scholar] [CrossRef]
- Alharbi, N.H.; Adhikari, K.N. Factors of yield determination in faba bean (Vicia faba). Crop Pasture Sci. 2020, 71, 305–321. [Google Scholar] [CrossRef]
- Bishop, J.; Potts, S.G.; Jones, H.E. Susceptibility of faba bean (Vicia faba L.) to heat stress during floral development and anthesis. J. Agro. Crop. Sci. 2016, 202, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.H.; Al-Khaishany, M.Y.; Al-Qutami, M.A.; Al-Whaibi, M.H.; Grover, A.; Ali, H.M.; Al-Wahibi, M.S.; Bukhari, N.A. Response of different genotypes of faba bean plant to drought stress. Int. J. Mol. Sci. 2015, 16, 10214–10227. [Google Scholar] [CrossRef] [PubMed]
- Temesgen, T.; Keneni, G.; Sefera, T.; Jarso, M. Yield stability and relationships among stability parameters in faba bean (Vicia faba L.) genotypes. Crop J. 2015, 3, 258–268. [Google Scholar] [CrossRef]
- Zhou, R.; Hyldgaard, B.; Yu, X.; Rosenqvist, E.; Ugarte, R.M.; Yu, S.; Wu, Z.; Ottosen, C.; Zhao, T. Phenotyping of faba beans (Vicia faba L.) under cold and heat stresses using chlorophyll fluorescence. Euphytica 2018, 214, 68. [Google Scholar] [CrossRef]
- Manning, B.K.; Adhikari, K.N.; Trethowan, R. Impact of sowing time, genotype, environment and maturity on biomass and yield components in faba bean (Vicia faba). Crop Pasture Sci. 2020, 71, 147–154. [Google Scholar] [CrossRef]
- El-Hendawy, S.; Shaban, W.; Sakagami, J.I. Does treating faba bean seeds with chemical inducers simultaneously increase chocolate spot disease resistance and yield under field conditions? Turk. J. Agric. For. 2010, 34, 475–485. [Google Scholar] [CrossRef]
- Brauna-Morževska, E.; Bankina, B.; Kaneps, J. Botrytis genus fungi as causal agents of legume diseases: A review. Res. Rural Dev. 2019, 2, 63–69. [Google Scholar] [CrossRef]
- Chang, K.F.; Conner, R.L.; Hwang, S.F.; Ahmed, H.U.; McLaren, D.L.; Gossen, B.D.; Turnbull, G.D. Effects of seed treatments and inoculum density of Fusarium avenaceum and Rhizoctonia solani on seedling blight and root rot of faba bean. Can. J. Plant Sci. 2014, 94, 693–700. [Google Scholar] [CrossRef]
- Ninou, E.; Tsialtas, J.T.; Dordas, C.A.; Papakosta, D.K. Effect of irrigation on the relationships between leaf gas exchange related traits and yield in dwarf dry bean grown under Mediterranean conditions. Agr. Water Manag. 2013, 116, 235–241. [Google Scholar] [CrossRef]
- Abid, G.; Hessini, K.; Aouida, M.; Aroua, I.; Baudoin, J.P.; Muhovski, Y.; Mergeai, G.; Sassi, K.; Machraoui, M.; Souissi, F.; et al. Agro-physiological and biochemical responses of faba bean (Vicia faba L. var. ‘minor’) genotypes to water deficit stress. Biotechnol. Agron. Soc. Environ. 2017, 21, 1–14. [Google Scholar]
- Liu, J.; Fan, Y.; Ma, Y.; Li, Q. Response of photosynthetic active radiation interception, dry matter accumulation, and grain yield to tillage in two winter wheat genotypes. Arch. Agron. Soil Sci. 2020, 66, 1103–1114. [Google Scholar] [CrossRef]
- Confalone, A.; Lizaso, J.I.; Ruiz-Nogueira, B.; Lopez-Cedron, F.X.; Sau, F. Growth, PAR use efficiency, and yield components of field-grown Vicia faba L. under different temperature and photoperiod regimes. Field Crop. Res. 2010, 115, 140–148. [Google Scholar] [CrossRef]
- Sandana, P.; Ramirez, M.; Pinochet, D. Radiation interception and radiation use efficiency of wheat and pea under different P availabilities. Field Crop. Res. 2012, 127, 44–50. [Google Scholar] [CrossRef]
- Barlog, P.; Grzebisz, W.; Lukowiak, R. Faba bean yield and growth dynamics in response to soil potassium availability and sulfur application. Field Crop. Res. 2018, 219, 87–97. [Google Scholar] [CrossRef]
- Statistics Lithuania. Official Statistics Portal: Agriculture. 2020. Available online: https://osp.stat.gov.lt/en_GB/zemes-ukis1 (accessed on 23 November 2020).
- FAOSTAT 2018. Database. Food and Agriculture Organization of the United Nations. Available online: www.fao.org/faostat/ (accessed on 23 November 2020).
- Rohaček, K.; Soukupova, J.; Bartak, M. Chlorophyll fluorescence: A wonderful tool to study plant physiology and plant stress. In Plant Cell Compartments—Selected Topisc; Schoefs, B., Ed.; Res Signpost: Kerala, India, 2008; pp. 41–104. [Google Scholar]
- Cosentino, S.L.; Patane, C.; Sanzone, E.; Testa, G.; Scordia, D. Leaf gas exchange, water status and radiation use efficiency of giant reed (Arundo donax L.) in a changing soil nitrogen fertilization and soil water availability in a semi-arid Mediterranean area. Europ. J. Agron. 2016, 72, 56–69. [Google Scholar] [CrossRef]
- Saeidi, M.; Abdoli, M. Effect of drought stress during grain filling on yield and its components, gas exchange variables, and some physiological traits of wheat cultivars. J. Agric. Sci. Tech. 2015, 17, 885–898. [Google Scholar]
- Liu, H.; Song, J.; Dong, L.; Wang, D.; Zhang, S.; Liu, J. Physiological responses of three soybean species (Glycine soja, G. gracilis, and G. max cv. Melrose) to salinity stress. J. Plant Res. 2017, 130, 723–733. [Google Scholar] [CrossRef]
- Dirsė, A.; Taparauskienė, L. Humidity fluctuations in plant vegetation periods and a comparison of its assessment methods. Žemės ūkio Moksl. 2010, 17, 9–17. (in Lithuanian). [Google Scholar]
- Anyia, A.O.; Herzog, H. Water-use efficiency, leaf area and leaf gas exchange of cowpeas under mid-season drought. Eur. J. Agron. 2004, 20, 327–339. [Google Scholar] [CrossRef]
- Zeleke, K.; Nendel, C. Growth and yield response of faba bean to soil moisture regimes and sowing dates: Field experiment and modelling study. Agric. Water Manag. 2019, 213, 1063–1077. [Google Scholar] [CrossRef]
- Girma, F.; Haile, D. Effects of supplemental irrigation on physiological parameters and yield of faba bean (Vicia faba L.) varieties in the highlands of Bale, Ethiopia. J. Agron. 2014, 1–6. [Google Scholar] [CrossRef]
- Mansour, E.; Desoky, E.M.; Ali, M.M.A.; Abdul-Hamid, M.I.; Ullah, H.; Attia, A.; Datta, A. Identifying drought-tolerant genotypes of faba bean and their agro-physiological responses to different water regimes in an arid Mediterranean environment. Agric. Water Manag. 2021, 247, 106754. [Google Scholar] [CrossRef]


| Variety | Country of Origin | Company | Growing Season Duration (Day) | Thousand Seed Weight (g) | Protein (%) |
|---|---|---|---|---|---|
| Bioro | Austria | Saatzuchtbetrieb Haus Gahleitner | 103 | 519 | 32.0 |
| Nida DS | Lithuania | Institute of Agriculture, Lithuanian Research Centre for Agriculture and Forestry | 103 | 531 | 30.3 |
| Reda DS | Lithuania | Institute of Agriculture, Lithuanian Research Centre for Agriculture and Forestry | 113 | 561 | 33.1 |
| Fuego | Germany | Norddeutsche Pflanzenzucht Hans–Georg Lembke KG | 114 | 654 | 32.7 |
| Julia | Austria | Saatzucht Gleisdorf GmbH | 103 | 545 | 29.5 |
| Alexia | Austria | Saatzucht Gleisdorf GmbH | 98 | 582 | 29.7 |
| Boxer | Sweden | Lantmännen ek. för. | 98 | 627 | 27.7 |
| Laura | Sweden | Lantmännen ek. för. | 107 | 591 | 27.7 |
| Isabell | Sweden | Lantmännen ek. för. | 106 | 596 | 28.2 |
| Indices | Seed Fungicide (A) | Growth Stage (B) | Variety (C) | A × B | A × C | B × C | A × B × C | Total |
|---|---|---|---|---|---|---|---|---|
| 1st season | ||||||||
| SPAD | 0.02 | 0.9 * | 13.2 ** | 0.3 | 2.1 | 6.3 ** | 1.1 | 23.9 |
| Fv/Fm | 1.4 * | 2.6 ** | 8.4 ** | 0.01 | 3.9 * | 2.3 | 3.7 | 22.3 |
| A | 6.2 ** | 0.0 | 9.1 * | 0.03 | 8.9 * | 22.7 ** | 20.0 ** | 66.9 |
| E | 0.9 | 0.6 | 22.1 ** | 3.2 ** | 20.7 ** | 18.7 ** | 9.6 ** | 75.9 |
| WUE | 0.4 | 3.7 * | 10.1 * | 0.02 | 17.6 ** | 11.2 * | 12.2 * | 55.8 |
| PWUE | 0.1 | 5.8 ** | 23.8 ** | 1.1 | 3.2 | 8.2 | 17.7 ** | 59.9 |
| gs | 0.2 | 2.3 ** | 70.7 ** | 0.3 | 9.2 ** | 4.7 ** | 2.6 * | 89.9 |
| Ci | 0.9 | 0.9 | 25.6 ** | 0.01 | 4.7 | 11.8 ** | 22.9 ** | 66.9 |
| gm | 42.0 ** | 0.4 | 14.5 ** | 0.04 | 8.3 * | 17.5 ** | 20.7 ** | 65.5 |
| Ls | 0.9 | 0.2 | 22.8 ** | 0.0 | 4.9 | 12.6 ** | 24.3 ** | 65.7 |
| 2nd season | ||||||||
| LAI | 0.3 ** | 85.9 ** | 1.5 ** | 0.8 ** | 2.2 ** | 1.8 ** | 2.2 ** | 94.6 |
| SPAD | 0.3 | 1.7 ** | 25.5 ** | 0.2 | 4.1 ** | 1.8 | 1.8 | 35.5 |
| Fv/Fm | 0.001 | 1.8 ** | 4.8 ** | 0.02 | 6.9 ** | 5.1 ** | 5.2 ** | 23.8 |
| A | 0.002 | 15.9 ** | 14.1 ** | 0.04 | 16.0 ** | 22.0 ** | 13.5 ** | 81.6 |
| E | 4.2 ** | 5.0 ** | 27.5 ** | 1.8 ** | 21.2 ** | 18.4 ** | 8.3 * | 86.5 |
| WUE | 1.2 | 3.4 ** | 23.2 ** | 3.2 ** | 18.9 ** | 15.2 ** | 8.6 ** | 73.7 |
| PWUE | 0.5 | 0.04 | 10.4 ** | 1.8 | 11.5 * | 16.4 ** | 22.4 * | 63.1 |
| gs | 0.2 | 24.9 ** | 5.1 | 0.6 | 15.4 ** | 14.9 ** | 15.9 ** | 77.0 |
| Ci | 1.8 ** | 31.4 ** | 23.2 ** | 0.1 | 12.7 ** | 14.6 ** | 9.3 ** | 93.1 |
| gm | 0.6 | 4.5 ** | 16.6 ** | 0.12 | 16.3 ** | 26.3 ** | 17.0 ** | 81.3 |
| Ls | 1.8 ** | 31.2 ** | 22.4 ** | 0.1 | 12.7 ** | 15.3 ** | 9.6 ** | 93.0 |
| Factor | SPAD | Fv/Fm | A | E | WUE | PWUE | gs | Ci | gm | Ls |
|---|---|---|---|---|---|---|---|---|---|---|
| 1st season | ||||||||||
| Averaged across GS and varieties a | ||||||||||
| Without SF | 41.0 b | 0.557 b | 2.46 b | 0.33 b | 17.9 b | 102 b | 0.049 b | 221 b | 0.012 b | 0.454 b |
| With SF | 40.9 b | 0.524 c | 3.08 a | 0.39 a | 22.9 b | 108 b | 0.046 b | 232 b | 0.016 a | 0.427 b |
| Averaged across SF treatments and varieties a | ||||||||||
| BBCH 62–63 | 41.4 b | 0.518 b | 2.77 b | 0.33 b | 28.5 b | 80 b | 0.054 b | 221 b | 0.013 b | 0.446 b |
| BBCH 69–71 | 40.5c | 0.563 a | 2.77 b | 0.38 b | 12.4 c | 129 a | 0.042 c | 232 b | 0.014 b | 0.435 b |
| Averaged across SF treatments and GS b | ||||||||||
| Bioro | 39.7 c | 0.517 b | 2.79 b | 0.26 b | 13.6 b | 21c | 0.143 a | 240 b | 0.013 b | 0.406 b |
| Nida DS | 39.7 c | 0.619 a | 2.69 b | 0.31 b | 17.4 b | 72 b | 0.045 b | 229 b | 0.014 b | 0.447 b |
| Reda DS | 37.4 c | 0.562 b | 2.68 b | 0.17 c | 25.6 b | 102 b | 0.035 c | 247 b | 0.011 b | 0.399 b |
| Fuego | 41.4 b | 0.572 b | 3.00 b | 0.42 b | 12.7 b | 127 b | 0.035 c | 283 a | 0.012 b | 0.311 c |
| Julia | 42.6 a | 0.483 c | 3.54 a | 0.22 c | 39.3 a | 160 a | 0.034 c | 194 c | 0.021 a | 0.518 a |
| Alexia | 43.0 a | 0.553 b | 2.35 b | 0.63 a | 7.6 b | 74 b | 0.035 c | 250 a | 0.011 b | 0.381 c |
| Boxer | 41.5 b | 0.539 b | 3.07 b | 0.44 b | 9.2 b | 201 a | 0.028 c | 199 c | 0.018 a | 0.499 a |
| Laura | 41.6 b | 0.535 b | 2.56 b | 0.53 a | 10.3 b | 95 b | 0.035 c | 209 b | 0.013 b | 0.474 b |
| Isabell | 41.6 b | 0.482 c | 2.25 c | 0.23 c | 48.2 a | 90 b | 0.041 c | 189 c | 0.013 b | 0.527 a |
| Trial mean | 41.0 | 0.540 | 2.77 | 0.36 | 20.4 | 105 | 0.048 | 227 | 0.014 | 0.440 |
| 2nd season | ||||||||||
| Averaged across GS and varieties a | ||||||||||
| Without SF | 45.4 b | 0.658 b | 3.06 b | 0.26 b | 21.2 b | 165 b | 0.018 b | 239 b | 0.014 b | 0.423 b |
| With SF | 44.9 b | 0.657 b | 3.04 b | 0.35 a | 15.9 b | 172 b | 0.018 b | 260 a | 0.013 b | 0.372 c |
| Averaged across SF treatments and varieties a | ||||||||||
| BBCH 62–63 | 45.8 b | 0.670 b | 3.93 b | 0.26 b | 22.8 b | 169 b | 0.022 b | 292 b | 0.016 b | 0.292 b |
| BBCH 69–71 | 44.5 c | 0.644 c | 2.16 c | 0.35 a | 14.2 c | 167 b | 0.014 c | 206 c | 0.011 c | 0.504 a |
| Averaged across SF treatments and GS b | ||||||||||
| Bioro | 46.2 b | 0.635 b | 4.59 a | 0.18 c | 36.0 a | 187 b | 0.023 a | 210 c | 0.022 a | 0.498 a |
| Nida DS | 42.0 c | 0.660 b | 3.10 b | 0.57 a | 6.2 c | 180 b | 0.018 b | 320 a | 0.011 b | 0.232 c |
| Reda DS | 43.0 c | 0.698 a | 2.06 c | 0.36 b | 8.3 c | 155 b | 0.015 c | 301 a | 0.007 c | 0.268 c |
| Fuego | 42.1 c | 0.675 b | 4.34 a | 0.21 c | 39.4 a | 199 a | 0.019 b | 246 b | 0.019 a | 0.411 b |
| Julia | 49.0 a | 0.674 b | 3.01 b | 0.33 b | 11.3 b | 178 b | 0.018 b | 221 c | 0.014 b | 0.466 a |
| Alexia | 48.4 a | 0.630 c | 3.05 b | 0.30 b | 16.4 b | 160 b | 0.019 b | 230 c | 0.014 b | 0.443 a |
| Boxer | 44.5 b | 0.640 b | 2.16 c | 0.28 b | 20.8 b | 147 b | 0.016 b | 206 c | 0.012 b | 0.498 a |
| Laura | 46.1 b | 0.642 b | 2.44 b | 0.18 c | 18.8 b | 158 b | 0.018 b | 264 a | 0.011 b | 0.360 c |
| Isabell | 45.1 b | 0.661 b | 2.69 b | 0.33 b | 9.7 c | 150 b | 0.018 b | 245 b | 0.011 b | 0.404 b |
| Trial mean | 45.1 | 0.657 | 3.05 | 0.30 | 18.5 | 168 | 0.018 | 249 | 0.013 | 0.398 |
| LAI | PAR Capture Ratio % | PAR Penetration Ratio % | |||||||
|---|---|---|---|---|---|---|---|---|---|
| BBCH 60 | BBCH 62–63 | BBCH 69–71 | BBCH 60 | BBCH 62–63 | BBCH 69–71 | BBCH 60 | BBCH 62–63 | BBCH 69–71 | |
| Averaged across varieties a | |||||||||
| Without SF | 1.29 b | 4.18 b | 3.63 b | 82.2 b | 92.7 b | 90.6 b | 15.4 b | 5.2 b | 5.3 b |
| With SF | 1.14 c | 3.76 c | 3.78 b | 79.7 c | 90.8 c | 91.3 b | 17.3 b | 7.9 a | 7.5 a |
| Averaged across SF treatments b | |||||||||
| Bioro | 1.27 b | 4.63 a | 3.98 a | 81.8 b | 94.5 a | 92.5 a | 15.2 b | 3.8 c | 4.4 c |
| Nida DS | 0.90 c | 3.63 c | 3.75 b | 77.5 b | 89.9 c | 91.3 b | 20.2 b | 8.1 a | 8.4 a |
| Reda DS | 1.13 b | 3.63 c | 3.60 b | 82.3 b | 89.7 c | 90.2 b | 14.2 b | 9.0 a | 7.0 a |
| Fuego | 1.20 b | 3.75 b | 3.57 b | 83.0 b | 90.9 b | 90.2 b | 15.1 b | 7.4 b | 7.2 b |
| Julia | 1.15 b | 3.93 b | 3.63 b | 79.3 b | 91.3 b | 90.7 b | 17.1 b | 6.8 b | 6.2 b |
| Alexia | 1.03 c | 4.43 a | 3.48 b | 73.9 c | 93.6 a | 89.8 b | 22.5 a | 4.8 c | 4.8 c |
| Boxer | 1.57 a | 3.95 b | 3.77 b | 86.9 a | 92.0 b | 91.1 b | 11.4 c | 6.7 b | 6.8 b |
| Laura | 1.28 b | 3.92 b | 3.55 b | 80.3 b | 92.1 b | 90.2 b | 17.2 b | 5.7 b | 6.7 b |
| Isabell | 1.42 a | 3.83 b | 4.02 a | 83.2 b | 91.4 b | 92.4 a | 13.4 b | 6.7 b | 6.1 b |
| Trial mean | 1.22 | 3.97 | 3.71 | 80.9 | 91.7 | 90.9 | 16.3 | 6.6 | 6.4 |
| Contribution (% of sum of square) of SF, variety and their interaction and significance | |||||||||
| SF (A) | 5.8 * | 9.4 ** | 3.0 ns | 6.1 * | 9.0 ** | 2.3 ns | 3.3 | 9.0 ** | 9.0 ** |
| Variety (B) | 35.0 ** | 21.8 ** | 20.1 * | 26.5 ** | 20.6 ** | 22.1 * | 26.5 ** | 20.6 ** | 20.6 ** |
| A × B | 15.9 * | 39.6 ** | 16.2 ns | 18.1 * | 43.4 ** | 15.7 ns | 18.1 * | 43.4 ** | 43.4 ** |
| 1st Season | 2nd Season | |||||||
|---|---|---|---|---|---|---|---|---|
| Factor | Plant Height (cm) | Seed Yield (t ha−1) | TSW (g) | Protein (%) | Plant Height (cm) | Seed Yield (t ha−1) | TSW (g) | Protein (%) |
| Average across varieties a | ||||||||
| Without SF | 116 b | 3.67 b | 482.0 b | 28.1 b | 94 b | 3.30 b | 518.4b | 25.9 b |
| With SF | 120 a | 3.70 b | 487.3 b | 28.6 a | 93 b | 3.23 b | 530.2a | 26.2 a |
| Average across SF treatments b | ||||||||
| Bioro | 134 a | 3.09 c | 420.1 c | 30.1 a | 109 a | 3.08 c | 482.7 c | 27.7 a |
| Nida DS | 114 c | 3.69 b | 457.8 c | 29.8 a | 91 b | 2.92 c | 488.9 c | 27.4 a |
| Reda DS | 123 a | 3.54 c | 463.0 c | 28.3 b | 94 b | 3.20 b | 533.1 b | 27.1 a |
| Fuego | 120 b | 4.13 a | 530.1 a | 28.5 b | 89 c | 3.57 a | 571.7 a | 25.3 c |
| Julia | 128 a | 3.64 b | 480.2 b | 28.6 b | 101 a | 3.38 b | 526.0 b | 26.6 a |
| Alexia | 101 c | 3.57 b | 436.1 c | 26.7 c | 84 c | 3.40 a | 483.3 c | 24.2 c |
| Boxer | 131 a | 3.65 b | 521.3 a | 28.3 b | 98 a | 3.38 b | 495.9 c | 24.8 c |
| Laura | 115 b | 3.76 b | 514.5 a | 26.0 c | 92 b | 3.13 c | 555.9 a | 24.4 c |
| Isabell | 98 c | 4.07 a | 539.0 a | 29.1 a | 87 c | 3.35 b | 581.9 a | 26.7 a |
| Trial mean | 118 | 3.68 | 484.7 | 28.4 | 94 | 3.27 | 524.4 | 26.0 |
| Contribution (% of sum of square) of SF, variety and their interaction and significance | ||||||||
| SF (A) | 1.5 ** | 0.1 ns | 0.3 ns | 1.9 ** | 0.1 ns | 1.2 ns | 1.8 ** | 0.7 ** |
| Variety (B) | 40.7 ** | 46.1 ** | 73.0 ** | 44.6 ** | 23.9 ** | 32.7 ** | 68.6 ** | 48.2 ** |
| A × B | 10.3 ** | 18.5 ** | 1.9 ns | 10.8 ** | 1.9 ns | 8.2 * | 0.8 ns | 6.4 ** |
| SPAD | Fv/Fm | A | E | WUE | PWUE | gs | Ci | gm | Ls | |
|---|---|---|---|---|---|---|---|---|---|---|
| SY | 0.348 ** | 0.504 ** | 0.060 | 0.072 | 0.138 | 0.263 * | −0.256 ** | 0.107 | 0.069 | 0.241 |
| Protein | 0.606 ** | 0.565 ** | 0.007 | 0.035 | 0.012 | 0.388 ** | −0.079 | 0.553 ** | 0.073 | 0.033 |
| TSW | 0.446 ** | 0.469 ** | 0.070 | 0.010 | 0.024 | 0.489 ** | 0.107 | 0.576 ** | 0.004 | −0.073 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janusauskaite, D.; Razbadauskiene, K. Comparison of Productivity and Physiological Traits of Faba Bean (Vicia faba L.) Varieties under Conditions of Boreal Climatic Zone. Agronomy 2021, 11, 707. https://doi.org/10.3390/agronomy11040707
Janusauskaite D, Razbadauskiene K. Comparison of Productivity and Physiological Traits of Faba Bean (Vicia faba L.) Varieties under Conditions of Boreal Climatic Zone. Agronomy. 2021; 11(4):707. https://doi.org/10.3390/agronomy11040707
Chicago/Turabian StyleJanusauskaite, Daiva, and Kristyna Razbadauskiene. 2021. "Comparison of Productivity and Physiological Traits of Faba Bean (Vicia faba L.) Varieties under Conditions of Boreal Climatic Zone" Agronomy 11, no. 4: 707. https://doi.org/10.3390/agronomy11040707
APA StyleJanusauskaite, D., & Razbadauskiene, K. (2021). Comparison of Productivity and Physiological Traits of Faba Bean (Vicia faba L.) Varieties under Conditions of Boreal Climatic Zone. Agronomy, 11(4), 707. https://doi.org/10.3390/agronomy11040707






